Abstract
Trait exaggeration through sexual selection will take place alongside other changes in phenotype. Exaggerated morphology might be compensated by parallel changes in traits that support, enhance or facilitate exaggeration: ‘secondary sexual trait compensation’ (SSTC). Alternatively, exaggeration might be realized at the expense of other traits through morphological trade-offs. For the most part, SSTC has only been examined interspecifically. For these phenomena to be important intraspecifically, the sexual trait must be developmentally integrated with the compensatory or competing trait. We studied developmental integration in two species with different development: the holometabolous beetle Onthophagus taurus and the hemimetabolous earwig Forficula auricularia. Male-dimorphic variation in trait exaggeration was exploited to expose both trade-offs and SSTC. We found evidence for morphological trade-offs in O. taurus, but not F. auricularia, supporting the notion that trade-offs are more likely in closed developmental systems. However, we found these trade-offs were not limited solely to traits growing close together. Developmental integration of structures involved in SSTC were detected in both species. The developmental integration of SSTC was phenotypically plastic, such that the compensation for relatively larger sexual traits was greater in the exaggerated male morphs. Evidence of intraspecific SSTC demands studies of the selective, genetic and developmental architecture of phenotypic integration.
Keywords: polyphenism, allometry, pleiotropy, correlational selection
1. Introduction
Sexual selection is an evolutionary force responsible for a huge diversity of extreme traits and displays. Alongside the traits that are the principal target for elaboration, there are a host of characters that are also selected, both for and against, as a consequence of sexual selection. Despite the importance of these phenomena in shaping the morphology, ecology and evolution of the species in question (e.g. Marden & Chai 1991; Emlen 2001), these traits that enhance the fitness returns derived from exaggerated secondary sexual characters have been largely neglected in intraspecific studies of sexual selection.
A number of recent studies have demonstrated that intra-organism competition occurs over resources devoted to somatic structures (Klingenberg & Nijhout 1998; Nijhout & Emlen 1998; Emlen 2001; Radwan et al. 2002; Moczek & Nijhout 2004). These morphological trade-offs have, in particular, been demonstrated in species where alternative reproductive tactics give rise to dramatically divergent morphologies among males, i.e. where there is extreme phenotypic plasticity (Nijhout & Emlen 1998; Emlen 2001; Radwan et al. 2002; Moczek & Nijhout 2004). These studies demonstrate that when resources are finite, as in the case of holometabolous insects (Nijhout & Emlen 1998; Emlen 2001; Moczek & Nijhout 2004) or quiescent mites (Radwan et al. 2002), investment in exaggerated morphology places demands on other traits reducing their final size or reducing overall body size. Resource competition of this kind is thought to be particularly acute in closed systems, and in the holometabola to be strongest between traits developing from spatially close areas of the epithelium (Nijhout & Emlen 1998; Emlen 2001). Resource competition is an important concept in the evolution of condition-dependent signalling (Tomkins et al. 2004), so the extent to which morphological trade-offs occur and affect life history is an important facet of sexual selection. Morphological trade-offs might be a property restricted to organisms developing structures in closed developmental systems since, where growth occurs continually or is punctuated by moulting, past growth does not necessarily influence the resources available for future growth. We nevertheless know of no tests of the hypothesized differences between these developmental systems.
The development of elaborate morphological traits undoubtedly does induce morphological trade-offs; nevertheless, some traits will be selected for in concert with elaborated traits. These traits might be selected for their compensatory role in bearing, displaying or using an elaborated trait (Hedenström & Møller 1992; Balmford et al. 1994; Swallow et al. 2000) or might be subject to selection for their role in enhancing the function of a trait that is the principal target of elaboration (Møller et al. 1995).
Traits that are subject to correlational selection, where the variance in the relationship between two traits is reduced by selection, are likely to become genetically correlated and to develop in an integrated manner (Cheverud 1996; Wagner 1996; Klingenberg 2004). Developmental integration of this type is not derived from a common modular developmental basis to the traits (West-Eberhard 2003; Klingenberg 2004) but through selection on pleiotropic variation (Cheverud 1996; Wagner 1996), and can therefore arise in traits that are developmentally distant and distinct (Cheverud 1996; Klingenberg 2004). Secondary sexual trait compensation is a situation in which the display trait and the compensatory trait are likely to be subject to correlational selection, e.g. when individuals with larger traits or more extravagant displays are selected because they also have the morphology to accomplish the extremes of display. Here, we test the hypothesis that, within species, in addition to morphological trade-offs associated with exaggerated structures, morphological integration means that individuals bearing larger traits will also possess greater compensatory or display-enhancing traits.
In species with male dimorphisms, often radically divergent phenotypes are expressed among males. In many such species, the dimorphism is an extreme case of phenotypic plasticity: a facultative consequence of the conditions under which the individual developed (Emlen 1994; Gross 1996; Hunt & Simmons 1997; Tomkins 1999; Radwan et al. 2002). These species are ideal for examining developmental trade-offs (Nijhout & Emlen 1998; Emlen 2001; Radwan et al. 2002) and also have great potential for exploring the developmental integration of traits that are selected alongside the principal target of elaboration. Where developmental reprogramming divides males between morphologies that differ in trait exaggeration, the adaptations individuals have to bearing exaggerated traits will become particularly apparent. Insects are useful model systems for examining developmental integration because we know with some precision when the adult morphology is determined. Consequently, the variation that we are able to measure is the result of developmental processes rather than the growth of compensatory structures in the adult.
Here, we examine two species of male-dimorphic insect, the dung beetle Onthophagus taurus and the European earwig Forficula auricularia. Adult morphology is determined at pupation in the holometabolous O. taurus and, in the case of the male dimorphism in F. auricularia, at the final moult (J. L. Tomkins, unpublished work). Onthophagus taurus has a dimorphism in the length of the horns carried on the males' head. This dimorphism arises from the extreme positive allometry of the horns (Tomkins et al. in press). The dimorphism is associated with alternative reproductive tactics; long horned ‘major’ males defend females in the tunnels in which they provision their brood, while short-horned minor males use side tunnels and try to sneak copulations (Moczek & Emlen 2000). Onthophagus taurus makes burrows beneath cattle dung in which mating takes place. Guarding major males block tunnels using their horns and by bracing their legs against the tunnel walls (Moczek & Emlen 2000). Major males tend to engage in these pushing contests and expel rivals from the tunnels they defend. The forelegs of majors are therefore likely to be important structures in these contests. Horns have also been shown to hinder major males in their passage through tunnels and hence the forelegs which provide the digging power are likely to be traits that compensate for this hindrance (Moczek & Emlen 2000).
The Dermaptera are hemimetabolous insects and F. auricularia develops through four nymphal instars prior to adult eclosion. The male morphs are indistinguishable in forceps length prior to the moult to adulthood (J. L. Tomkins, unpublished work). The male dimorphism in forceps length is consistent with a developmental reprogramming event (Tomkins et al. in press). The forceps in F. auricularia are used for fighting between males, which involves both pinching and twisting opponents from their footing. In F. auricularia, the reprogramming of forceps length means that for a similar body plan, males may be carrying forceps that are relatively much longer. The trait principally responsible for bearing the additional weight of the forceps in macrolabic males are the hind-legs. The hind-legs will also be important determinants of a male's ability to wield the forceps and wrestle other males.
Under the hypothesis that sexual selection for extreme phenotypes in these species is likely to result in the developmental integration of compensatory traits, as well as trade-offs, we predicted that characters that were important in determining the efficacy of the sexual traits in question would show developmental integration. Using these species, we were able to compare the processes of integration and competition in different developmental systems. The dimorphic nature of trait expression also allowed us to investigate the phenotypic plasticity of developmental integration.
2. Methods
The O. taurus were a sample of 150 males collected from the field in southwestern Western Australia. We measured the male's pronotum width; left and right horn length; left and right elytra length; the length of the femur, tibia and tarsus of the left and right front leg; the femur of the left and right hind-leg; the left and right wing length; and the length of the large sternite on the animal's abdomen. From the paired characters, we used the mean of both sides in the analysis, and in both species we used pronotum width as a measure of body size.
The F. auricularia were a sample of 150 males collected from the island of West Wideopen in the Farne Islands group in Northumbria in the United Kingdom. In this sample, we measured head width, pronotum width, right fore-femur length, right hind-femur length, right elytron length and right forceps length.
All measurements were made using a binocular microscope and eyepiece graticule for O. taurus and Scion image analysis software in the case of F. auricularia.
The model of Kotiaho & Tomkins (2001) was used to discriminate male morphs. The same model was used to detect the limits to the distribution of intermediate males in F. auricularia (Tomkins et al. in press).
3. Results
(a) Onthophagus taurus
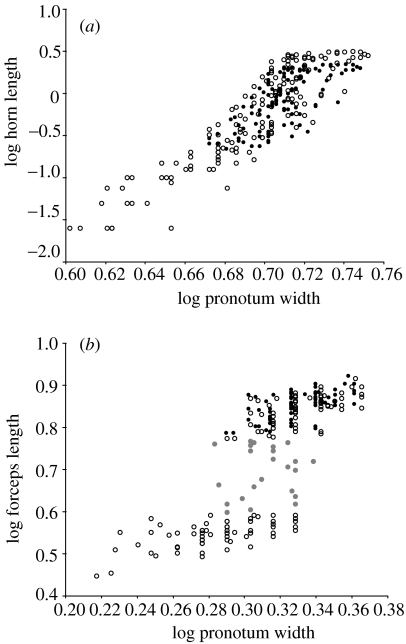
Onthophagus taurus from Western Australia have a few major males that reach the asymptote of horn length, and the horn allometry can be linearized by taking the log transformation of the x and y variables (Tomkins et al. in press; figure 1a). To examine the developmental integration of the traits measured in O. taurus, we first estimated the correlation coefficients between the various morphological traits and horn length. Horn length was most strongly correlated with the length of the femur and tibia of the forelegs (table 1).
Figure 1.
Allometric relationships between body size measured as pronotum width and the length of (a) the male head horn of Onthophagus taurus, minor males=open circle, major males=filled circle, and (b) the forceps of Forficula auricularia. In F. auricularia, brachylabic males=open circle, intermediate males=filled grey circle and macrolabic males=filled circle.
Table 1.
Pearson correlations of log transformed horn length and log transformed morphological traits.
| pronotum | head | elytra | fore-femur | fore-tibia | fore-tarsus | hind-femur | sternite | wing | |
|---|---|---|---|---|---|---|---|---|---|
| r | 0.902 | 0.878 | 0.875 | 0.929 | 0.921 | 0.810 | 0.911 | 0.889 | 0.800 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 |
| n | 146 | 146 | 146 | 145 | 144 | 146 | 144 | 146 | 145 |
The strength of the correlations between horn length and the femur and tibia of the forelegs suggests that these traits are developmentally linked to the length of the horns in these beetles. Nevertheless, these traits are highly correlated with one another and multiple regression cannot be performed because of the high multicollinearity. We used a principal components (PCA) analysis to partition the variance attributable to the various traits. From the nine traits, we derived eight principal components (PCs), six of which were informative (table 2).
Table 2.
Factor loadings for the principal components analysis of the morphological traits in O. taurus. (Variables that provide the greatest weight in each component are in bold.)
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
|---|---|---|---|---|---|---|
| pronotum | 0.990 | −0.053 | −0.014 | 0.022 | −0.032 | 0.018 |
| head | 0.968 | −0.053 | −0.102 | 0.205 | 0.074 | 0.042 |
| elytra | 0.972 | −0.093 | 0.094 | −0.001 | −0.159 | 0.097 |
| fore-femur | 0.988 | −0.052 | −0.080 | −0.021 | −0.031 | −0.046 |
| fore-tibia | 0.981 | −0.012 | −0.118 | −0.030 | −0.026 | −0.126 |
| fore-tarsus | 0.907 | 0.419 | 0.018 | 0.007 | −0.014 | 0.020 |
| hind-femur | 0.991 | −0.036 | −0.032 | −0.062 | −0.021 | −0.030 |
| sternite | 0.973 | −0.044 | −0.044 | −0.142 | 0.135 | 0.097 |
| wing | 0.951 | −0.047 | 0.287 | 0.026 | 0.076 | −0.070 |
The PCs were weighted such that PC1 accounted for overall size, PC2 fore-tarsus, PC3 head width and fore-tibia length but mostly wing length, PC4 head width and sternite, PC5 elytra and sternite and PC6 fore-tibia (table 2). There was no PC that accounted for variation in fore-femur length.
To determine which traits interacted with relative horn length we conducted a general linear model with log horn length as the dependent variable and male morph and the six remaining informative PCs as predictor variables. PC1 was included in the model so that the remaining PC effects were on relative horn length; non-significant interactions were removed from the model (table 3). This analysis demonstrated that there was no relationship between PC2 (fore-tarsus length) and relative horn length. PC3 was negatively related to relative horn length (table 2); PC3 is weighted positively, by wing length, hence wing length is negatively related to relative horn length (figure 2). PC3 is also weighted negatively by fore-tibia length, suggesting that fore-tibia length is positively related to residual horn length. PC4 was negatively related to relative horn length (table 3); PC4 is weighed positively by head width (table 2) and therefore horns appear to get relatively larger to some degree at the expense of head width. PC5 is negatively related to relative horn length (table 3); elytra length is negatively related and sternite positively related to PC5 (table 2). This suggests that the abdominal sternite gets smaller and elytra larger as relative horn size increases. Finally, there was a marginally non-significant negative regression coefficient for PC6 (table 3). The length of the fore-tibia was negatively related to PC6 (table 2), which supports the conclusion derived from PC3 that as horn length increases, so too does the length of the fore-tibia (figure 3). There were two significant interactions, the first between morph and PC1 shows that majors have a steeper horn allometry than minors (Tomkins et al. in press). The second between morph and PC3 reveals that the relationship between relative horn length and relative wing length is different in the two male morphs (figure 2). This analysis shows that, once overall size has been accounted for, elytra length and fore-tibia length are the only traits to be positively related to relative horn length, while those other traits for which identifiable variance could be partialled were negatively related to horn length. The only exception is fore-tarsus length, which was not related to horn length.
Table 3.
General linear model of traits influencing relative horn size in O. taurus. (The dependent variable is log horn length, PC1 controls for the size dependence of horn length. The partial regression slopes and amount of variance in horn size explained are also reported (r2).)
| source | df | MS | F | p | B±s.e. | partial r2 |
|---|---|---|---|---|---|---|
| corrected model | 9 | 4.484 | 148.72 | 0.000 | 0.911 | |
| intercept | 1 | 5.006 | 166.03 | 0.000 | 0.559 | |
| morph | 1 | 0.953 | 31.61 | 0.000 | 0.194 | |
| PC1 (size) | 1 | 8.737 | 289.76 | 0.000 | 0.689 | |
| PC2 (fore-tarsus) | 1 | 0.006 | 0.20 | 0.655 | −0.07±0.01 | 0.002 |
| PC3 (wing/fore-tibia) | 1 | 1.132 | 37.53 | 0.000 | 0.223 | |
| PC4 (head) | 1 | 0.285 | 9.45 | 0.003 | −0.04±0.01 | 0.067 |
| PC5 (elytra/sternite) | 1 | 0.380 | 12.60 | 0.001 | −0.05±0.01 | 0.088 |
| PC6 (fore-tibia) | 1 | 0.091 | 3.02 | 0.085 | −0.03±0.01 | 0.023 |
| morph×PC1 (size) | 1 | 0.217 | 7.18 | 0.008 | 0.052 | |
| morph×PC3 (wing/foretib) | 1 | 0.132 | 4.38 | 0.038 | 0.032 | |
| error | 131 | 0.030 |
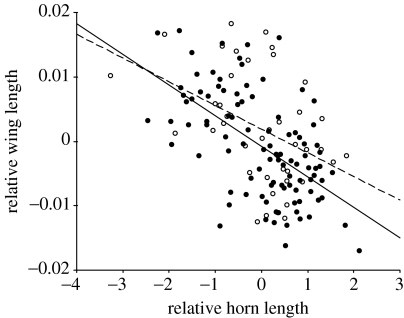
Figure 2.
Scatterplot and least squares regression line of the relationship between relative wing length and relative horn length. Residual wing length was calculated from least squares regression of the log transformed trait on body size measured as PC1, minor males=open circle and broken line (β=−0.004±0.001; t39=2.89, p=0.006), major males=filled circle and solid line (β=−0.005±0.001; t98=7.125, p<0.001).
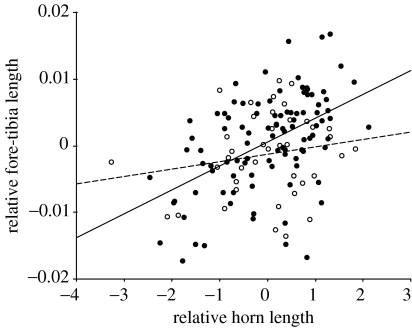
Figure 3.
Scatterplot and least squares regression lines for residual fore-tibia length and residual horn length in O. taurus. Residual fore-tibia length was calculated from least squares regression of the log transformed trait on body size measured as PC1, minor males=open circle and broken line (β=0.001±0.001; t39=1.24, p=0.26), major males=filled circle and solid line (β=0.004±0.001; t98=5.67, p<0.001).
We expected that wing length would be positively related to horn length as a trait that would compensate for the presence of large horns; however, the analysis above suggests that this is not the case and that horns and wings in fact trade-off. An additional PCA was performed in order to obtain a measure of relative horn investment. The second PC from a PCA of log horn length and PC1 (body size in the previous PCA) was used to measure horn length variation independent of size variation. We have called this PC relative horn length. Relative horn length was calculated in this way separately for each morph to account for the slight difference in horn allometry. In support of the PCA above, further examination of the trade-off with a GLM with residual wing length (from log wing length on PC1(size)) as a dependent variable, and morph (main effect) and relative horn length (covariate), revealed evidence of phenotypic plasticity in the trade-off associated with the dimorphism, i.e. there was a significant morph effect (F1,137=4.137, p=0.04). The interaction was not significant (p=0.4), but the effect of relative horn length was highly significant (F1,137=40.6, p<0.001; figure 2).
A similar GLM with relative fore-tibia length as the dependent variable was performed to address the phenotypic plasticity of the apparent integration of relative horn length and relative fore-tibia length. This analysis revealed a significant interaction between morph and relative horn length (F1,137=4.46, p=0.036; figure 3), indicating that the developmental integration of the horns and fore-tibia scaled differently between the morphs (figure 3). The effect of morph was non-significant (p=0.101).
(b) Forficula auricularia
The dimorphism in F. auricularia is characterized by two linear functions of forceps length on pronotum width, separated by a step that indicates a reprogramming event in development (Nijhout & Wheeler 1996; Tomkins et al. in press; figure 1b). The analysis of the developmental integration in F. auricularia requires that the relationship between forceps length and body size is accounted for separately within each morph. Table 4 shows the correlation coefficients of forceps length against the morphological traits of interest for the brachylabic, intermediate and macrolabic males. The correlation coefficients are much lower than in the dung beetle. The correlation coefficients are highest for pronotum width and lowest for fore-femur length. In the few intermediate individuals, the strongest correlation (albeit not significant to Bonferroni correction) is with hind-femur length.
Table 4.
Pearson correlation coefficients of the relationship between log forceps length and log transformed values for other body parts.
| head width | pronotum width | fore-femur | hind-femur | elytron | |
|---|---|---|---|---|---|
| brachylabic | |||||
| r | 0.510 | 0.610 | 0.447 | 0.503 | 0.555 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| n | 64 | 64 | 64 | 64 | 64 |
| intermediate | |||||
| r | 0.003 | 0.196 | 0.229 | 0.406 | −0.061 |
| p | 0.988 | 0.358 | 0.283 | 0.049 | 0.777 |
| n | 24 | 24 | 24 | 24 | 24 |
| macrolabic | |||||
| r | 0.553 | 0.617 | 0.226 | 0.554 | 0.597 |
| p | 0.000 | 0.000 | 0.077 | 0.000 | 0.000 |
| n | 62 | 62 | 62 | 62 | 62 |
The lower correlation coefficients between traits in F. auricularia allow the examination of each trait without using PCA. The efficacy of this is demonstrated by the individual significance of a number of the traits in the model (table 5) and the low variance inflation factors (all<4.7). Table 5 shows a general linear model for brachylabic and macrolabic males only; intermediate males were excluded to be conservative in the discrimination of male types (the results are qualitatively the same when intermediate individuals are included among the macrolabic males). In F. auricularia, the relationship between forceps length and the length of the hind-femur differs between macrolabic and brachylabic males (table 5). Figure 4 shows that there is developmental integration between hind-femur length and forceps length, such that relative forceps length and relative hind-femur length increase together but only in macrolabic males, and hence this developmental effect is phenotypically plastic and dependent on male morph. There was a significant relationship between pronotum width and forceps length as expected, and also between elytron length and forceps length, independent of the other traits; however, neither of these differed between the male morphs (table 5). Unlike O. taurus, all of the partial correlations were positive.
Table 5.
General linear model for the relationship between male forceps length, morph and the body parts of brachylabic and macrolabic F. auricularia. (Partial correlation coefficients are derived from a multiple regression and are not presented where the interaction was significant (see figure 3). Non-significant interactions p>0.1 were sequentially removed from the model.)
| source | df | mean square | F | p | partial r |
|---|---|---|---|---|---|
| model | 7 | 0.402 | 668.311 | 0.000 | |
| intercept | 1 | 0.029 | 47.428 | 0.000 | |
| morph | 1 | 0.000 | 0.465 | 0.496 | |
| head width | 1 | 0.000 | 0.000 | 0.987 | 0.050 |
| pronotum width | 1 | 0.006 | 9.883 | 0.002 | 0.208 |
| fore-femur length | 1 | 0.000 | 0.098 | 0.755 | 0.077 |
| hind-femur length | 1 | 0.003 | 4.746 | 0.031 | |
| elytron length | 1 | 0.008 | 12.606 | 0.001 | 0.151 |
| morph×hind-femur | 1 | 0.005 | 8.442 | 0.004 | |
| error | 118 | 0.001 |
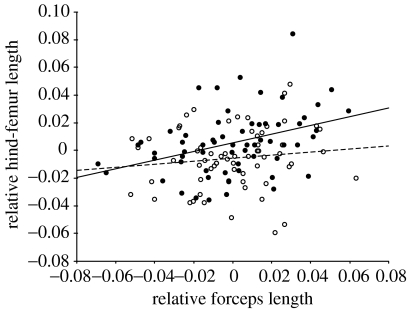
Figure 4.
Scatterplot and least squares regression lines for relative hind-femur length and relative forceps length in F. auricularia. Brachylabic males=open circle and broken line (β=0.114±0.112; t62=1.02, p=0.313) and macrolabic males=filled circle and solid line (β=0.313±0.099; t60=3.16, p=0.002). Residuals were calculated from least squares regression of the log transformed trait on log pronotum width. For forceps length, the residuals were calculated separately for each morph.
4. Discussion
Our data show developmental integration between morphological and secondary sexual traits in two species with extreme phenotypic plasticity. Morphological trade-offs have been suggested to have an important role in shaping the evolution and ecology of onthophagine dung beetles (Emlen 2001). Here, we show that these morphological trade-offs can extend beyond the traits in close proximity to the area from which horns develop. Furthermore, we find that, in contrast to the prevalent trade-off, positive covariance between secondary sexual and ordinary morphological traits also occurs within the closed system of the O. taurus prepupa. In F. auricularia, no trade-offs were apparent, consistent with the notion that acute resource competition is less likely in an open developmental system. We found evidence for parallel integration of the forceps and elytra length. Furthermore, we found evidence for a developmental switch in the integration of the forceps and the hind-femur of F. auricularia and in the fore-tibia and horns of O. taurus. This is evidence for phenotypic plasticity in developmental integration and enables intraspecific secondary sexual trait compensation to occur even in species with intrasexual dimorphisms.
In O. taurus, the pattern that emerges from the analysis is that the relative size of the males' horns has a negative effect on the size of the head and wings. A reduction in head width is expected, given that somatic structures are known to cause local competition for resources (Nijhout & Wheeler 1996; Klingenberg & Nijhout 1998; Emlen 2001), a phenomenon elegantly demonstrated in the trade-offs between horns at the rear of the head and eyes in O. acuminatus, and horns at the front of the head and antennae in O. sharpi (Emlen 2001). Developmental integration of traits that have pleiotropic effects on one another should be revealed as correlated responses when one trait is selected for. Correlated responses to selection on horn length in O. acuminatus were only significant for relative eye size and not for antennae or mouthparts (palps), further suggesting that there is a hierarchy of competition within the head (Nijhout & Emlen 1998). Nevertheless, our data suggest that the competition exerted within individuals that grow larger than expected horns also has a negative effect on the overall width of the head. A reduction in head size with increased relative horn size will exert further constraints on the sizes of eyes and antennae.
Unlike previous studies, we found that there was evidence of a trade-off between relative head-horn size and relative wing length, and that this trade-off was perhaps more extreme in major males (table 3). The spatial separation of the horns and wings suggests that they are less likely to be influenced by local resource competition than, for example, eyes and head horns. Hunt et al. (1999) found that wing length was positively related to relative horn length; however, this analysis did not use principal components to address the multicollinearity between the traits, perhaps accounting for the discrepancy in these outcomes. Other evidence is also equivocal; although not significant, directional selection on horn length in O. acuminatus did result in a correlated response in wing length of considerable magnitude (p=0.067; Nijhout & Emlen 1998). However, the direction of the response is not reported and whether it supports the current findings or those of Hunt et al. (1999) is unclear. Recent evidence for a trade-off between horns and genitalia in O. taurus has suggested that resource competition might occur on an organism-wide basis in these beetles, dependent on the timing of the development of the traits (Moczek & Nijhout 2004). The nature of the trade-off was such that the removal of the imaginal disk from which genitalia are derived caused male beetles to express horns at a smaller body size (Moczek & Nijhout 2004). This is equivalent to increasing the elevation of the horn length–body size (log–log) allometry, i.e. creating individuals with a positive residual horn length. Our data for wings show a comparable effect: when horns are relatively long, competition is apparently increased and wings are relatively short. The mechanism of resource competition in these species remains to be determined, but the evidence is consistent with the notion that growing traits act as a sink for the growth promoting hormones, perhaps juvenile hormone (JH), and that competition is mediated to some extent through the availability of JH to different tissues (Moczek & Nijhout 2004; Tomkins et al. in press).
In contrast to the trade-offs found between the horns and other traits expressed by the beetle, we found that there was also positive covariance between the relative size of the fore-tibia and the relative length of the horns. This positive correlation indicates that relatively large horns are developed in concert with relatively large fore-tibia. The positive covariance between relative horn size and relative fore-tibia length is evidence for the developmental integration of these two traits, and the interaction term is evidence of phenotypic plasticity in this integration. The forelegs and head horns are derived from spatially separate areas of epidermis and therefore the integration observed is not likely to be the consequence of modular development, but rather of correlational selection acting on pleiotropic variation (Cheverud 1996; Wagner 1996; Klingenberg 2004). A genetic correlation ought to exist between traits that are developmentally integrated (Cheverud 1996; Wagner 1996; West-Eberhard 2003; Klingenberg 2004), but we have no such evidence for O. taurus. There was no correlated response in foreleg length in O. acuminatus selected for relatively long and relatively short horns; although this study did detect correlated responses in eyes (Nijhout & Emlen 1998), because there were only two lines, the ability to detect weaker genetic correlations will have been very low (Roff 1997; Unrug et al. 2004).
Our results show that horn length is integrated with the length of the fore-tibia. The interaction between relative horn length and morph on relative fore-tibia length is evidence that there is plasticity in this integration. This is intuitive since residual horn length is likely to be a much more significant factor in males with absolutely larger horns than those with absolutely smaller ones. This is borne out in the slopes of the interaction in which minor males' relative horn length is not significantly related to fore-tibia length, while in major males it is (figure 3). The cost of horn carrying in terms of agility in tunnels has been demonstrated in O. taurus majors (Moczek & Emlen 2000). Males used their powerful forelegs for excavating tunnels and males with relatively large horns would likely be handicapped if they did not also have apparatus for digging tunnels commensurate with their horn size. Alternatively, if forelegs are selected in majors for blocking tunnels, they may be selected alongside horns as a trait that increases the success of guarding males. Why, under such a role, relative foreleg length would be selected to be integrated with relative horn size is not clear. These data suggest that the integration of the two traits increases with absolute horn size such that the integration is more apparent in major males. The increase in integration may reflect the degree to which horns affect manoeuvrability, or their role in enhancing the efficacy of blocking tunnels. How the patterns we have found in this Australian population of O. taurus compare with other populations of this beetle in which there are many more major males (Moczek & Nijhout 2003) would be intriguing to know.
Dermaptera are hemimetabolous insects; therefore, unlike O. taurus, there is not a single point at which the morphology of the adult is determined, but rather growth occurs through a series of instars. The developmental environment is therefore not a closed system during the period when final adult size and shape is determined. In the context of the male dimorphism in F. auricularia, macrolabic males only diverge from the ontogenetic allometry of brachylabic males at the final moult, so there is a sudden developmental transition between morphs (J. L. Tomkins, unpublished work). This is consistent with the pattern of the log–log allometry in which there is a step function between two linear sections, rather than the continuum seen in O. taurus.
The hemimetabolous growth of the earwigs is reflected in the much lower correlations between forceps length and the morphological traits than was the case for horn length in O. taurus. This is likely to be because the growth of structures that influence adult shape occurs over a much longer period of time in F. auricularia, and that therefore the timing of the growth of different body parts will vary; e.g. forceps only begin to become sexually dimorphic in the third instar (J. L. Tomkins, unpublished work). The correlation coefficients between forceps and body traits were similar for both macrolabic and brachylabic males. There is no strong relationship between body size and forceps length in intermediate males (figure 1b); however, despite this, and the small sample, there was a significant correlation between forceps length and hind tibia length. The statistical significance of this is less important than the indication that the integration between hind-femur length and forceps length that is a feature of macrolabic males is present in these intermediates.
The general linear model of the morphological traits in F. auricularia and their partial regression coefficients revealed that, unlike O. taurus, there were no trade-offs between the exaggerated secondary sexual trait and other traits. The relatively high partial regression coefficient between pronotum width and forceps length was unsurprising; however, there was also a strong and significant partial correlation with elytra length. This means that relative forceps length and relative elytra length are developmentally integrated. A post hoc exploration of this result showed that when log elytron length is entered into a GLM as the dependent variable, log pronotum width and log forceps length as covariates, and male morph as a factor; morph is significant (F1,122=11.83, p=0.001), demonstrating that elytron length is reprogrammed in the same manner as forceps length. In addition, however, pronotum width (F1,122=20.152, p<0.001) and forceps length (F1,122=17.31, p<0.001) are also significant. This analysis shows that, in these dimorphic earwigs, in addition to the reprogramming of forceps length, elytron length is reprogrammed and, furthermore, is also integrated developmentally with forceps length. Although we did not measure the wings owing to the extreme difficulty in unfolding and measuring them, the elytra do cover the wings and it seems likely that the increased elytron length is related to increases in wing size. If wing area showed the same patterns as elytron length, it would suggest that the developmental linkage between forceps and these traits was in compensation for carrying larger forceps during flight. Intraspecific variation in the degree of sexual dimorphism across species of stalk-eyed flies is associated with a reduction in flight performance and traits potentially associated with reducing this cost were increased wing length, increased thorax weight and reduced abdominal weight (Swallow et al. 2000), suggesting that exaggerated traits in insects also have implications for flight performance (cf. Hedenström & Møller 1992; Balmford et al. 1994).
The dimorphism in forceps length in our earwig sample is one in which mean body size differs between the morphs by 11%, whereas mean forceps length differs by 37%. This means that larger individuals are carrying disproportionately more weight at the end of their abdomen. The pattern of developmental integration between forceps length and hind-femur length reported here is consistent with a compensation for the mechanical disadvantage imposed by long forceps in macrolabic males. What is intriguing about our results is that the hind-femur length is only related to residual forceps length in macrolabic males (table 5 and figure 3). The difference in the relationships between these traits across the morphs indicates that, as part of the reprogramming that results in the macrolabic phenotype, hind-femur length becomes developmentally integrated with forceps length. Evidently, developmental integration is strongly phenotypically plastic in this species, generating a pattern very similar to the dung beetle.
The plasticity of the integration between forceps and hind-femur length, and horn length and fore-tibia length, in O. taurus is more intriguing than the parallel patterns of elytron length and forceps length in the earwig. The developmental integration does not appear to be a consequence of a modular developmental unit in either species because of the physical distance between the traits, and because plasticity is expected between, but not within, developmental modules (West-Eberhard 2003; Klingenberg 2004). Hence, the developmental integration is likely to reflect a genetic correlation between the two traits that have been subject to a history of correlational selection (Cheverud 1996; Wagner 1996; Klingenberg 2004). The observation of plastic integration where the integration is based on genetic correlation is more difficult to explain than plasticity involving localized modular units (West-Eberhard 2003). First, if the integration is derived from a genetic correlation, the phenotypic manifestation of this correlation is somehow obscured in the brachylabic and minor males. Evidence suggests that the alternative male morphs in these species do not reflect a genetic polymorphism and, instead, morph depends largely on body size, and body size variation has a large environmental component (Hunt & Simmons 1997; Moczek & Emlen 1999; Tomkins 1999). Consequently, the integration is unlikely to be linked to an allele for a particular morph. Second, any genetic correlation in macrolabic or major males is maintained despite relaxed selection on the covariance among brachylabic and minor males. This relaxed selection will increase recombination and disrupt the selective process that generates the genetic correlation between the traits. The nature of the genetic correlation between forceps and hind-femur length, and horn and fore-tibia length, remains to be determined. Until this has been done, it remains possible that the phenotypic correlations we have observed in major and macrolabic males might conceivably arise from a different developmental process (West-Eberhard 2003; Klingenberg 2004).
The patterns of morphological trade-off and integration in the species we have examined are likely to be found in intraspecific studies of other species with extreme variation in morphology. For example, it seems most likely that where extreme positive allometry occurs, compensatory structures will accompany the exaggerated trait, and that other structures will be depleted by the resource competition (cf. Knell et al. 2004). In taxa where exaggerated structures become fixed at a particular moment in development, and in particular those developing adult morphology in a closed system such as holometabolous insects, the stage is set for resource competition (Klingenberg & Nijhout 1998; Nijhout & Emlen 1998; Emlen 2001; Radwan et al. 2002; Moczek & Nijhout 2004). Equally, in such systems, the opportunity for selection to act on precise integration of traits that enable exaggerated structures to be borne and displayed is also clear. Nevertheless, as we have shown here for a hemimetabolous insect, trait compensation through developmental integration can also occur and should not be dismissed. Evidence for correlational sexual selection of the type likely to increase developmental integration has been demonstrated recently in the empidid dance fly Rhamphomyia sulcata, in which sexual selection on nuptial gift-carrying males increased the strength of the correlation between wing length and hind-femur length (LeBas et al. 2004). In contrast, selection decoupling the correlation between two tightly correlated morphological traits was found in another empidid R. tarsata: selection that would act to reduce developmental integration (LeBas et al. 2003). The influence of sexual selection on non-sexual morphology, either through trade-offs or developmental integration/disintegration fuelled by the type of correlational selection demonstrated in empidid flies, highlights the importance of indirect sexual selection in determining phenotypes. Sexual selection pressures have consequences for morphology reaching beyond the secondary sexual traits usually considered. Studies that incorporate the developmental, genetic and selective architecture of phenotypic variation will be required to increase our understanding of the roles of trade-offs and developmental integration in the evolution and development of morphology.
Acknowledgments
We thank J. Hunt for providing the O. taurus, and K. Lahtinen and C. Benskin for assistance measuring the beetles and earwigs. J.L.T. was funded by a BBSRC David Phillips Research Fellowship, J.S.K. by the Academy of Finland and N.R.L by NERC Fellowship.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Balmford A, Jones I.L, Thomas A.L.R. How to compensate for costly sexually selected tails: the origin of sexually dimorphic wings in long-tailed birds. Evolution. 1994;48:1062–1070. doi: 10.1111/j.1558-5646.1994.tb05293.x. [DOI] [PubMed] [Google Scholar]
- Cheverud J.M. Developmental integration and the evolution of pleiotropy. Am. Zool. 1996;36:44–50. [Google Scholar]
- Emlen D.J. Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae) Proc. R. Soc. B. 1994;256:131–136. [Google Scholar]
- Emlen D.J. Costs and the diversification of exaggerated animal structures. Science. 2001;291:1534–1536. doi: 10.1126/science.1056607. [DOI] [PubMed] [Google Scholar]
- Gross M.R. Alternative reproductive tactics: diversity within sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- Hedenström A, Møller A.P. Morphological adaptations to song flight in passerine birds: a comparative study. Proc. R. Soc. B. 1992;247:183–187. [Google Scholar]
- Hunt J, Simmons L.W. Patterns of fluctuating asymmetry in beetle horns: an experimental test of the honest signalling hypothesis. Behav. Ecol. Sociobiol. 1997;41:109–114. [Google Scholar]
- Hunt J, Kotiaho J.S, Tomkins J.L. Dung pad residence time covaries with male morphology in the dung beetle Onthophagus taurus. Ecol. Entomol. 1999;24:174–180. [Google Scholar]
- Klingenberg C.P. Integration, modules and development: molecules to morphology to evolution. In: Pigliucci M, Preston K, editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Oxford University Press; New York: 2004. pp. 213–230. [Google Scholar]
- Klingenberg C.P, Nijhout H.F. Competition among growing organs and developmental control of morphological asymmetry. Proc. R. Soc. B. 1998;265:1135–1139. [Google Scholar]
- Knell R.K, Pomfret J.C, Tomkins J.L. The limits of elaboration: curved allometries reveal the constraints on mandible size in stag beetles. Proc. R. Soc. B. 2004;271:523–528. doi: 10.1098/rspb.2003.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiaho J.S, Tomkins J.L. The discrimination of alternative male morphologies. Behav. Ecol. 2001;12:553–557. [Google Scholar]
- LeBas N.R, Hockham L.R, Ritchie M.G. Nonlinear and correlational sexual selection on ‘honest’ female ornamentation. Proc. R. Soc. B. 2003;270:2159–2165. doi: 10.1098/rspb.2003.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBas N.R, Hockham L.R, Ritchie M.G. Sexual selection in the gift-giving dance fly, Rhamphomyia sulcata, favours small males carrying small gifts. Evolution. 2004;58:1763–1772. doi: 10.1111/j.0014-3820.2004.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Marden J.H, Chai P. Aerial predation and butterfly design: how palatability, mimicry and the need for evasive flight constrain mass allocation. Am. Nat. 1991;138:15–36. [Google Scholar]
- Moczek A.P, Emlen D.J. Proximate determination of male horn dimorphism in the beetle Onthophagus taurus (Coleoptera: Scarabaeidae) J. Evol. Biol. 1999;12:27–37. [Google Scholar]
- Moczek A.P, Emlen D.J. Male horn dimorphism in the scarab beetle Onthophagus taurus: do alternative tactics favour alternative phenotypes? Anim. Behav. 2000;59:459–466. doi: 10.1006/anbe.1999.1342. [DOI] [PubMed] [Google Scholar]
- Moczek A.P, Nijhout H.F. Rapid evolution of a polyphenic threshold. Evol. Dev. 2003;5:259–268. doi: 10.1046/j.1525-142x.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Moczek A.P, Nijhout H.F. Trade-offs during the development of primary and secondary sexual traits in a horned beetle. Am. Nat. 2004;163:184–191. doi: 10.1086/381741. [DOI] [PubMed] [Google Scholar]
- Møller A.P, Linden M, Soler J.J, Moreno J. Morphological adaptations to an extreme sexual display, stone-carrying in the black wheatear, Oenanthe leucura. Behav. Ecol. 1995;6:368–375. [Google Scholar]
- Nijhout H.F, Emlen D.J. Competition among body parts in the development and evolution of insect morphology. Proc. Natl Acad. Sci. USA. 1998;95:3685–3689. doi: 10.1073/pnas.95.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout H.F, Wheeler D.E. Growth models of complex allometries in holometabolous insects. Am. Nat. 1996;148:40–56. [Google Scholar]
- Radwan J, Unrug J, Tomkins J.L. Status dependence and morphological trade-offs in the expression of a sexually selected character in the mite, Sancassania berlesei. J. Evol. Biol. 2002;15:744–752. [Google Scholar]
- Roff D.A. Chapman and Hall; New York: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Swallow J.G, Wilkinson G.S, Marden J.H. Aerial performance of stalk-eyed flies that differ in eye-span. J. Comp. Physiol. B. 2000;170:481–487. doi: 10.1007/s003600000124. [DOI] [PubMed] [Google Scholar]
- Tomkins J.L. Environmental and genetic determinants of the male forceps length dimorphism in the European earwig Forficula auricularia. L. Behav. Ecol. Sociobiol. 1999;47:1–8. [Google Scholar]
- Tomkins J.L, Radwan J, Kotiaho J.S, Tregenza T. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 2004;19:323–328. doi: 10.1016/j.tree.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Tomkins, J. L., Kotiaho, J. S., LeBas, N. R. In press. 2005 Matters of scale: positive allometry and the evolution of male dimorphisms. Am. Nat.165 [DOI] [PubMed]
- Unrug J, Tomkins J.L, Radwan J. Alternative phenotypes and sexual selection: can dichotomous handicaps honestly signal quality? Proc. R. Soc. B. 2004;271:1401–1406. doi: 10.1098/rspb.2004.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G.P. Homologues, natural kinds and the evolution of modularity. Am. Zool. 1996;36:36–43. [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York: 2003. Developmental plasticity and evolution. [Google Scholar]