Abstract
Structures responsible for the onset, propagation, and cessation of generalized seizures are not known. Lesion and microinfusion studies suggest that the substantia nigra pars reticulata (SNR) seizure-controlling network could play a key role. However, the expression of neural activity within the SNR and its targets during discrete pre- and postictal periods has not been investigated. In rats, we used flurothyl to induce generalized seizures over a controlled time period and 2-deoxyglucose autoradiography mapping technique. Changes in neural activity within the SNR were region-specific. The SNRposterior was selectively active during the pre-clonic period and may represent an early gateway to seizure propagation. The SNRanterior and superior colliculus changed their activity during progression to tonic-clonic seizure, suggesting the involvement in coordinated regional activity that results in inhibitory effects on seizures. The postictal suppression state was correlated with changes in the SNR projection targets, specifically the pedunculopontine tegmental nucleus and superior colliculus.
Keywords: Autoradiography, Basal ganglia, Deoxyglucose, Flurothyl-induced primary generalized seizures, Seizure stages, Substantia nigra
Introduction
The basal ganglia are involved in many types of seizures. A large body of evidence suggests that specifically the substantia nigra pars reticulata (SNR) plays a major role in the modulation and propagation of seizures (Garant and Gale, 1983; Iadarola and Gale, 1982). Earlier studies using microinfusions or lesions suggested that the GABAergic SNR projections to the superior colliculus (SC) and pedunculopontine tegmental nucleus (PPTg) may be especially influential; interruption of nigral inhibitory effects on these projections by SNR lesions or muscimol (an agonist at GABAA receptor sites) infusions is anticonvulsant, and accordingly bicuculline (an antagonist at GABAA receptor sites) injection into the SC or PPTg has an anticonvulsant effect (Depaulis et al., 1990; Okada et al., 1989; Redgrave et al., 1992). Thus, blockade of nigral inhibitory effects on these structures hinders seizure development.
However, investigators also found that there are two anatomically discrete regions in the SNR of adult rats, the SNRanterior and the SNRposterior (Fan et al., 1997; Moshé et al., 1994; Shehab et al., 1996; Thompson et al., 2000; Velíšková and Moshé, 2001). These two regions mediate separate facilitatory or inhibitory effects on seizures in response to localized microinfusions of agents that modulate GABAA receptor neurotransmission (Moshé et al., 1994; Thompson et al., 2000; Velíšková and Moshé, 2001; Velíšková et al., 1996), and glutamatergic or dopaminergic systems (Fan et al., 1997; Velíšková et al., 2001). The factors responsible for the differential SNR effects on seizures may be intrinsic cell type and receptor differences between the two regions (Galanopoulou et al., 2003; Hedberg et al., 2003; Ravizza et al., 2003; Velíšek et al., 2005; Velíšková et al., 1998) or distinct efferent targets of the SNRanterior or SNRposterior cells (Moshé et al., 1994). The differentiation is important because it suggests inhibitory and excitatory mechanisms controlled from two different subregions of a basal ganglia nucleus that could have a large influence on seizure suppression or progression.
To determine that two SNR regions do, indeed, play a significant role in seizures, we asked: Do the neural events during individual seizure stages show that the SNRanterior and SNRposterior are separable regions? Although others have examined seizure stages in pilocarpine seizures or maximal electroshock using [14C]2-deoxyglucose (2 DG) autoradiography, advanced seizure stages developed quickly, making it difficult to discriminate individual seizure stages (Andre et al., 2002; Handforth and Treiman, 1995a,b). Therefore, we used the flurothyl seizure model, which permitted us to control the onset of individual seizure stages by prolonging the pre-clonic and pre-tonic-clonic stages and 2 DG autoradiography (Ackermann et al., 1986; Sokoloff et al., 1977). Flurothyl is a volatile convulsant (Prichard et al., 1969). By changing the rate of flurothyl delivery, the onset of seizures can be modulated (Lánský et al., 1997). The 2 DG procedure allowed us to identify changes in neural activity, not only in the SNR, but also in nigral afferent and efferent targets and in other basal ganglia nuclei potentially involved in seizures. We focused on the deep layers of the SC, PPTg, and ventromedial nucleus of the thalamus (VM), which are projection targets of the SNRanterior and SNRposterior (Bolam et al., 2000).
Materials and methods
Animals
Forty-nine adult male Sprague-Dawley rats (Taconic Farm, NY) were used. Rats were housed at a constant temperature (23°C) and relative humidity (60%) with a fixed 12-h light-dark cycle (light on at 7:00) and free access to food and water in our AAALAC-approved animal facility. On the day before the 2 DG experiment, food but not water was withdrawn to keep plasma glucose levels relatively low and to maximize 2 DG uptake. All experimental procedures were approved by our institutional animal care committee.
Flurothyl-induced seizures
Flurothyl is a volatile convulsant agent established as an effective tool to measure the brain’s threshold to primary generalized seizures (Prichard et al., 1969). Continuous administration into a closed chamber and thus inhalation of flurothyl produces two types of sequential seizures. First, a clonic seizure occurs consisting of facial and forelimb clonus with preservation of the righting reflex; the seizure lasts 15-45 s. The rat may experience several clonic seizures. These clonic seizures originate in the forebrain structures (Browning, 1985). Subsequently, a tonic-clonic seizure occurs consisting of loss of righting reflex, tonic flexion, or extension of all four limbs lasting 5-10 s and followed by clonus of all four limbs lasting as long as flurothyl is delivered (Sperber et al., 1999; Velíšek et al., 1995). Development of tonic-clonic seizures suggests spread of the ictal activity into the brainstem (Browning, 1985). Cessation of flurothyl administration at any point leads to the termination of behavioral seizures including EEG discharges (Sperber et al., 1999).
To induce seizures, each rat was placed in an airtight chamber(9.38 l), and flurothyl was delivered via a Harvard pump. Delivery rate was adjusted for each experimental group so that the duration of the testing period was always 20 min. The 20-min time period was chosen because there was a high mortality rate in adult rats during the ictal state when seizures were maintained longer.
Deoxyglucose autoradiography
To examine functional neural activity (Sokoloff et al., 1977) over the entire 20 min studied, we injected the rats subcutaneously with 2 DG (0.05 μCi/g, Amersham) as described previously by others and us (Simmons et al., 1998; Velíšek et al., 2005). The advantage of the subcutaneous injection for seizure development studies is that uptake is continuous and therefore samples the entire post-injection time period compared to an i.v. bolus injection, which is more suitable for stable states (Simmons et al., 1998). In addition, the subcutaneous administration is less invasive compared to the intravenous injection, thus minimizing the stress of the animals. At the end of the experiment, we injected an overdose of sodium pentobarbital (100 mg/kg, i.p.), removed the brain, immediately froze it in methylbutane chilled to -35°C, and then stored at -70°C. Coronal sections 30 μm thick were cut in a cryostat, mounted on slides, and dried on a hot plate at 60°C. Five sections were collected every 300 μm through the entire brain. Slides were placed in contact with X-ray film (Kodak, BioMax, MR) in a cassette. The films were developed after 10 days of exposure.
Experimental groups
The choice of experimental groups was based on the behavioral expression of motor seizures. In a pilot study, we determined the correlation of behavioral patterns of flurothyl seizures and ictal EEG activity. The clonic seizure onset was preceded by bursts of short episodes of rhythmic EEG discharges in the motor cortex. Cessation of any behavioral motor seizure was associated with simultaneous termination of EEG discharges (see also Sperber et al., 1999).
2 DG controls not exposed to flurothyl (n = 10)
Each rat was injected with 2 DG and placed in the chamber without any flurothyl administration and sacrificed 20 min later. The behavior included occasional grooming, rearing, and sniffing during the first 5 min, and then the rat usually sat calmly in the rear corner.
2 DG controls for pre-clonic/tonic-clonic stages exposed to flurothyl with no seizures (n = 6)
2 DG was injected. Rats were placed in the chamber. The rate of flurothyl delivery was set to 5 μl/min so that the rats did not develop any seizure behavior for 20 min. The behavior did not differ from the previous control group, which was not exposed to flurothyl. Rats were sacrificed immediately after removal from the chamber.
Pre-clonic (n = 6)
2 DG was injected. Rats were placed in the chamber. The rate of flurothyl delivery was set to 10 μl/min so that the first clonic seizure occurred in approximately 20 min. For the first 19 min, the behavior of the rats did not differ from controls. Then, occasional twitches began about 1 min before the clonic seizure evolved. As soon as the seizure started, the rat was sacrificed.
Pre-tonic-clonic (n = 6)
2 DG was injected. Rats were placed in the chamber. The rate of flurothyl delivery was set to 20 μl/min so that the rats developed a tonic-clonic seizure in 20 min. As soon as the tonic-clonic seizure began, rats were sacrificed. These rats also experienced 1-3 clonic seizures prior to the tonic seizure. The first clonic seizure occurred after about 7 min.
Ictal (n = 7)
2 DG was injected. Rats were placed in the chamber. The rate of flurothyl delivery was set to 50 μl/min so that the rats would undergo tonic-clonic seizures within 3-5 min. Then, the flurothyl rate was set to 20 μl/min, a sufficient dose of flurothyl to maintain continuous seizures for the rest of the 20 min period until the rats were sacrificed.
Controls for post-clonic/tonic-clonic stage, exposed to flurothyl, no seizures (n = 8)
Rats were placed in the chamber. The rate of flurothyl delivery was set to 5 μl/min so that the rats did not develop any seizure behavior for 20 min. Rats were removed from the chamber, injected with 2 DG, and sacrificed 20 min later.
Post-clonic (n = 6)
Rats were placed in the chamber. The rate of flurothyl delivery was set to 20 μl/min. A clonic seizure was induced. As soon as the seizure began, the rat was removed from the chamber. The seizure spontaneously stopped within 15-25 s, and the rat was injected immediately with 2 DG. No additional seizure occurred. During the testing period, the rat was sitting without any movement. Rats were sacrificed 20 min after the 2 DG injection.
Post-tonic-clonic (n = 6)
Rats were placed in the chamber. The rate of flurothyl delivery was set to 20 μl/min. A tonic-clonic flurothyl seizure was induced. As soon as the seizure began, the rats were removed from the chamber. After 3-4 min, the seizure spontaneously stopped, and rats were injected immediately with 2 DG. No additional seizure occurred. During the testing period, the rat was lying without any movement. Rats were sacrificed 20 min after the 2 DG injection.
Plasma glucose measurements
Seizures are accompanied by a significant increase in plasma glucose levels (Handforth and Treiman, 1995b; Schwechter et al., 2003). What can appear as decreased metabolism postictally may be largely the competitive effects of high plasma glucose on 2 DG uptake. To help us with our estimates of change in neural activity following a seizure, we measured plasma glucose levels. Rats were placed in the flurothyl chamber to induce clonic (n = 3) or tonic-clonic (n = 3) seizures. Controls with (n = 5) or without flurothyl exposure (n = 5) were used. Glucose level measurements were taken prior to a seizure, immediately following the seizure and 20 min after seizure induction. Blood samples were obtained from tip of the tail (∼10 μl) as described previously (Schwechter et al., 2003). The baseline value for all groups was 46 ± 4 mg/100 ml (mean ± SEM), which is relatively low because the animals did not receive food for up to 20 h before the experiment. The postictal values did not exceed 150 mg/100 ml, suggesting that hyperglycemia does not account for the large decrease in uptake we observed postictally.
Autoradiogram analysis
The analysis was performed as described in detail previously (Velíšek et al., 2005). Briefly, brain samples from rats from all groups were exposed on the same film. This allowed us visually to compare the global metabolic activity between individual stages. Autoradiograms were transilluminated on a light box and scanned using a CCD video camera and NIH image software (Wayne Rasband, NIH). To assess the regional changes in neural activity during different seizure stages, we compared the patterns of the 2 DG uptake by visual inspection, and we quantified changes in selected regions. For group comparisons, we used normalized 2 DG uptake because subcutaneous administration of 2 DG precludes absolute quantification of glucose uptake possible only if indwelling catheters are used (Sokoloff et al., 1977). Data were acquired in calibrated optical density values. For each region of interest (ROI) within a slice, we quantified the patterns of the 2 DG uptake by using densitometric ratios of the ROI compared to the whole brain slice density. We used these ratios to correct for variability among animals and films. These ratios were then used for group comparisons. We first calculated the mean radioactivity value for the entire brain slice at each level we measured. There was no difference among the pre-seizure groups and controls, and thus the group comparisons were valid without further consideration. How-ever, the mean radioactivity value for the entire brain slice was significantly different in the post-seizure groups compared to their controls (P < 0.05). We expected this effect because other studies have shown global decreases in glucose utilization in the postictal state (Ackermann et al., 1986). Thus, the ratios for the post-seizure groups were (1) considered as a general indicator of within-group consistency for conclusions drawn by visual inspection, and (2) decreases compared to controls were considered significant because the whole brain was decreased in the post-seizure groups, and any further decrease in the ratio from a ROI is most likely meaningful.
Regions measured
Our analysis was limited to regions of interest within the basal ganglia and its projection targets. We measured the dorsal part of caudate-putamen (CP); the globus pallidus interna (GPi; endopeduncular nucleus) (level: -0.92 mm from bregma); the globus pallidus externa (GPe) and VM (level: -2.3 mm from bregma); the STN (level: -3.8 mm from bregma); the SNRanterior (level: 4.8 mm from bregma); the SNRposterior and intermediate layer of SC (level: -6.04 mm from bregma); the PPTg (level: 8.0 mm from bregma).-on the atlas The levels for individual structures are based of Paxinos and Watson (1998).
Statistical analysis
Data were tested for normality using Kolmogorov-Smirnoff test. If normality was confirmed for all subgroups, ratios were compared within each group by either one-way ANOVA with post-hoc Fisher Protected Least Significant Difference test or with a Student’s t test (two-group comparisons). If Kolmogorov-Smirn-off test revealed non-Gaussian distribution, data were first transformed using logarithmic transformation and then compared as described above. For clarity, the results are presented as a percentage of increased or decreased 2 DG uptake compared to the identical ROI in appropriate controls. All values are mean ± SEM. Statistical significance was preset at P < 0.05.
Results
Each seizure state produced a characteristic pattern of 2 DG uptake and metabolism indicative of changes in neural activity. During the ictal state, autoradiographs showed a general increase in uptake and significant increases in the SNR as expected from earlier studies, while during the postictal states, we observed a general decrease similarly to others (Ackermann et al., 1986). In addition, controls with flurothyl exposure, but no seizure behavior, did not show any significant changes in any brain area studied when compared to rats without exposure to flurothyl. Thus, the effects shown are not caused by flurothyl alone rather by seizurerelated activity.
The substantia nigra pars reticulata
Pre-clonic/pre-tonic-clonic/ictal states
Visual inspection suggested that the SNRanterior and SNRposterior were differentially affected in the pre-clonic and pre-tonic-clonic stages (Fig. 1). Furthermore, one-way ANOVA of the ratio measurements revealed significant differences from controls during individual seizure states within the SNR [SNRanterior: F(3,19) = 13.92, P < 0.0001; SNRposterior: F(3,19) = 9.65, P = 0.0004]. Pairwise post-hoc comparisons showed that, during the pre-clonic state, only the SNRposterior increased 2 DG uptake significantly compared to controls (+8%; P < 0.05; Fig. 3A). During the pre-tonic-clonic state, both SNR regions showed a significant increase in 2 DG uptake (+15.3%, in the SNRanterior P < 0.05 and +10.5% for SNRposterior P < 0.05; Fig. 3A). During the ictal state, 2 DG uptake was significantly elevated in both SNR regions compared to controls (+31.5% in the SNRanterior, P < 0.05 and +20% for SNRposterior, P < 0.05; Fig. 3B).
Fig. 1.
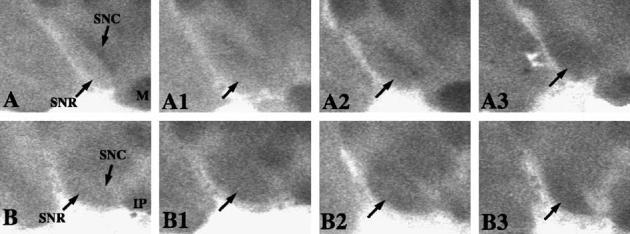
Preictal and ictal regional SNR activity. Coronal section autoradiograms depict changes in 2 DG accumulation and glucose utilization during preictal and ictal seizure stages. Top panels are examples of activity in the SNRanterior. Bottom panels are examples of activity in the SNRposterior. (A) Control SNRanterior. (A1) Pre-clonic SNRanterior (arrow). Compared to the control in panel (A), there is little difference in gray levels. (A2) Pre-tonic-clonic SNRanterior (arrow). Activity increased compared to control. (A3) Ictal SNRanterior (arrow). Uptake and glucose utilization increased over control (bars in inset). (B) Control SNRposterior. (B1) Pre-clonic SNRposterior. Note the increased 2 DG uptake in the reticulata region (arrow) compared to the control in panel (B). (B2) Pre-tonic-clonic SNRposterior (arrow). Note the increase in 2 DG uptake in the reticulata region compared to control in panel (B). (B3) Ictal SNRanterior (arrow). Note the increased 2 DG uptake in the reticulata region compared to the control in panel (B). IP, interpeduncular nucleus. M, mammillary bodies. SNC, substantia nigra pars compacta. SNRa, substantia nigra pars reticulata anterior. SNRp, substantia nigra pars reticulata posterior.
Fig. 3.
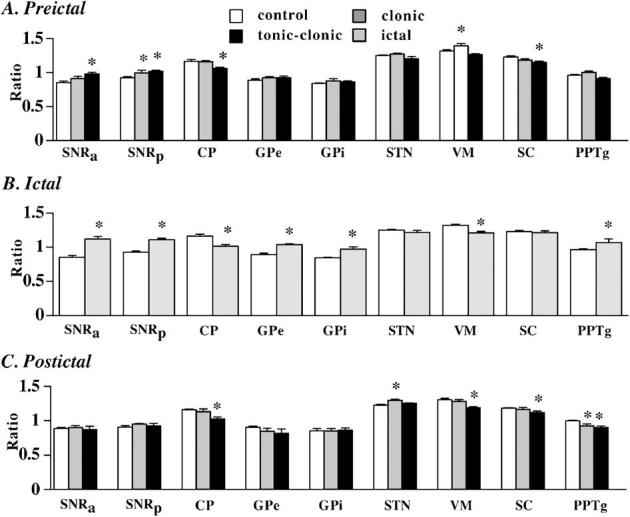
Quantitative changes in 2 DG relative uptake in preictal and postictal states. Shown is the optical density ratio of the ROI for the SNR and its main input (CP, GPe, STN) and output (VM, SC, PPTg) structures. (A) Preictal states for motor seizure expression. The SNRanterior was activated during pre-tonic-clonic stage, but during pre-clonic stage, the SNRposterior was activated prior to both seizures. The reduction in the SC is consistent with increased inhibitory activity in the SNRanterior. (B) Ictal state. We show the activation of the SNR and its input and output structures for comparison. Our data show similar activation pattern within the basal ganglia circuitry during the ictal state as shown previously by others. (C) Postictal states. The SNR played more of a role prior to seizures than postictally, while the STN and PPTg played more of a role postictally than preictally. The CP, SC, and VM were affected both pre- and postictally. CP, caudate-putamen. GPe, globus pallidus externa. GPi, globus pallidus interna (endopeduncular nucleus). PPTg, pedunculopontine tegmental nucleus. SC, intermediate layers of the superior colliculus. STN, subthalamic nucleus. VM, ventromedial nucleus of the thalamus. *P < 0.05 compared to controls.
Postictal states
During postictal clonic or tonic-clonic states, no visual changes within the SNR were observed (Fig. 2). This was confirmed by calculating the 2 DG uptake ratios, which did not differ from control ratios in both SNR regions (Fig. 3C).
Fig. 2.
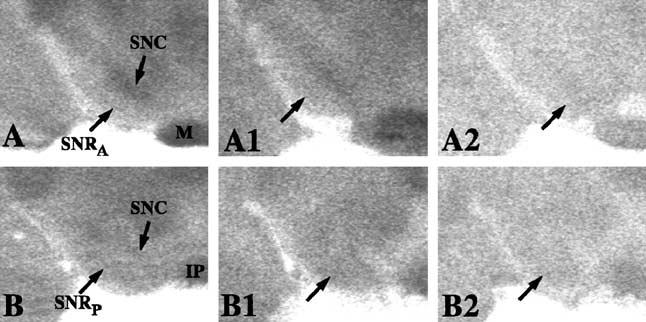
Postictal regional SNR activity. Coronal section autoradiograms depict changes in 2 DG accumulation and glucose utilization during postictal seizure states in the SNRanterior and SNRposterior. (A and B) Control SNRanterior and SNRposterior, respectively (arrows). (A1 and B1) Postictal to a clonic seizure in the SNRanterior and SNRposterior, respectively. Compared to the control in panels (A) and (B) (arrows), there is little difference in gray levels in the SNR. (A2 and B2) Postictal to a tonic-clonic seizure. There was a generalized decrease in brain 2 DG uptake and glucose utilization throughout the brain. However, there was no detectable relative change in the SNR (arrows).
Other basal ganglia nuclei and substantia nigra projection regions
Pre-clonic/pre-tonic-clonic/ictal states
Visual inspection of autoradiograms revealed distinct regional pattern in 2 DG utilization depending on the seizures stage. We observed the most dramatic changes in the majority of structures studied during the ictal period. Overall one-way ANOVA of the ratio measurements revealed significant differences from controls within individual structures (Figs. 3A and B; CP: F(3,19) = 12.3, P = 0.0001; GP: F(3,19) = 12.96, P < 0.0001; VM: F(3,17) = 14.27, P < 0.0001; SC: F(3,18) = 3.22, P = 0.047; PPTg: F(3,20) = 3.7, P = 0.03). The number of involved structures increased from the early pre-clonic and pre-tonic-clonic states to the ictal period (Figs. 3A and B). Pairwise post-hoc comparisons of the ratios showed that, during the preictal period prior to a clonic seizure, the 2 DG uptake significantly increased compared to control in the VM (+5.9%, P < 0.05, Fig. 3A). During the pre-tonic-clonic state (Fig. 3A), neural activity decreased in the SC (-6.5%, P < 0.05) and CP (9%, P < 0.05). During the ictal state (Fig. 3B), most structures-studied showed changes in activity. The 2 DG uptake and glucose utilization ratio was significantly elevated in the GP (+16.7%; P < 0.05;) and PPTg-(+11.1%, P < 0.05). Neural activity decreased in the CP (13%, P <0.05) and VM (8%, P <0.05).
postictal states
Visual inspection of the autoradiograms showed a general decrease in uptake and activity throughout the entire brain. Nevertheless, at the level of individual structure ratios, one-way ANOVA revealed significant differences from controls (Fig. 3C) during individual postictal states [CP: F(2,14) = 7.83, P = 0.005; STN: F(2,14) = 8.99, P = 0.004; VM: F(2,16) = 7.12, P = 0.007; SC: F(2,16) = 4.35, P = 0.03; PPTg: F(2,17) = 6.6, P = 0.009]. During the postictal state following a clonic seizure (Fig. 3C), the ratio index of neural activity was significantly increased within the subthalamic nucleus (+5.86%, P < 0.05) and decreased in PPTg (-7.1%, P < 0.05). During the postictal state to a tonic-clonic-seizure (Fig. 3C), neural activity was decreased in CP (11.7%, P0.05), and 5.6%, P < < 0.05), VM (-9.1%, P < 0.05), SC (PPTg (-9.5%, P < 0.05).
Discussion
The results show that SNRanterior and SNRposterior can be differentiated in terms of their local neural activity in the pre-clonic period. As the clonic seizure progresses into a tonic-clonic seizure, now both SNR regions become activated. The findings confirm previous local injection, histological, and receptor subtype studies suggesting that the two regions are different (Fan et al., 1997; Galanopoulou et al., 2003; Hedberg et al., 2003; Moshéet al., 1994; Ravizza et al., 2003; Shehab et al., 1996; Thompson et al., 2000; Velíšek et al., 2005; Velíšková et al., 1998) and provide new information regarding the SNR projection targets and other basal ganglia activity during the pre-clonic/tonic-clonic and postictal period. Importantly, the differentiation between two subregions within the SNR was detected without any lesion or local drug infusion (Garant and Gale, 1983; Moshé et al., 1994; Shehab et al., 1996; Velíšková and Moshé, 2001). The observation that the early-stage flurothyl-induced seizure events use these regions and their pathways identifies them as possible experimental and clinical interventions.
In addition, we confirmed the findings of others that the entire SNR was metabolically active during the ictal period (Ben-Ari et al., 1981; Engel et al., 1978; Nehlig et al., 1992; Pazdernik et al., 1985). We also investigated the possibility that GPe and GPi (endopeduncular nucleus) act differentially because they may have opposing effects on movement (DeLong, 1990; Gerfen et al., 1990). The GP was detectably activated only during the ictal stage, and the GPe and GPi did not show any differential involvement. One new finding regarding the ictal period in our model is that the PPTg was also involved.
Flurothyl induces primary generalized motor seizures (Velíšek et al., 1995). In adult rats, flurothyl first causes a clonic seizure involving forelimb clonic movements, presumably originating within the forebrain structures (Browning and Nelson, 1986), and then a tonic-clonic seizure ensues affecting all limbs. The changes represent activity involved during the initiation/propagation of a seizure. Neuronal activity changed selectively in the SNRposterior. Others have suggested that the SNR is involved in seizure propagation, especially during clonic seizures (Bonhaus et al., 1991; Garant and Gale, 1983; Handforth and Ackermann, 1995), however, these studies did not identify the region-specific effects within the SNR probably because of rapid progression of seizure states, and thus both SNR regions were already involved. A study by Collins et al. (1986) demonstrated involvement of the SNR during forelimb movements induced by electrical stimulation of motor cortex. The authors showed that during forelimb movements both SNRposterior and SNRanterior showed increase in 2 DG uptake. In accordance with this, our finding of selective 2 DG changes in the SNRposterior prior to motor seizure expression suggests its role during seizure initiation and propagation, while the SNRanterior becomes involved later, when a motor seizure occurs (either as induced forelimb movement by an electrical stimulation or following a fully developed clonic seizure seen in the pre-tonic-clonic stage). Pharmacological studies support the differential role of the SNRanterior and SNRposterior since enhancement of GABA neurotransmission is anticonvulsant in the SNRanterior and proconvulsant in the SNRposterior in different seizure models (Moshé et al., 1994; Thompson et al., 2000; Velíšková et al., 1996). Furthermore, a recent preliminary report of nigral electrophysiological activity during a clonic seizure induced by kindling also shows that neuronal discharge rate increases selectively in the SNRposterior but not the SNRanterior (Gernert et al., 2004). Thus, our findings are consistent with the interpretation that the SNRposterior could act as a “gateway” for seizure propagation.
The co-activation of the VM during the pre-clonic state could be caused by changes in the SNRposterior, perhaps reflecting GABA or acetylcholine release through abundant GABAergic and cholinergic projection neurons (Di Chiara et al., 1979; Kha et al., 2001). However, pharmacological and lesion studies showed no involvement of VM in seizure control (Garant et al., 1993; Moshé et al., 1985).
During the pre-tonic-clonic period in our study, when clonic seizures had occurred, glucose uptake and metabolism increased in both the SNRposterior and SNRanterior. In addition, metabolism decreased significantly in the SC, which was not present in the pre-clonic period. This suggests an anticonvulsant action through the SNRanterior comprising the following series of events: GABAergic strionigral projections inhibit SNR GABAergic projections (but axon terminal activity increased glucose utilization in the SNRanterior (Ackermann et al., 1984; Sokoloff, 1999); the inhibition in the SNR leads to decreased presynaptic activity, less GABA release and 2 DG uptake in the SC. This is consistent with anticonvulsant effects of local injections of GABA receptor agonists in the SNR (Depaulis et al., 1989) and nigral lesions resulting in suppression of its inhibitory effects on SC (Garant and Gale, 1983). Accordingly, increased neural firing in the SC by local infusions of bicuculline has anticonvulsant effects (Depaulis et al., 1990; Redgrave et al., 1992).
Postictally, 2 DG uptake and glucose metabolism decreased in PPTg, VM, and SC. As the changes in 2 DG uptake reflect the activity at the synapses and not the activity of cell bodies within a structure, low 2 DG uptake indicates a decrease in afferent activity to several regions that are SNR targets. The persistent changes in activity during both postictal states in the PPTg may be important. Our previous study showed that behavioral motor seizure cessation in the flurothyl model also corresponds to rapid termination of EEG discharges (Sperber et al., 1999). Thus, the changes in 2 DG uptake within the PPTg cannot be attributed to any remaining ictal activity. Moreover, the activity in the PPTg decreased during both postictal states, while it increased during the ictal state. The PPTg is one of the main players in the process of movement and behavioral arrest (Klemm, 2001). Postictal states can be compared to movement arrest, using similar suppressive mechanisms during motor seizure termination. Decreased uptake in the PPTg is consistent with lack of inhibitory GABAergic regulation, e.g., from the SNR, which represents one of most important afferent projections of the PPTg (Rye et al., 1987; Spann and Grofova, 1991). This in turn results in an increase of cholinergic or glutamatergic activity within the PPTg, which is reflected by increased activity in the STN, one of the main ascending projections of PPTg (Nakano et al., 2000). Although we cannot be sure that the increase in the ratio for the STN is a true increase in metabolism, it is known that STN is also involved in movement arrest, which would be in accordance with our assumption.
Summary and conclusions
The basal ganglia are known to have profound inhibitory and excitatory effects on behavior (DeLong, 1990; Mink, 2003). Furthermore, they receive a large cortical input (Bolam et al., 2000; Maurice et al., 1998; McGeorge and Faull, 1989; Parent and Hazrati, 1995). Thus, these nuclei and their efferent projections are excellent candidates for the control of generalized seizures. Our study shows that two SNR regions act separately during distinct seizure stages. The most important finding is that the SNRposterior is selectively affected during an early pre-clonic stage. We suggest that interventions within the SNRposterior have a greater chance of preventing seizures before they start than those of the SNRanterior and that the SNRposterior seems to be an early gateway to seizure propagation.
Acknowledgments
We gratefully acknowledge Dr. L. Velíšek for his help with statistical evaluation and Drs. S.L. Moshé, R.F. Ackermann, and A. Nehlig for help with experimental design and helpful comments during manuscript preparation. Supported by the grants NS-20253, NS-36238, and NS-21356 from NIH and the CURE grant. The procedures for animal experimentation utilized in this report were reviewed and approved by the Institutional Animal Care and Use Committee.
References
- Ackermann RF, Finch DM, Babb TL, Engel J., Jr. Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J. Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann RF, Engel J, Jr., Phelps ME. Identification of seizure-mediating brain structures with the deoxyglucose method: studies of human epilepsy with positron emission tomography, and animal seizure models with contact autoradiography. Adv. Neurol. 1986;44:921–934. [PubMed] [Google Scholar]
- Andre V, Henry D, Nehlig A. Dynamic variations of local cerebral blood flow in maximal electroshock seizures in the rat. Epilepsia. 2002;43:1120–1128. doi: 10.1046/j.1528-1157.2002.17702.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J. Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus DW, Russel RD, McNamara JO. Activation of substantia nigra pars reticulata neurons: role in the initiation and behavioral expression of kindled seizures. Brain Res. 1991;545:41–48. doi: 10.1016/0006-8993(91)91267-5. [DOI] [PubMed] [Google Scholar]
- Browning RA. Role of the brain-stem reticular formation in tonic-clonic seizures: lesion and pharmacological studies. Fed. Proc. 1985;44:2425–2431. [PubMed] [Google Scholar]
- Browning RA, Nelson DK. Modification of electroshock and pentylenetetrazol seizure patterns in rats after precollicular transections. Exp. Neurol. 1986;93:546–556. doi: 10.1016/0014-4886(86)90174-3. [DOI] [PubMed] [Google Scholar]
- Collins RC, Santori EM, Der T, Toga AW, Lothman EW. Functional metabolic mapping during forelimb movement in rat: I. Stimulation of motor cortex. J. Neurosci. 1986;6:448–462. doi: 10.1523/JNEUROSCI.06-02-00448.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong M. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Snead OCI, Marescaux C, Vergnes M. Suppressive effects of intranigral injection of muscimol in three models of generalized non-convulsive epilepsy induced by chemical agents. Brain Res. 1989;498:64–72. doi: 10.1016/0006-8993(89)90399-5. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Liu Z, Vergnes M, Marescaux C, Micheletti G, Warter JM. Suppression of spontaneous generalized non-convulsive seizures in the rat by microinjection of GABA antagonists into the superior colliculus. Epilepsy Res. 1990;5:192–198. doi: 10.1016/0920-1211(90)90038-w. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 1979;176:273–284. doi: 10.1016/0006-8993(79)90983-1. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr., Wolfson L, Brown L. Anatomical correlates of electrical and behavioral events related to amygdaloid kindling. Ann. Neurol. 1978;3:538–544. doi: 10.1002/ana.410030615. [DOI] [PubMed] [Google Scholar]
- Fan XD, Zhang X, Yu PH, Li XM, Juorio AV. Induction of preconvulsive behavior and Fos expression by dopamine-induced nigral lesion in the rat. Brain Res. 1997;751:31–36. doi: 10.1016/s0006-8993(96)01386-8. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp. Neurol. 2003;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Garant D, Gale K. Lesions of substantia nigra protect against experimentally induced seizures. Brain Res. 1983;273:156–161. doi: 10.1016/0006-8993(83)91105-8. [DOI] [PubMed] [Google Scholar]
- Garant DS, Xu SG, Sperber EF, Moshé SL. The influence of thalamic GABA transmission on the susceptibility of adult rats to flurothyl induced seizures. Epilepsy Res. 1993;15:185–192. doi: 10.1016/0920-1211(93)90055-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gernert M, Fedrowitz M, Wlaz P, Löscher W. Subregional changes in discharge rate, pattern, and drug sensitivity of putative GABAergic nigral neurons in the kindling model of epilepsy. Eur. J. Neurosci. 2004;20:2377–2386. doi: 10.1111/j.1460-9568.2004.03699.x. [DOI] [PubMed] [Google Scholar]
- Handforth A, Ackermann RF. Mapping of limbic seizure progressions utilizing the electrogenic status epilepticus model and the 14C-2-deoxyglucose method. Brain Res. Brain Res. Rev. 1995;20:1–23. doi: 10.1016/0165-0173(94)00003-8. [DOI] [PubMed] [Google Scholar]
- Handforth A, Treiman DM. Functional mapping of the early stages of status epilepticus: a 14C-2-deoxyglucose study in the lithiumpilocarpine model in rat. Neuroscience. 1995a;64:1057–1073. doi: 10.1016/0306-4522(94)00376-g. [DOI] [PubMed] [Google Scholar]
- Handforth A, Treiman DM. Functional mapping of the late stages of status epilepticus in the lithiumpilocarpine model in rat: a 14C-2-deoxyglucose study. Neuroscience. 1995b;64:1075–1089. doi: 10.1016/0306-4522(94)00377-h. [DOI] [PubMed] [Google Scholar]
- Hedberg TG, Velíšková J, Sperber EF, Nunes ML, Moshé SL. Age-related differences in NMDA/metabotropic glutamate receptor binding in rat substantia nigra. Int. J. Dev. Neurosci. 2003;21:95–103. doi: 10.1016/s0736-5748(02)00125-9. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by g-aminobutyric acid. Science. 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Kha HT, Finkelstein DI, Tomas D, Drago J, Pow DV, Horne MK. Projections from the substantia nigra pars reticulata to the motor thalamus of the rat: single axon reconstructions and immunohistochem-ical study. J. Comp. Neurol. 2001;440:20–30. doi: 10.1002/cne.1367. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Behavioral arrest: in search of the neural control system. Prog. Neurobiol. 2001;65:453–471. doi: 10.1016/s0301-0082(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Lánský P, Velíšková J, Velíšek L. An indirect method for absorption rate estimation: flurothyl-induced seizures. Bull. Math. Biol. 1997;59:569–579. doi: 10.1007/BF02459466. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J. Neurosci. 1998;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch. Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Okada R, Albala BJ. Ventromedial thalamic lesions and seizure susceptibility. Brain Res. 1985;337:368–372. doi: 10.1016/0006-8993(85)90077-0. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Brown LL, Kubová H, Velíšková J, Zukin RS, Sperber EF. Maturation and segregation of brain networks that modify seizures. Brain Res. 1994;665:141–146. doi: 10.1016/0006-8993(94)91164-9. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J. Neurol. 2000;247(Suppl 5):V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Vergnes M, Marescaux C, Boyet S. Mapping of cerebral energy metabolism in rats with genetic generalized non-convulsive epilepsy. J. Neural Transm. 1992;35:141–153. doi: 10.1007/978-3-7091-9206-1_10. [DOI] [PubMed] [Google Scholar]
- Okada R, Nagishi N, Nagaya H. The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol induced seizures. Brain Res. 1989;480:383–387. doi: 10.1016/0006-8993(89)90212-6. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia: I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pazdernik TL, Cross RS, Giesler M, Samson FE, Nelson SR. Changes in local cerebral glucose utilization induced by convulsants. Neuroscience. 1985;14:823–835. doi: 10.1016/0306-4522(85)90146-0. [DOI] [PubMed] [Google Scholar]
- Prichard JW, Gallagher BB, Glaser GH. Experimental seizure-threshold testing with flurothyl. J. Pharmacol. Exp. Ther. 1969;166:170–178. [PubMed] [Google Scholar]
- Ravizza T, Friedman LK, Moshé SL, Velíšková J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int. J. Dev. Neurosci. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Simkins M, Overton P, Dean P. Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy—I. Mapping of dorsal midbrain with bicuculline. Neuro-science. 1992;46:379–390. doi: 10.1016/0306-4522(92)90059-b. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J. Comp. Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Schwechter EM, Velíšková J, Velíšek L. Correlation between extracellular glucose and seizure susceptibility in adult rats. Ann. Neurol. 2003;53:91–101. doi: 10.1002/ana.10415. [DOI] [PubMed] [Google Scholar]
- Shehab S, Simkins M, Dean P, Redgrave P. Regional distribution of the anticonvulsant and behavioural effects of muscimol injected into the substantia nigra of rats. Eur. J. Neurosci. 1996;8:749–757. doi: 10.1111/j.1460-9568.1996.tb01260.x. [DOI] [PubMed] [Google Scholar]
- Simmons JM, Ackermann RF, Gallistel CR. Medial forebrain bundle lesions fail to structurally and functionally disconnect the ventral tegmental area from many ipsilateral forebrain nuclei: implications for the neural substrate of brain stimulation reward. J. Neurosci. 1998;18:8515–8533. doi: 10.1523/JNEUROSCI.18-20-08515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem. Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedures and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Spann BM, Grofova I. Nigropedunculopontine projection in the rat: an anterograde tracing study with phaseolus vulgaris-leucoagglutinin (PHA-L) J. Comp. Neurol. 1991;311:375–388. doi: 10.1002/cne.903110308. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Romero MT, Stanton PK. Flurothyl status epilepticus in developing rats: behavioral, electrographic histological and electrophysiological studies. Brain Res. Dev. Brain Res. 1999;116:59–68. doi: 10.1016/s0165-3806(99)00075-9. [DOI] [PubMed] [Google Scholar]
- Thompson K, Anantharam V, Behrstock S, Bongarzone E, Campagnoni A, Tobin AJ. Conditionally immortalized cell lines, engineered to produce and release gaba, modulate the development of behavioral seizures. Exp. Neurol. 2000;161:481–489. doi: 10.1006/exnr.1999.7305. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Velíšková J, Ptachewich Y, Shinnar S, Moshé SL. Effects of MK-801 and phenytoin on flurothyl-induced seizures during development. Epilepsia. 1995;36:179–185. doi: 10.1111/j.1528-1157.1995.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Velíšková J, Ravizza T, Giorgi FS, Moshé SL. Circling behavior and [14C]2-deoxyglucose mapping in rats: possible implications for autistic repetitive behaviors. Neurobiol. Dis. 2005;18(2):346–355. doi: 10.1016/j.nbd.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann. Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Velísek L, Nunes ML, Moshé SL. Developmental regulation of regional functionality of substantia nigra GABAA receptors involved in seizures. Eur. J. Pharmacol. 1996;309:167–173. doi: 10.1016/0014-2999(96)00341-x. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Kubová H, Friedman LK, Wu R, Sperber EF, Zukin RS, Moshé SL. The expression of GABAA receptor subunits in the substantia nigra is developmentally regulated and region-specific. Ital. J. Neurol. Sci. 1998;19:205–210. doi: 10.1007/BF02427602. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Liptáková S, Hussain S. The effects of N-methyl-d-aspartate antagonist 2-amino-7-phosphonoheptanoic acid microinfusions into the adult male rat substantia nigra pars reticulata are site-specific. Neurosci. Lett. 2001;316:108–110. doi: 10.1016/s0304-3940(01)02379-5. [DOI] [PubMed] [Google Scholar]


