Abstract
When the kidney is subjected to acute increases in blood pressure (BP), renal blood flow (RBF) and glomerular filtration rate (GFR) are observed to remain relatively constant. Two mechanisms, tubuloglomerular feedback (TGF) and the myogenic response, are thought to act in concert to achieve a precise moment-by-moment regulation of GFR and distal salt delivery. The current view is that this mechanism insulates renal excretory function from fluctuations in BP. Indeed, the concept that renal autoregulation is necessary for normal renal function and volume homeostasis has long been a cornerstone of renal physiology. This article presents a very different view, at least in regard to the myogenic component of this response. We suggest that its primary purpose is to protect the kidney against the damaging effects of hypertension. The arguments advanced take into consideration the unique properties of the afferent arteriolar myogenic response that allow it to protect against the oscillating systolic pressure, and the accruing evidence that when this response is impaired the primary consequence is not a disturbed volume homeostasis, but rather an increased susceptibility to hypertensive injury. It is suggested that redundant and compensatory mechanisms are capable of achieving volume regulation despite considerable fluctuations in distal delivery and the assumed moment-by-moment regulation of renal hemodynamics is questioned. Evidence is presented suggesting that additional mechanisms may exist to maintain ambient levels of RBF and GFR within normal range despite chronic alterations in BP and severely impaired acute responses to pressure. Finally the implications of this new perspective on the divergent roles of the renal myogenic response to pressure versus the TGF response to changes in distal delivery are considered and it is proposed that, in addition to TGF-induced vasoconstrictor responses, vasodepressor responses to reduced distal delivery may play a more critical role in modulating afferent arteriolar reactivity, in order to integrate the regulatory and protective functions of the renal microvasculature.
Keywords: Renal Microcirculation, Afferent Arteriole, Myogenic, Tubuloglomerular Feedback, Renal Autoregulation
Introduction
One of the most striking characteristics of the renal circulation is the ability of the kidney to maintain a constant renal blood flow (RBF) and glomerular filtration rate (GFR) as renal perfusion pressure is altered. The dual regulation of both RBF and GFR is achieved by proportionate changes in the preglomerular resistance and is believed to be mediated by two mechanisms, tubuloglomerular feedback (TGF) and the renal myogenic response. TGF involves a flow-dependent signal that is sensed at the macula densa, and alters tone in the adjacent segment of the afferent arteriole via a mechanism that remains controversial, but likely involves adenosine and/or ATP (30, 80, 144). The myogenic response involves a direct vasoconstriction of the afferent arteriole when this vessel is presented with an increase in transmural pressure. The current view is that these two mechanisms act in concert and that their primary role is to stabilize renal function by preventing pressure-induced fluctuations in RBF, GFR and the delivery of filtrate to the distal tubule (“distal delivery”).
Over the last two decades, evidence has accrued to indicate that this “autoregulatory” response plays a concurrent role in protecting the kidney from hypertensive injury (14, 15). This view is based on the strong link between autoregulatory capacity and susceptibility to hypertensive injury. In the presence of intact autoregulation, minimal injury is observed despite substantial hypertension. However, when blood pressure (BP) is elevated beyond the upper limit of normal autoregulatory capacity, renal damage develops rapidly. Conversely, if autoregulatory capacity is diminished, susceptibility to hypertensive renal damage is greatly enhanced and injury is observed with even moderate hypertension. Nevertheless, the primary function of the renal vascular responses to pressure, and of the myogenic and TGF mechanisms, is believed to be “regulatory”, as reflected in the very term “autoregulation”. Thus “renal protection” is lost when “renal autoregulation” fails. However, as discussed below, the requirements for maintaining a constant GFR and for protecting the glomerulus from hypertensive injury differ, even though both involve a regulation of glomerular capillary pressure (PGC). Moreover, the myogenic response and TGF system clearly sense different signals and, therefore, may play distinct roles in “protection” and “regulation”. This review presents the authors' perspective on the role of vascular responses to pressure in regulating renal function and in protecting the kidney against the adverse effects of elevated systemic BP.
Historical Perspectives
Renal autoregulation may have first been described by Rein in 1931 (125). However as early as 1902, Bayliss observed that the renal vasculature exhibits a profound vasoconstriction when the kidney was subjected to elevated pressure (12). Bayliss viewed the renal response as an example of the “myogenic” response of vascular beds. In regard to the purpose of this general response, he suggested that “The peripheral powers of reaction possessed by the arteries is of such a nature as to provide as far as possible for the maintenance of a constant flow of blood through the tissues supplied by them, whatever may be the height of the general blood-pressure…” (12). The concept that renal vascular responses to pressure might also serve to regulate function in the kidney was further advanced by the observation of Forster and Maes in 1947 (49) that not only RBF but also GFR remained constant with acute elevations in BP. From the outset, it was recognized that the dual regulation of GFR and RBF could only be achieved if pressure-induced vasoconstriction was restricted to preglomerular resistance vessels.
It was generally accepted that, in the kidney, the need for volume preservation required that the capacity of the tubules to reabsorb the filtrate not be overwhelmed by excessive glomerular filtration rates. Specifically, the delivery of filtrate to the distal segment which has a more limited reabsorptive capacity needed to be precisely regulated. The unique anatomical relationship between the early distal nephron and its glomerular vascular pole was recognized by Goormaghtigh to provide a potential site for such regulation (53). Thus in the vast majority of mammalian nephrons, the early distal tubule makes direct contact with the vascular pole of its originating glomerulus. The early observations of Hársing that inhibition of proximal fluid reabsorption decreased both GFR and RBF, led to his suggestion that increased filling of the distal tubule might evoke signaling via the macula densa to regulate vascular resistance (68). The subsequent demonstrations that alterations in the composition of the fluid presented to this early distal site caused reductions in the up-stream proximal stop-flow pressure (154) and that increased early distal tubular flow reduced the GFR of the affected nephron (136) established the presence of such a “tubulo-glomerular feedback” coupling distal filtrate delivery to preglomerular vascular responses. These observations supported the hypothesis, first proposed in 1963 (64, 152), that the autoregulation of GFR and RBF involved a unique mechanism in the kidney whereby preglomerular vasoconstriction was triggered by increased distal delivery. This concept was consistent with the prevailing view that, in addition to a general myogenic response (e.g., 7, 51), the differing physiologic and metabolic requirements of tissues needed to be achieved by organ-specific vascular regulatory mechanisms. Subsequent approaches, including mathematical modeling, led to the consensus that both TGF and myogenic vasoconstriction are essential for normal autoregulation (8, 74, 108, 116), though their relative contributions remain controversial. Thus the current view is that when BP is elevated, these two mechanism act in concert to achieve a precise regulation of GFR and RBF. The underlying assumption throughout has been that this response reflects a phenomenon whose primary purpose is to insulate renal sodium and volume regulation from fluctuations in BP (e.g., 75, 153, 114).
During this same period, Wilson and Byrom conducted their pioneering investigations into the pathogenesis of target organ damage seen in the 2 kidney/1 clip model of hypertension (2K/1C) and the involvement of autoregulatory or myogenic mechanisms (171, 172). Based on the local vasospasm observed in the cerebral vasculature using the cranial window approach, it was initially thought that an exaggerated myogenic vasoconstriction and tissue ischemia led to the manifestation of “hypertensive encephalopathy” (28). However, subsequent studies by these and other investigators indicated that an overwhelming of the myogenic capacity in some vascular segments by excessive BP led to focal vasodilatation, increased wall tension and, ultimately, hypertensive cerebral vascular injury (reviewed in 27, 50). Similar mechanisms were postulated for the renal injury seen in this hypertensive model. Studies in the uninephrectomized deoxycorticosterone acetate (DOCA)/salt model of malignant nephrosclerosis by Hill and Heptinstall confirmed the enhanced susceptibility of a dilated renal vascular bed to hypertensive injury (72). These investigators additionally suggested that the severity of such damage may depend not only on the severity of the hypertension but also on the renal autoregulatory or myogenic capacity. The importance of local myogenic mechanisms in protecting against hypertensive injury was formally recognized in the concept proposed in 1972 that hypertensive encephalopathy may develop only when BPs exceed the upper limit of cerebral blood flow autoregulation (94). A great deal of experimental and clinical evidence has since been obtained in support of the concept (86, 93). Moreover, although the concept was initially proposed in the context of target organ damage observed with severe or malignant hypertension, an association between preglomerular vasodilatation, increased PGC and progressive glomerulosclerosis even with moderate hypertension, was subsequently recognized in chronic kidney disease (CKD) models (9, 10, 77, 118). The direct demonstration that, in addition to being vasodilated, the preglomerular vasculature of the 5/6 renal ablation model of CKD also exhibits impaired renal autoregulation provided a potential explanation for the greatly enhanced glomerular susceptibility to hypertensive injury seen in this model (21).
Collectively, such observations suggest that the same mechanisms responsible for renal autoregulation play a critical role in protecting the kidney from the damaging effects of hypertension. Since PGC is a primary determinant of GFR and an elevation in PGC is thought to be an initiating event in the sequence leading to glomerular injury, renal protection might be viewed as simply as an ancillary consequence of the regulation of GFR. Indeed, despite the clear linkage of the loss of autoregulatory capacity and glomerular injury, the primary importance of the “regulatory” role of renal autoregulation and its requirement for volume homeostasis has remained a cornerstone of renal physiology.
BP Variability and the Requirements for Protection versus Regulation
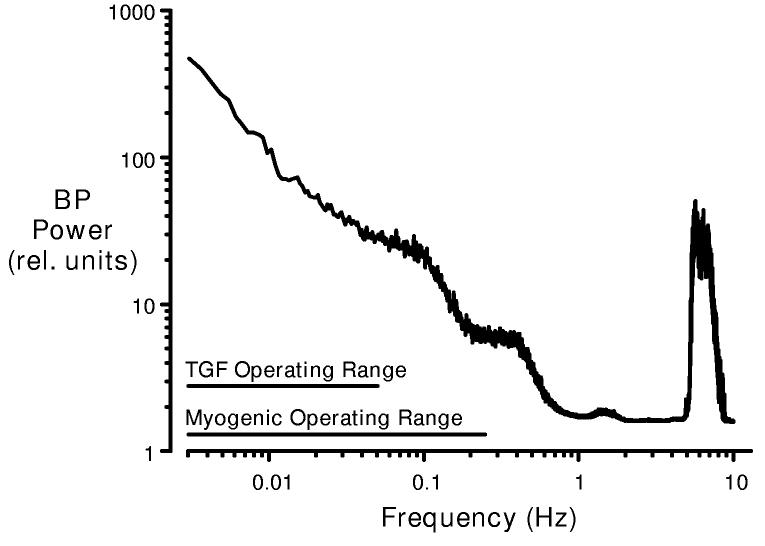
A fundamental consideration in regard to both the “regulatory” and the “protective” functions of the renal vasculature is the fact that BP spontaneously fluctuates at multiple frequencies. This is illustrated in figure 1, which depicts the BP power spectrum of the conscious rat. Because the amplitude of the BP fluctuation varies with frequency, the BP power (energy/unit time, proportional to the square of the amplitude) is also a function of frequency. In general, slow events exhibit larger amplitudes than more rapid signals (73, 103). The exception to this well-described 1/frequency relationship is the very rapid BP oscillation due to the pulse, which manifests as the power peak observed at the heart rate (∼6 Hz in the rat). These various frequencies summate to form the complex BP signals that are delivered to the preglomerular vasculature in vivo. Thus the BP signals that evoke renal autoregulatory responses are always oscillatory in nature, and the kinetic attributes of TGF and the myogenic mechanism determine the frequency range over which both autoregulation and renal protection can manifest.
Figure 1.
Blood pressure (BP) power spectrum in the conscious rat (mean data, n=10). The BP signal is a complex wave form derived from various fluctuations that oscillate at different frequencies. BP power is proportional to the square of the amplitude of these fluctuations (from the mean BP) and is plotted as a function of oscillation frequency (f). Note the 1/f relationship seen at frequencies below 1 Hz and the natural frequencies of TGF and the myogenic response. A major BP power peak is produced at the heart rate frequency (6 Hz in the rat). By current interpretations, this signal is beyond the myogenic operating range and, accordingly, is handled passively by the renal vasculature.
Dynamic autoregulatory studies, employing transfer function and frequency domain analyses, have revealed the natural frequency of the TGF mechanism in the rat to be in the range of 0.05 Hz (2, 32, 36, 38, 56, 76, 122, 165,166). The myogenic response is much faster, with a natural frequency of 0.1-0.2 Hz in the anaesthetized rats and 0.2-0.3 Hz in conscious animals (ibid). Essentially similar data regarding the kinetics of these mechanisms have been obtained through analyses of RBF responses to step changes in BP (84, 85, 102,175). These natural frequencies imply that the myogenic response can prevent changes in RBF in response to BP fluctuations that occur at intervals greater than 3-4 seconds, whereas TGF responds to slower BP fluctuations, over intervals of 20 seconds or longer. Given the differences in their mechanisms, it is not surprising that these two systems exhibit markedly different response times. To elicit a TGF response, a pressure increase must be transmitted and elicit an increase in the flow rate through the thick ascending limb. This, in turn, alters the composition of the fluid presented to the macula densa, stimulating the secretion of a vasoconstrictor near the afferent arteriole, ultimately increasing preglomerular resistance. In contrast, the myogenic mechanism involves an intrinsic smooth muscle response to increased transmural pressure. The underlying mechanisms, though not fully resolved, involve depolarization, activation of voltage-gated L-type Ca+2 channels and Ca+2 entry triggering a rapid vasoconstriction (39).
The observation that fluctuations in BP occurring faster than 0.3 Hz are accompanied by parallel RBF fluctuations, without attenuation, has been interpreted as indicating that the renal vasculature responds passively to such high frequency signals (e.g., 75). This interpretation is reasonable if one considers a regulation of function to be the primary role of this response. As illustrated in figure 1, major variations in BP occur primarily at frequencies well below 0.3 Hz and the natural frequencies of the myogenic and TGF mechanisms are sufficient to attenuate their effects on renal function. The focus on BP fluctuations occurring exclusively at low frequencies (<1 Hz) is also appropriate when considering only the regulation of function. Perturbations in BP which persist for only a fraction of a second would have insignificant effects on mean RBF and GFR (41). Conversely, to be effective, renal protection must be achieved over the full range of BP frequencies. Indeed, it is most critical to provide protection against the rapidly oscillating systolic BP, as this component has been shown to correlate most closely with end organ hypertensive injury (22, 71, 82, 174). Yet the emphasis on the regulation of function as the end-point of pressure-dependent renal vascular responses has led to the concept that the high frequency systolic BP signal (occurring at 6 Hz in the rat) is handled passively by the renal vasculature. Clearly, from the viewpoint of renal protection, this cannot be true.
The Renal Myogenic Response to the Oscillating Systolic Pressure Signal
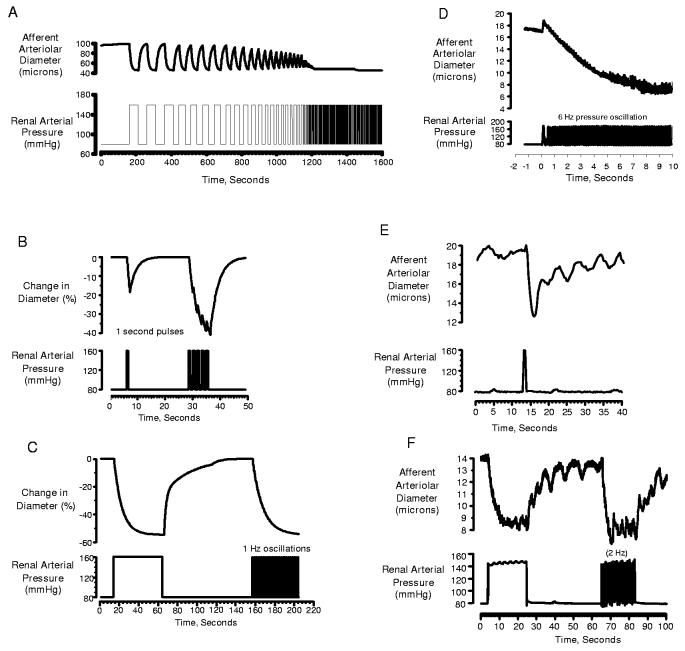
The considerations presented above illustrate that the requirements on the vasculature to achieve renal protection are quite distinct from the requirements to achieve autoregulation. Our current concepts, which primarily address the latter, cannot explain how the renal vasculature normally protects the kidney from the damaging effects of the oscillating systolic BP. Our recent studies using the in vitro perfused hydronephrotic kidney provide a potential resolution of this issue. As discussed in detail in references 97 and 98, the afferent arteriole exhibits a very short delay in activation (200-300 ms) and rapid constriction kinetics when exposed to a sudden pressure increase. When pressure is subsequently reduced, vasodilation is evoked after a much longer delay (∼1 s). Using these kinetic parameters, we developed a simple mathematical model that produced myogenic transfer functions similar to those revealed by frequency domain analysis in the intact rat kidney in vivo (97). Thus the myogenic response of the model exhibited an operating frequency of 0.3 Hz. As shown in figure 2A, the model predicted an ability of the afferent arteriole to track pressure changes presented at low frequencies, but not when oscillations exceeded 0.3 Hz. However, rather than exhibiting a passive response to high frequencies, the model exhibits a sustained vasoconstriction. The kinetic determinants of the sustained response are further illustrated by figure 2B. When the model is presented with a pressure transient of short duration (1 sec), it responds with a transient vasoconstriction that is slightly out of phase with the pressure signal. When presented with a train of such pulses, the responses merge into a sustained vasoconstriction (figures 2B&C). As illustrated in figures 2D-F, the actual responses of the afferent arteriole replicated these predictions of the mathematical model. Moreover as shown in figure 2D, this ability of the afferent arteriole to respond to oscillating pressure signals extended to oscillations occurring at the heart rate (6 Hz).
Figure 2.
Examples of responses to pressure transients and oscillating pressure signals predicted by mathematical model based on kinetics of afferent arteriole (A-C) and actual responses observed in the hydronephrotic rat kidney (D-F). As shown in panel D, pressure oscillations presented at the rat heart rate (6 Hz) elicit a sustained afferent arteriolar vasoconstriction. Panels B,C,E &F reproduced with permission from reference 97.
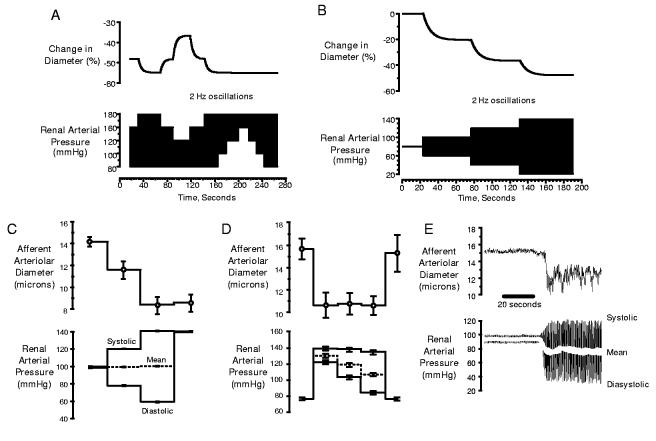
As discussed above, BP signals present to the afferent arteriole in vivo as complex wave forms, consisting of a summation of oscillations occurring at each frequency. The rapidly oscillating systolic BP is an incessant component of this signal and is always superimposed on the slower oscillations. Which pressure is sensed by the myogenic mechanism? As figure 3A illustrates, the model predicted that the magnitude of the responses evoked under such conditions is exclusively determined by the systolic signal. Changes in diastolic and mean pressures had no effect, whereas elevations in systolic pressure evoked responses even if mean pressure was held constant (figure 3B). As shown in figures 3C-E, the actual afferent arteriolar responses faithfully mirrored those predicted by the model. Thus, not only is the afferent arteriole capable of responding to oscillating systolic BP, it is this signal that would provide the primary stimulus for setting myogenic tone under in-vivo conditions.
Figure 3.
Predicted dependency of myogenic tone on systolic BP signal by mathematical model (A&B) and actual afferent arteriolar responses observed in hydronephrotic rat kidney preparation, confirming this prediction (C, D& E). Reproduced with permission from reference 97.
These findings await confirmation by investigations in the intact kidney. Early studies examining the effects of pulsatile versus non-pulsatile perfusion on renal function suggested similar autoregulatory responses to both static and pulsatile pressure signals (126, 140), in contradiction with the above predictions. These investigations were hampered by the indirect methods available to assess blood flow and focused on the role of pulsatile perfusion in regard to organ preservation and extracorporeal perfusion, rather than the specific effects of systolic versus mean pressures in setting autoregulatory tone. Moreover, depulsation per se has effects that may confound interpretations. Many early studies have shown this maneuver to reduce renal cortical blood flow and GFR and to stimulate renin (52, 101, 104), observations confirmed by recent studies (e.g., 111, 149, 155). Investigations specifically designed to critically evaluate the influences of mean versus systolic BP signals on myogenic tone in vivo are needed and currently lacking. In considering this problem, it should be noted that anesthetics may alter myogenic kinetics (e.g., 36) and dynamic autoregulatory studies in conscious rats consistently demonstrate faster myogenic responses than those using anesthetized animals.
Modeling results clearly indicate that the ability of the afferent arteriole to respond to oscillating signals and the dominant role of the systolic BP in setting tone are both determined by its kinetic attributes. The dynamic signature of the myogenic component of autoregulation of our in vitro model has been shown to be identical to that of the normal in vivo kidney in the conscious rat (35), suggesting normal myogenic kinetics. Moreover, transfer function modeling demonstrated that our mathematical construct mimics the normal myogenic signature (97). The time course for myogenic vasoconstriction in our model is nearly identical to that reported for the normal kidney. Young and Marsh found that, in response to an acute BP increase, renal vascular resistance increased after a delay of <1 s, achieved 50% of the response within 3-4 s and the response reached completion by 15-20 s (175). More recently, Just and Arendshorst (85) reported a delay in the onset of pressure-induced vasoconstriction in the intact kidney of 390 ms and a time constant of 5.1 s. These parameters, reflecting the global response of the renal vasculature, correspond closely to those we observed at the single arteriole level (200-300 ms delay and 4 s time constant (97, 98)). In vitro studies have not demonstrated such rapid kinetics in the juxtamedullary nephron preparation (163), perhaps suggesting that such mechanisms play a more important role in protecting cortical nephrons. However, the pressure signals evoked were relatively slow in onset (>2 s to achieve peak), impacting on an evaluation of the delay. A critical characteristic underlying the response to pressure oscillations is the longer delay in the onset of the vasodilation observed when pressure is reduced. Just and Arendshorst (85) reported a delay in pressure-induced vasodilation of 530 ms, considerably shorter than the 1 s delay we found in the hydronephrotic kidny (97), but nevertheless significantly longer than the delay in vasoconstriction in the intact kidney. Longer delays in the offset versus onset of vasoconstrictor responses induced by changes in loop of Henle flow are also reported (29, 37). The dilation observed at the afferent arteriolar level was best fit to a bi-exponential function, but achieved 66% of the maximal response within ∼3 s (97), similar to overall rate constant reported by Just and Arendshorst (2.6 s (85)). Further investigations are needed to confirm these kinetic findings and to critically determine whether the systolic or mean pressure is the primary determinant of myogenic tone in the intact in vivo setting.
Implications for the protective and regulatory roles of the renal autoregulatory response
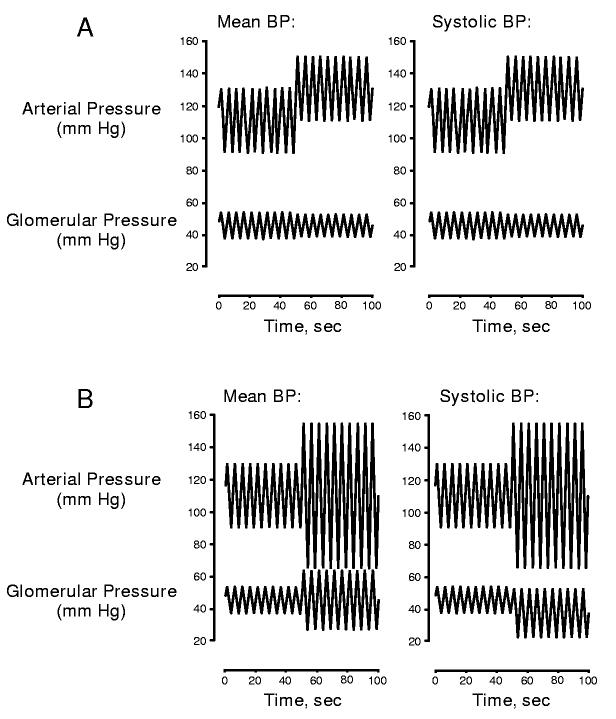
Obviously RBF and GFR are determined by mean, not systolic BP. Accordingly, a myogenic mechanism that responds exclusively to the systolic BP could contribute autoregulation only to the extent changes in mean BP parallel changes in systolic BP. However in regard to renal protection, the dominant influence of the systolic BP is quite logical. Figure 4 illustrates a mathematical treatment comparing how myogenic responses triggered either by mean or systolic BP would attenuate the transmission of pressure transients to down-stream glomerular capillaries. Note that when mean and systolic BP change in concert, the two models elicit a similar regulation of PGC (figure 4A). However, as shown in figure 4B, increases in pulse pressure or episodes of isolated systolic hypertension result in a transmission of the peak pressure transient when mean BP determines myogenic tone, but not when the myogenic response is linked to the systolic BP signal.
Figure 4.
Modeling study illustrating the consequences, in regard to the regulation of glomerular capillary pressure, of two differing myogenic mechanisms, one in which the level of tone is dependent on mean BP (left), the second in which tone is dependent on the systolic BP (right). Note that a similar regulation of PGC is seen when mean and systolic pressure change in concert (A). However, isolated transients in systolic pressure are transmitted to the glomerulus when mean pressure sets myogenic tone, but not when tone is set by the systolic signal (B).
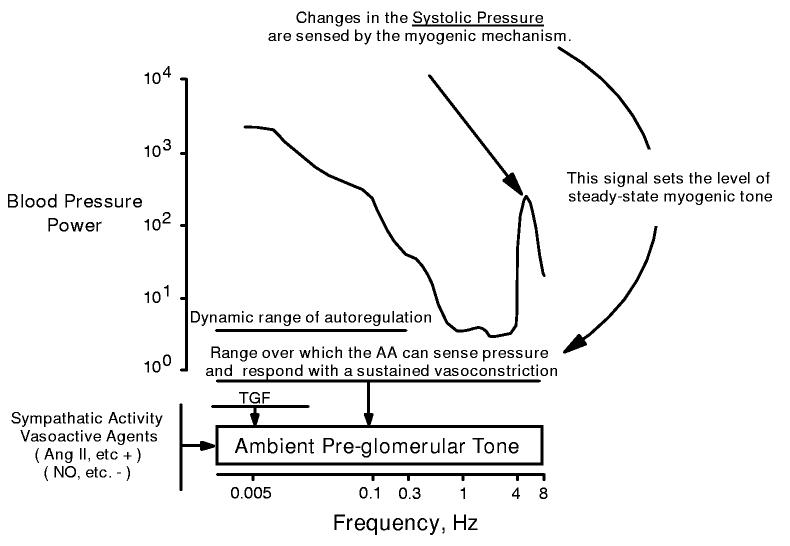
The above considerations suggest an alternate view of the role of the myogenic response in regard to autoregulation and renal protection (illustrated in figure 5). The systolic BP signal determines a level of sustained ambient myogenic tone. Increases in this signal trigger increases in tone, thereby imposing increased impedance to limit the transmission of BP transients to the downstream glomerulus. To the extent that changes in mean BP mirror changes in the systolic BP signal, such alterations in myogenic tone would also result in autoregulation. Nevertheless, the primary goal achieved by this response is a protection against glomerular transmission of the pulsatile systolic BP. The natural frequencies of the myogenic response and TGF would determine the dynamic range over which autoregulation would manifest. However, renal protection would be achieved over the full range of pressure oscillations by the sustained increase in myogenic tone. This modified view may explain how a myogenic mechanism operating at 0.3 Hz normally protects the kidney from the more rapidly oscillating systolic BP.
Figure 5.
Alternate view of pressure-induced activation of the renal vasculature. Changes in the oscillating systolic pressure are sensed by the myogenic mechanism and it is this signal that sets the level of steady-state myogenic tone. This response provides protection over the full range of BP frequencies by limiting the transmission of pressure transients to the glomerular capillaries (see figure 4). Thus the myogenic response would contribute to a steady-state ambient level of pre-glomerular tone. Dynamic autoregulation of RBF and GFR occur at frequencies below the myogenic operating range as a consequence of this myogenic response and, at lower frequencies, as mediated by TGF.
If the myogenic mechanism protects the glomerulus by responding exclusively to the systolic BP, it must be recognized that an inherent corollary to this hypothesis is that autoregulation of GFR or RBF, at least as it relates to the myogenic mechanism, would be a secondary consequence. This point is clearly illustrated by the myogenic response depicted in figure 3E. In this experiment an oscillating pressure signal was imposed in which the systolic pressure increased, while mean pressure was reduced. If the myogenic response exists to preserve GFR, the vessel should dilate to maintain PGC as mean pressure is reduced. It actually constricts. This response is consistent with a primary role in protecting against increases in the systolic BP, but is clearly “counter regulatory” in regard to the control of GFR. The responses to mean pressures that are depicted in figure 3 B&C would similarly fail to regulate GFR.
It has been argued that since GFR is influenced by factors such as plasma colloid osmotic pressure, proximal tubular pressure and the filtration coefficient (Kf), a myogenic mechanism responding to changes in transmural pressure alone would not be not sufficient for its regulation (e.g., 112). If the pressure signal that is being sensed is the systolic BP signal, rather than mean BP, the role of this mechanism in regulating GFR might be further questioned. Thus TGF, by responding to alterations in distal delivery, might play a more prominent role. Conversely, since TGF does not directly sense and respond to pressure, it is less suited for renal protection. Whether these systems evolved primarily to protect against hypertensive injury or to insulate function from BP fluctuations is a difficult question. However, one way of addressing this issue is to examine the consequences when these autoregulatory mechanisms are impaired.
Consequences of impaired renal autoregulation on renal protection
The kidney appears to normally be protected from hypertensive injury as long as the BP remains within the autoregulatory range. However, when autoregulation is impaired, the vulnerability to such injury is markedly augmented. The use of BP radiotelemetry has allowed an assessment of the quantitative relationships between BP and renal damage. This is illustrated in figure 6, which depicts individual measures of BP and renal injury obtained using the most commonly employed rodent models for essential hypertension (spontaneously hypertensive rat, SHR), malignant nephrosclerosis (stroke-prone SHR, SHRsp) and CKD (5/6 ablation model). The SHR exhibits efficient autoregulation (6) and, despite significant elevations in BP, shows little renal damage even when BP is further increased by salt supplementation (55). The SHRsp, which also has intact renal autoregulation, develops vascular and glomerular damage, but only when exposed to more severe elevations in BP (54, 55). It is of note, that the autoregulatory responses of the SHR are shifted to higher pressures (81), an adaptation that extends the range of renal protection, but reduces the ability to regulate RBF and GFR at lower pressures. When injury is observed in the aging SHR, it is often restricted to juxtamedullary glomeruli (48). This may relate to the observation that the intermediate segments of the interlobular artery (ILA) in this strain exhibit an enhanced myogenic response (69), which would provide added protection for the distal superficial glomeruli, but not the juxtamedullary nephrons. Autoregulatory responses are also reported to be slower in the juxtamedullary cortex of the aged SHR (127) and this could contribute to the pattern of injury. Finally, when autoregulatory capacity in the SHR is reduced by 5/6 renal ablation, these animals rapidly develop malignant nephrosclerosis (17).
Figure 6.
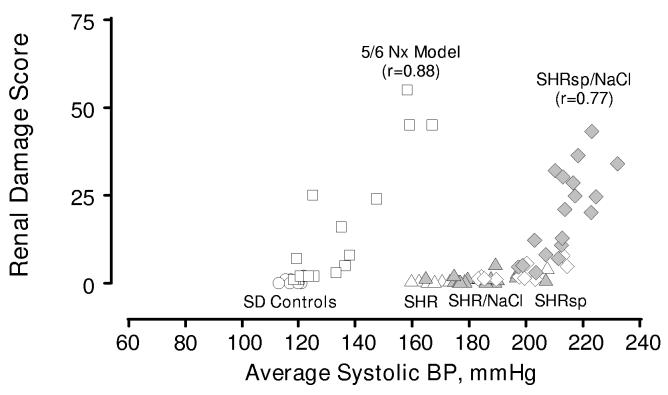
Relationships between renal injury and systolic BP in normotensive Sprague Dawley rats (SD, circles), SHR (triangles), stroke-prone SHR (SHRsp, diamonds) and 5/6 remnant kidney model (squares) and effects of increased dietary salt on SHR (grey triangles) and SHRsp (grey diamonds). Data reproduced with permission from references 16 & 55. Pattern of injury parallels that of renal autoregulation. Thus, the injury seen in the SHRsp/NaCl occurs at BPs that exceed the myogenic limit. The remnant kidney exhibits impaired autoregulation, and exhibits a much lower BP threshold for hypertensive injury than normal or SHR kidneys. (modified with permission from reference 14).
As shown in figure 6, the BP threshold for injury in the 5/6 ablation model of CKD, which exhibits impaired autoregulation, is much lower than that of the SD or SHR (16. 21). The pattern of injury in this model is predominantly that of focal and segmental glomerulosclerosis, consistent with elevated glomerular pressures (14, 15,117). The role of hypertension in this lesion is further demonstrated by the fact that lowering BP results in proportionate reductions in injury (20,59). Similar relationships between impaired renal autoregulation and increased renal susceptibility to hypertensive injury have been noted in the DOCA/salt model of hypertension (72,109), and in the non-clipped kidney of the 2K/1C model (124,133). Moreover, interventions that alter autoregulatory capacity, such as dietary protein restriction or calcium channel blockers (CCBs), produce corresponding changes in susceptibility to hypertensive injury in models of CKD (57, 60, 62) and in the DOCA/salt and 2K/1C models of hypertension (92,133).
Observations in genetic rat strains provide further evidence linking impaired renal autoregulatory mechanisms to increased susceptibility to hypertensive injury. An example is the Fawn-Hooded rat, which spontaneously develops hypertension, proteinuria and focal and segmental glomerular sclerosis at a young age (160,161). This animal exhibits impaired renal autoregulation and severely diminished afferent arteriolar myogenic reactivity, although TGF is reported to be intact (160,161,162). The Brown Norway rat (BNR) also exhibits impaired myogenic responses, but does not develop hypertensive renal injury as it normally exhibits a relatively low BP (33,164). However, as shown by Churchill and coworkers (33), when exposed to hypertension by transplantation into the SHR, the BNR kidneys develop substantially more severe injury as compared to the SHR kidneys.
While the above findings demonstrate a link between reduced autoregulatory capacity and hypertensive injury, the specific contributions of impairments in myogenic versus TGF mechanisms are not fully known. The difficulties involve the same problems impeding attempts to assess the individual contributions of these two interacting systems when autoregulation is intact. In the DOCA/salt, 2K/1C, and the 5/6 ablation models, TGF responses of the affected kidney are reported to be blunted or reset (109,124,128,133), but a concurrent defect in myogenic reactivity is not excluded (e.g., 70). Indeed, dynamic autoregulation studies in the conscious 5/6 ablation model suggest an attenuation of the myogenic component (18). Moreover, in the Fawn-Hooded, the Dahl salt-sensitive and the BNR, the genetic defect in autoregulation seems to primarily involve the myogenic mechanism, while TGF is intact or even enhanced (87, 88, 145). In this context, an examination of the susceptibility to hypertensive injury in the gene deletion models with absent or impaired TGF (30, 80, 135, 144) would be illuminating. Regardless of the pathogenesis of impaired autoregulation in these animal models, it is important to note that qualitatively similar data have been obtained in humans (reviewed in 14, 15). Thus, most patients with primary uncomplicated essential hypertension who exhibit intact renal autoregulation lack significant renal injury. By contrast, patients with diabetes or CKD exhibit impaired renal autoregulation and a greatly increased susceptibility to renal damage with even modest hypertension. Indeed, the deleterious effects of co-existent hypertension are believed to play a major role in the progression of both diabetic and non-diabetic CKD. Recognition of the lower BP threshold for renal damage in these patients is evidenced by the progressively lower goals for optimal BP control that are recommended for CKD patients by recent guidelines (83).
Consequences of impaired renal autoregulation on volume homeostasis
In contrast to the unambiguous evidence linking impaired autoregulation to hypertensive renal injury, there little if any evidence to suggest that impaired autoregulation is accompanied by disturbed volume homeostasis. Hypertension, a potential manifestation of impaired volume regulation, is not clearly linked to a loss of autoregulation. In the rat remnant kidney model, hypertension develops following conventional 5/6 renal ablation by infarction, but not when renal mass is reduced by surgical excision (16, 58, 61). In the BNR, BP is not only typically reduced, but manipulations such as DOCA/salt, have minimal effects (33). Similarly, there is no evidence to date of detectable disturbances in volume homeostasis in the murine gene-deletion models lacking TGF (e.g., 159). This may reflect compensatory adaptations or impaired TGF may cause volume disturbances only when the system is exposed to specific stresses. Given the critical importance of volume control for survival and the redundancy of the mechanisms regulating salt excretion (1,40,45), these findings may not be too surprising.
A compelling argument concerning the potential role of autoregulation in volume regulation is that the impact of overwhelming the distal reabsorptive capacity would be catastrophic. However, compensatory mechanisms appear to accommodate increased distal delivery under most circumstances. A common example is the compensatory response to the chronic administration of loop diuretics, which not only increase distal delivery but also block TGF (43, 89). After an initial loss of volume, proximal and distal compensatory adaptations achieve a new steady-state within three to four days, despite continued use of the diuretic (ibid). A similar time-course is observed in response to large perturbations in salt intake. Demonstrating that, in addition to the rapid TGF response, chronic adaptations effectively regulate volume. The observed resetting of TGF in settings associated with persistent changes in distal salt delivery is consistent with such interpretations (5,151,167). Finally, mechanisms other than TGF, including tubuloglomerular balance (66) contribute to the regulation of distal delivery.
Clearly TGF has the potential to counteract inappropriate swings in distal delivery, regardless of BP signals, and is likely critical in acute tubular injury. Indeed, impaired proximal reabsorption coupled with impaired distal mechanisms would be life threatening, if filtration were not curbed. However, acute increases in BP normally evoke a proportionate pressure-induced natriuresis, despite autoregulation. While TGF may modulate this response, a prevention of BP-induced increases in distal delivery would interfere with this mechanism (47, 65, 105). Pressure-induced natriuresis is an essential component of BP regulation and experimental hypertension is exacerbated when this mechanism is prevented by servo-null control of renal perfusion pressure (67). Its modulatory role in human hypertension is also evident by the chronic volume depletion commonly seen in patients with pheochromocytoma (25).
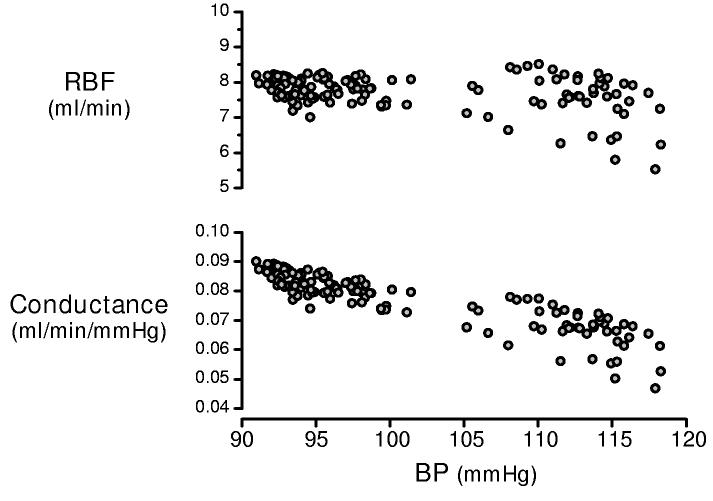
Thus while it may be argued that compensatory mechanisms mask an appreciation of the normal contribution of renal autoregulation to volume homeostasis, it is clear that a precise acute stabilization of renal hemodynamics is not an a priori requirement for volume control. Indeed, in contrast to observations made in anesthetized animals, considerable time-dependent variability is seen when one monitors spontaneous RBF and BP in conscious animals. This is illustrated by figure 7, which depicts the variations in RBF and BP seen over a two hour period in a conscious unrestrained rat. Similar observations are reported by other laboratories (120, 123, 142, 143). Such temporal variability may may reflect the influence of neurohormonal and metabolic inputs (figure 5), but clearly does not support the concept of moment-by-moment autoregulation. It is, however, not inherently inconsistent with a modulatory role for TGF or other mechanisms, acting on slower time scales, to provide an approximate stability of RBF and GFR.
Figure 7.
Spontaneous variations in RBF in the conscious unrestrained Sprague Dawley rat over a two hour period (day-time). The RBF and conductance values are 100 second moving averages with 50% overlap of the segments. Note that although autoregulation is evident from the pressure-dependent conductance responses (lower panel), RBF values exhibit marked variability (top).
Regulation of RBF and GFR independent of autoregulatory responses
As experimentally defined, autoregulation concerns the rapidly acting mechanisms that defend against imposed perturbations in BP from causing acute changes in RBF and GFR. An underlying assumption to the postulate that such acute responses are required for normal renal function is that these same mechanisms are required to prevent chronic elevations in BP from altering ambient GFR and RBF. Thus without autoregulation, BP differences would be expected to influence basal renal hemodynamics. Curiously, this is typically not the case.
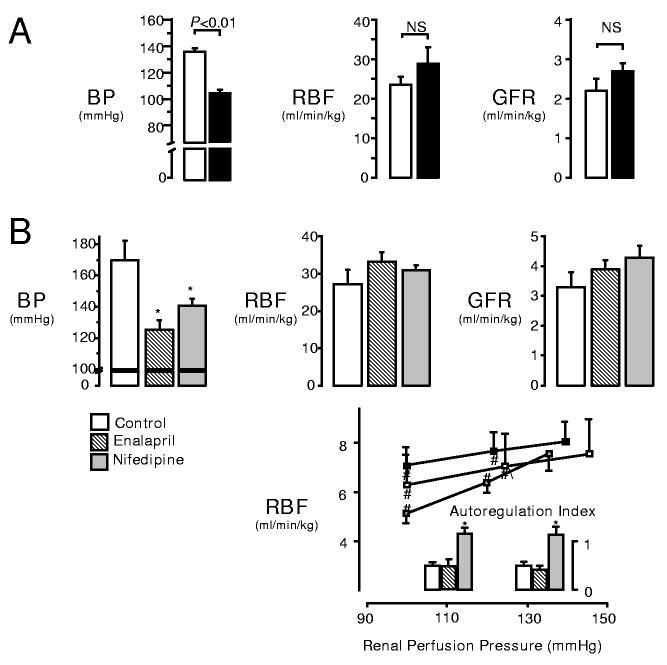
A clear example relates to the ambient levels of RBF and GFR observed in different CKD models. In the “infarction model” (RK-I) and “surgical excision model” (RK-NX), 5/6 of the renal mass is removed, but by two procedures that have different effects on BP. During the initial compensatory phase (2-3 weeks), minimal renal injury is seen and each model exhibits a marked impairment in autoregulation (58, 61). However, as depicted in figure 8A, the basal GFR and RBF are very similar, despite marked differences in BPs. Similarly, the therapeutic reduction in chronic BP (RK-I model) does not alter ambient GFR or RBF. This is seen even with nifedipine, which abolishes all residual autoregulatory capacity (60). Thus, GFR and RBF are similar in untreated, and nifedipine- and enalapril-treated RK-I (figure 8B) despite differing BPs and autoregulatory capacities. The underlying mechanisms are not clear. Reductions in Kf often accompany PGC elevations and this is reversed when PGC is lowered (4, 157).
Figure 8.
A: Ambient RBF and GFR of CKD “infarction model” (RK-I, white bars) and “surgical excision model” (RK-NX, black bars) remain similar despite significantly different BP and impaired autoregulation. B: Effects of 2 weeks of anti-hypertensive treatment on ambient BP, RBF and GFR in infarction CKD model (RK-I). Lower panel, illustrates impaired autoregulatory capacity (autoregulatory index of 0 or 1 indicate perfect or no autoregulation). Chronic reduction in BP with either enalapril (striped) or nifedipine (solid grey) did not alter ambient GFR or RBF, even though nifedipine completely abolished any residual autoregulatory capacity. *indicates P<0.05 versus control. # indicates P<0.05 versus basal. (Data reproduced with permission from references 60 & 61).
The lack of influence of chronic BP elevations, despite impaired acute autoregulatory capacity, suggests the presence of other mechanisms capable of regulating basal GFR and RBF over a longer time course. Clearly, chronic alterations in GFR and RBF occur in response to metabolic and excretory needs. Examples include the hyperfiltration observed with protein feeding (90) and uninephrectomy (19,24) and in pregnancy (11). Evidence implicates a chronic resetting of TGF in such settings and in the hyperfiltration seen in diabetes (141, 158). Longer acting adaptations to elevated pressure may also exist, and such mechanisms could contribute to the observed lack of impact of acute autoregulatory impairment on volume homeostasis. Just and Arendshorst (84) recently reported that when both the myogenic mechanism and TGF were inhibited, a third mechanism that exhibited a very slow time course was discerned. The relationship of this putative third mechanism to GFR and RBF regulation in the CKD models remains to be examined. However, it should be pointed out that without the existence of such a compensatory mechanism, antihypertensive therapy in patients with CKD and impaired renal autoregulation would not be feasible, as the resultant reductions in BP would cause acute, proportional, and persistent declines in renal function.
Interactions between myogenic and TGF mechanisms
The myogenic and TGF responses share the same effector site, the afferent arteriole and interactions between these two systems are unavoidable. Each response is capable of modulating the other. The prevailing view is that these two mechanisms act in concert to accomplish the same end, a stabilization of renal function when BP is altered. This has led to a focus on synergistic interactions. If TGF and myogenic mechanisms play distinct roles in regulating function and protection, their interactions might be more complex. Macula densa triggered responses because of their slower time course, could modulate the more rapid operation of a protective myogenic mechanism. Moreover, both synergistic and antagonistic interactions could occur, based on physiologic needs. Thus, in addition to TGF-mediated vasoconstriction, macula densa-mediated vasodepressor responses could limit myogenic reactivity, when protective responses disrupt renal function.
The maintenance of an adequate GFR and/or distal delivery is clearly important for normal volume homeostasis. Compensatory mechanisms may accommodate increases in distal delivery, but severe reductions are generally associated with volume retention. This is seen in clinical settings such as congestive heart failure and cirrhosis (46,115,138). When BP is low, the reduced stimulation of TGF and myogenic mechanisms evoke vasodilation. However, additional macula densa mechanisms contribute to preserving GFR when renal perfusion is impaired (132). A clinical example is renal arterial stenosis in which increased renin release evokes angiotensin II-dependent efferent arteriolar tone to maintain GFR (78,148). The local formation of PGE2 is essential in such settings, as illustrated by the critical role of cyclooxygenase (COX) in congestive heart failure and cirrhosis (44, 168, 169). The renin and COX pathways interact in a complex manner. Angiotensin II stimulates COX activity and PGE2 is critical in macula densa signaling of renin release (13, 26, 96,137). At the microvascular level, PGE2 attenuates afferent arteriolar responses to angiotensin II, while preserving the efferent vasoconstriction (42,147). The resultant increase in glomerular outflow resistance maintains PGC and preserves GFR when renal perfusion is compromised.
The macula densa constitutively expresses high levels of both neuronal (NO) synthase and COX2 (13). The roles of these two vasodilator pathways in classic TGF signaling are not fully resolved, but both are implicated in TGF “re-setting” (23, 110, 134, 150, 151). PGE2 and NO have also been shown to modulate the strength and kinetics of the myogenic component of autoregulation (85, 147, 165,166). Could the expression of these two pathways in the JGA also reflect the existence of vasodepressor mechanisms that might attenuate afferent arteriolar myogenic reactivity in settings in which distal delivery is impaired? Several investigators have demonstrated that signals reflecting reduced distal delivery, such as reduced osmolality or chloride concentration, trigger the release of NO and PGE2 from the JGA and/or macula densa and extraglomerular mesangial cells (121, 156, 170,173), and direct, microelectrode measurements demonstrate an elevation in distal tubular NO concentration in response to furosemide (95). If severe reductions in distal delivery trigger the combined release of NO and PGE2, this would be a powerful vasodilator signal. PGE2 and NO elicit afferent arteriolar vasodilation through cAMP and cGMP, respectively. These two cyclic nucleotides and their associated kinases act by divergent and redundant signaling pathways (99, 100, 119) and exert synergistic interactions via phosphodiesterase III (119, 130), further enhancing the vasodilator capacity of this combination. Could PGE2 and NO modulate myogenic reactivity when a “protective” vasoconstriction exerted untoward effects on distal delivery in the affected nephron? The myogenic responses depicted in figures 3C-E might trigger such a mechanism. In these cases, the “protective” responses to the systolic BP would result in a further decrease in mean glomerular perfusion pressure, potentially causing an inappropriate reduction in GFR and distal delivery. Under such conditions, a macula densa-triggered depressor mechanism could attenuate myogenic reactivity, preserving distal delivery, and thereby integrating the protective and regulatory functions of the renal vasculature.
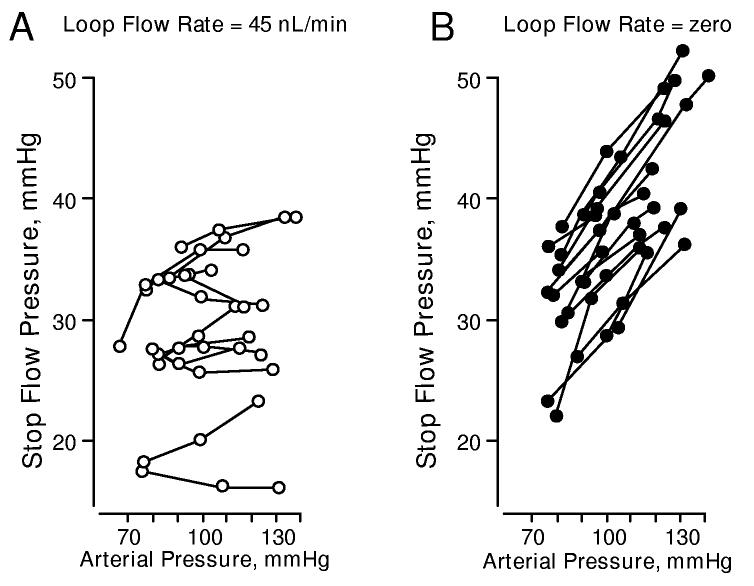
It is generally accepted that an active TGF system (normal or increased distal delivery) is necessary for the full expression of the myogenic response and severe reductions in distal delivery have been shown to impair myogenic responses. For example, in an elegant study Schnerrman and Briggs (131) demonstrated that effective PGC regulation was observed when distal tubular flow rate was held constant (eliminating pressure-dependent TGF signaling), but maintained at a high flow rate (40 nl/min, figure 9A). Presumably, PGC regulation was achieved by the myogenic response. However, as shown in figure 9B, pressure regulation was disrupted when the macula densa was exposed to zero flow. Similarly, Navar and coworkers (113) demonstrated that a cessation in distal delivery disturbs the regulation of PGC in the dog. Others have shown, using the juxtamedullary nephron preparation that myogenic vasoconstriction is attenuated when TGF is blocked by furosemide or papillectomy (107, 129, 146, 163). Such observations are generally interpreted as demonstrations of the “permissive” role of TGF response on myogenic reactivity. However, it must be emphasized in the present context that these observations could equally be interpreted as suggesting the presence of a vasodepressor mechanism that reduces myogenic reactivity when flow signaling at the macula densa is depressed. Does TGF-induced vasoconstriction actually play an obligate role in myogenic signaling? The robust myogenic responses seen in the hydronephrotic kidney, which has no TGF mechanism, suggests this is perhaps not the case. Studies evaluating the possibility the attenuated myogenic reactivity seen when distal delivery is reduced or TGF signaling is blocked reflects a macula densa mediated vasodepressor mechanism would be of great interest.
Figure 9.
An example of PGC regulation during a constant early distal flow rate of 40 nl/min (A, left) and the disturbance of pressure regulation under zero flow conditions (B, right) in the anesthetized rat. PGC was estimated by micropuncture measurements of early proximal tubule “stop-flow” pressure. Renal perfusion pressure was altered by an aortic clamp placed proximal to the renal artery. Flow rate was altered by microperfusion of the loop of Henle. (data reproduced with permission from reference 131).
Finally, the unique anatomy suggests a primary importance of macula densa signaling in settings associated with internephron heterogeneity. Many renal diseases are characterized by heterogeneity in single nephron GFR, tubular function and distal salt delivery (3, 31, 79). Moreover, in the diseased kidney, the focal distribution of vascular/glomerular lesions suggests a heterogeneity in regard to the pressure signals to these sites (31,117). It is not difficult to envision a need for individual nephron control under such conditions. Internephron heterogeneity in perfusion patterns and renin release may contribute to the pathogenesis of hypertension in the 5/6 renal ablation model and other settings (34, 61, 106, 139). In this regard it should be noted that angiotensin II and endothelin-1 potently enhance myogenic reactivity (91) and a stimulation of either of these pathways could promote localized pre-glomerular vasospasm. A macula densi-mediated vasodilatory response could play an important regulatory role in affected nephrons. Clearly, there is a need for additional investigations in this area.
Conclusions and Perspectives
The observed autoregulation of RBF and GFR has long been interpreted as reflecting a mechanism that is required for normal renal excretory function and volume homeostasis (e.g., 63). Nevertheless, observations in diverse animal models indicate that the when renal autoregulation is impaired, there is no evidence of disturbed volume regulation. While it may be argued that redundant compensatory mechanisms mask the impact of impaired autoregulation, these observations clearly demonstrate that intact renal autoregulatory mechanisms are not an obligate requirement for adequate volume control.
Intact autoregulation does appear to be absolutely essential for normal renal protection, as impaired renal autoregulatory capacity is invariably associated with an increase in the susceptibility to hypertensive injury. Of the two underlying mechanisms, the myogenic response is uniquely suited to this protective role. Its unusual kinetic attributes allow the afferent arteriole to sense elevations in the rapidly oscillating systolic BP and adjust tone to this signal. While it is important to emphasize that this postulate awaits critical evaluations using in vivo preparations and other experimental models, an important question concerns the determinants of this adaptation. The rapid onset in vasoconstriction, which is also observed in vivo (85,175), is critical in regard to the response to oscillating signals. What novel smooth muscle mechanisms are involved? Elevations in the systolic BP correlate most closely with end organ damage (22, 71, 82,174). Is the afferent arteriole unique or do terminal arterioles of other vascular beds provide protection against this oscillating pressure through similar adaptations?
Normal autoregulation requires both myogenic and TGF mechanisms and a myogenic constriction triggered by the systolic BP would contribute to autoregulation when this signal changes in concert with mean BP. Similarly, any mechanism elevating preglomerular tone, including TGF, could be viewed as contributing to renal protection. However, it is also possible that the myogenic and TGF responses play distinct roles in regard to protection and regulation of function and considerations of their potential interactions should be expanded. Macula densa-mediated vasodepressor mechanisms, triggered by reduced distal delivery, could protect GFR by attenuating inappropriate pre-glomerular vasoconstriction. An interesting possibility is that such a response may modulate myogenic reactivity when a “protective” vasoconstriction to elevated systolic BP disrupts the “regulation” of distal delivery. Such interactions would serve to integrate the protective and regulatory functions of the renal vasculature. Studies evaluating this possibility would be of interest. Finally observations that ambient GFR and RBF remain normal, despite hypertension, in animal models with impaired acute autoregulatory responses suggest the existence of previously unappreciated long-term adaptations. The nature of the underlying mechanisms deserves investigation.
Acknowledgements
The authors wish to acknowledge support from the NIH (AKB, KAG), the Veteran's Administration (KAG) and the CIHR (RL). RL is a Scientist of the Alberta Heritage Foundation for Medical Research. We wish to thank Dr. Michael Walsh for his careful reading of the manuscript and his suggestions. We also wish to thank two anonymous and thorough reviewers, whose comments and suggestions led to significant improvements in the final version of this review.
References
- 1.Abraham WT, Schrier RW. Body fluid volume regulation in health and disease. Adv Intern Med. 1994;39:23–47. [PubMed] [Google Scholar]
- 2.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005 doi: 10.1152/ajprenal.00349.2004. In press. [DOI] [PubMed] [Google Scholar]
- 3.Allison MEM, Wilson CB, Gottschalk CW. Pathophysiology of experimental glomerulonephritis in rats. J Clin Invest. 1974;53:1402–1423. doi: 10.1172/JCI107689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendshorst WJ. Altered reactivity of tubuloglomerular feedback. Annu Rev Physiol. 1987;49:295–317. doi: 10.1146/annurev.ph.49.030187.001455. [DOI] [PubMed] [Google Scholar]
- 6.Arendshorst WJ, Beierwaltes WH. Renal and nephron hemodynamics in spontaneously hypertensive rats. Am J Physiol. 1979;236:F246–F251. doi: 10.1152/ajprenal.1979.236.3.F246. [DOI] [PubMed] [Google Scholar]
- 7.Aukland K. Myogenic mechanisms in the kidney. J Hypertens Suppl. 1989;7:S71–S76. [PubMed] [Google Scholar]
- 8.Aukland K, Oien AH. Renal autoregulation: models combining tubuloglomerular feedback and myogenic response. Am J Physiol. 1987;252:F768–F783. doi: 10.1152/ajprenal.1987.252.4.F768. [DOI] [PubMed] [Google Scholar]
- 9.Azar S, Johnson MA, Hertel B, et al. Single-nephron pressures, flows and resistances in hypertensive kidneys with nephrosclerosis. Kidney Int. 1977;12:28–40. doi: 10.1038/ki.1977.76. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin DS, Neugarten J. Hypertension and renal disease. Am J Kidney Dis. 1987;10:186–191. doi: 10.1016/s0272-6386(87)80173-7. [DOI] [PubMed] [Google Scholar]
- 11.Baylis C. Glomerular filtration rate in normal and abnormal pregnancies. Semin Nephrol. 1999;19:133–139. [PubMed] [Google Scholar]
- 12.Bayliss WM. On the local reaction of the arterial wall to changes in intraluminal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003;65:481–500. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- 14.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens. 2002;11:73–80. doi: 10.1097/00041552-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension. 2004;44:1–7. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 16.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric BP monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 17.Bidani AK, Griffin KA, Plott W, Schwartz MM. Renal ablation acutely transforms “benign” hypertension to “malignant” nephrosclerosis in hypertensive rats. Hypertension. 1994;24:309–316. doi: 10.1161/01.hyp.24.3.309. [DOI] [PubMed] [Google Scholar]
- 18.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. ‘Step’ vs ‘Dynamic’ autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol. 2003;285:F113–F120. doi: 10.1152/ajprenal.00012.2003. [DOI] [PubMed] [Google Scholar]
- 19.Bidani AK, Mitchell KD, Schwartz MM, Navar LG, Lewis EJ. Absence of glomerular injury or nephron loss in a normotensive rat remnant kidney model. Kidney Int. 1990;38:28–38. doi: 10.1038/ki.1990.163. [DOI] [PubMed] [Google Scholar]
- 20.Bidani AK, Picken MM, Bakris G, Griffin KA. Lack of evidence of BP independent protection by renin-angiotensin system blockade after renal ablation. Kidney Int. 2000;57:1651–1661. doi: 10.1046/j.1523-1755.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 21.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:1003–1010. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 22.Black HR. The paradigm has shifted to systolic blood pressure. J Hum Hypertens. 2004;18(Suppl 2):S3–S7. doi: 10.1038/sj.jhh.1001795. [DOI] [PubMed] [Google Scholar]
- 23.Blantz RC, Deng A, Lortie M, Munger K, Vallon V, Gabbai FB, Thomson SC. The complex role of nitric oxide in the regulation of glomerular ultrafiltration. Kidney Int. 2002;61:782–785. doi: 10.1046/j.1523-1755.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- 24.Bock HA, Bachofen M, Landmann J, Thiel G. Glomerular hyperfiltration after unilateral nephrectomy in living kidney donors. Transpl Int. 1992;5(Suppl 1):S156–S159. doi: 10.1007/978-3-642-77423-2_50. [DOI] [PubMed] [Google Scholar]
- 25.Bravo EL. Pheochromocytoma: new concepts and future trends. Kidney Int. 1991;40:544–556. doi: 10.1038/ki.1991.244. [DOI] [PubMed] [Google Scholar]
- 26.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
- 27.Byrom FB. The hypertensive vascular crisis. Grune & Stratton; New York: 1969. [Google Scholar]
- 28.Byrom FB. Pathogenesis of hypertensive encephalopathy and its relation to the malignant phase of hypertension: Experimental evidence from the hypertensive rats. Lancet. 1954;2:201–211. doi: 10.1016/s0140-6736(54)91821-8. [DOI] [PubMed] [Google Scholar]
- 29.Casellas D, Moore LC. Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol. 1990;258:F660–F669. doi: 10.1152/ajprenal.1990.258.3.F660. [DOI] [PubMed] [Google Scholar]
- 30.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114. 2004:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chagnac A, Kiberd BA, Farinas MC, Strober S, Sibley RK, Hoppe R, Myers BD. Outcome of the acute glomerular injury in proliferative lupus nephritis. J Clin Invest. 1989;84:922–930. doi: 10.1172/JCI114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chon KH, Chen YM, Holstein-Rathlou NH, Marsh DJ, Marmarelis VZ. On the efficacy of linear system analysis of renal autoregulation in rats. IEEE Trans Biomed Eng. 1993;40:8–20. doi: 10.1109/10.204766. [DOI] [PubMed] [Google Scholar]
- 33.Churchill PC, Churchill MC, Bidani AK, Griffin KA, Picken M, Pravenec M, Kren V, Lezin E, St., Wang J-M, Wang N, Kurtz TW. Genetic susceptibility to hypertension-induced renal damage in the rat: evidence based on kidney specific genome transfer. J Clin Invest. 1997;100:1373–1382. doi: 10.1172/JCI119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correa-Rotter R, Hostetter TH, Manivel JC, Rosenberg ME. Renin expression in renal ablation. Hypertension. 1992;20:483–490. doi: 10.1161/01.hyp.20.4.483. [DOI] [PubMed] [Google Scholar]
- 35.Cupples WA, Loutzenhiser RD. Dynamic autoregulation in the in vitro perfused hydronephrotic rat kidney. Am J Physiol. 1998;275:F126–F130. doi: 10.1152/ajprenal.1998.275.1.F126. [DOI] [PubMed] [Google Scholar]
- 36.Cupples WA, Novak P, Novak V, Salevsky FC. Spontaneous blood pressure fluctuations and renal blood flow dynamics. Am J Physiol. 1996;270:F82–F89. doi: 10.1152/ajprenal.1996.270.1.F82. [DOI] [PubMed] [Google Scholar]
- 37.Daniels FH, Arendshorst WJ. Tubuloglomerular feedback kinetics in spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol. 1990;259:F529–F534. doi: 10.1152/ajprenal.1990.259.3.F529. [DOI] [PubMed] [Google Scholar]
- 38.Daniels FH, Arendshorst WJ, Roberds RG. Tubuloglomerular feedback and autoregulation in spontaneously hypertensive rats. Am J Physiol. 1990;258:F1479–F1489. doi: 10.1152/ajprenal.1990.258.6.F1479. [DOI] [PubMed] [Google Scholar]
- 39.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 40.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 41.Drumond MC, Deen WM. Analysis of pulsatile pressures and flows in glomerular filtration. Am J Physiol. 1990;261:F409–F419. doi: 10.1152/ajprenal.1991.261.3.F409. [DOI] [PubMed] [Google Scholar]
- 42.Edwards RM. Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Physiol. 1985;248:F779–F784. doi: 10.1152/ajprenal.1985.248.6.F779. [DOI] [PubMed] [Google Scholar]
- 43.Ellison DH. Adaptation to Diuretic Drugs. Diuretic Agents: Clinical Physiology and Pharmacology. Academic; San Diego, CA: 1997. pp. 209–232. [Google Scholar]
- 44.Epstein M. Renal prostaglandins and the control of renal function in liver disease. Am J Med. 1986;80:46–55. doi: 10.1016/0002-9343(86)90931-9. [DOI] [PubMed] [Google Scholar]
- 45.Epstein M. Renal effects of head-out water immersion in humans: a 15-year update. Physiol Rev. 1992;72:563–621. doi: 10.1152/physrev.1992.72.3.563. [DOI] [PubMed] [Google Scholar]
- 46.Epstein M. Hepatorenal syndrome: emerging perspectives of pathophysiology and therapy. J Am Soc Nephrol. 1994;4:1735–1753. doi: 10.1681/ASN.V4101735. [DOI] [PubMed] [Google Scholar]
- 47.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: What we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 48.Feld LG, VanLiew JB, Galaske RG, Boyland JW. Selectivity of renal injury and proteinuria in the spontaneously hypertensive rat. Kidney Int. 1977;12:332–343. doi: 10.1038/ki.1977.120. [DOI] [PubMed] [Google Scholar]
- 49.Forster RP, Maes JP. Effect of experimental neurogenic hypertension on renal blood flow and glomerular filtration rates in intact denervated kidneys of unanesthetized rabbits with adrenal glands demedullated. Am J Physiol. 1947;150:534–540. doi: 10.1152/ajplegacy.1947.150.4.534. [DOI] [PubMed] [Google Scholar]
- 50.Giese J. The Pathogenesis of Hypertensive Vascular Disease. Muskgaard; Copenhagen, Denmark: 1966. [Google Scholar]
- 51.Gilmore JP, Cornish KG, Rogers SD, Joyner WL. Direct evidence for myogenic autoregulation of the renal microcirculation in the hamster. Circ Res. 1980;47:226–230. doi: 10.1161/01.res.47.2.226. [DOI] [PubMed] [Google Scholar]
- 52.Goodman TA, Gerard DF, Bernstein EF, Dilley RB. The effects of pulseless perfusion on the distribution of renal cortical blood flow and on renin release. Surgery. 1976;80:31–39. [PubMed] [Google Scholar]
- 53.Goormaghtigh N. L'appareil neuro-myo-artériel juxta-glomérulaire du rein: ses réactions en pathologie et ses rapports avec le tube urinifère. C R Seances Soc Biol Fil. 1937;124:293–296. [Google Scholar]
- 54.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure dependent. Hypertension. 2003;41:201–206. doi: 10.1161/01.hyp.0000049881.25304.73. [DOI] [PubMed] [Google Scholar]
- 55.Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens. 2001;14:311–320. doi: 10.1016/s0895-7061(00)01282-6. [DOI] [PubMed] [Google Scholar]
- 56.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol. 2004;286:F1136–F1143. doi: 10.1152/ajprenal.00401.2003. [DOI] [PubMed] [Google Scholar]
- 57.Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium blockers in the rat remnant kidney model. Kidney Int. 1999;55:1849–1860. doi: 10.1046/j.1523-1755.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 58.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical determinant of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 59.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int. 1994;46:1010–1018. doi: 10.1038/ki.1994.361. [DOI] [PubMed] [Google Scholar]
- 60.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffin KA, Picken MM, Churchill M, Churchill P, Bidani AK. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol. 2000;11:497–506. doi: 10.1681/ASN.V113497. [DOI] [PubMed] [Google Scholar]
- 62.Griffin KA, Picken M, Giobbie-Hurder A, Bidani AK. Low protein diet mediated renoprotection in remnant kidneys: renal autoregulatory vs hypertrophic mechanisms. Kidney Int. 2003;63:607–616. doi: 10.1046/j.1523-1755.2003.00759.x. [DOI] [PubMed] [Google Scholar]
- 63.Guyton AC, Hall JE. Textbood of Medical Physiology. 11th edition Saunders; 2006. p. 323. [Google Scholar]
- 64.Guyton AC, Langston JB, Navar G. Theory for renal autoregulation by feedback at the juxtaglomerular apparatus. Circ Res. 1964;15(Suppl):187–197. [PubMed] [Google Scholar]
- 65.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 66.Haberle DA, von Baeyer H. Characteristics of glomerulotubular balance. Am J Physiol. 1983;244:F355–F366. doi: 10.1152/ajprenal.1983.244.4.F355. [DOI] [PubMed] [Google Scholar]
- 67.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15:547–559. doi: 10.1161/01.hyp.15.6.547. [DOI] [PubMed] [Google Scholar]
- 68.Hársing L, Fonyódi S, Kabát M, Kövér G. Effect of phlorizin and of mercurial diuretics on renal haemodynamics. Acta Physiol Hung. 1957;12:363–371. [PubMed] [Google Scholar]
- 69.Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in Spontaneously Hypertensive Rats. Hypertension. 1992;19:153–160. doi: 10.1161/01.hyp.19.2.153. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi K, Epstein M, Saruta T. Altered myogenic responsiveness of the renal microvaculature in experimental hypertension. J Hypertens. 1996;14:1387–1401. doi: 10.1097/00004872-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 71.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 72.Hill GS, Heptinstall RH. Steroid-induced hypertension in the rat. A microangiographic and histologic study on the pathogenesis of hypertensive vascular and glomerular lesions. Am J Pathol. 1968;52:1–39. [PMC free article] [PubMed] [Google Scholar]
- 73.Holstein-Rathlou NH, He J, Wagner AJ, Marsh DJ. Patterns of blood pressure variability in normotensive and hypertensive rats. Am J Physiol. 1995;269:R1230–R1239. doi: 10.1152/ajpregu.1995.269.5.R1230. [DOI] [PubMed] [Google Scholar]
- 74.Holstein-Rathlou NH, Marsh DJ. A dynamic model of renal blood flow autoregulation. Bull Math Biol. 1994;56:411–429. doi: 10.1007/BF02460465. [DOI] [PubMed] [Google Scholar]
- 75.Holstein-Rathlou NH, Marsh DJ. Renal blood flow regulation and arterial pressure fluctuations: a case study in nonlinear dynamics. Physiologic Rev. 1994;74:637–681. doi: 10.1152/physrev.1994.74.3.637. [DOI] [PubMed] [Google Scholar]
- 76.Holstein-Rathlou NH, Wagner AJ, Marsh DJ. Tubuloglomerular feedback dynamics and renal blood flow autoregulation in rats. Am J Physiol. 1991;260:F53–F68. doi: 10.1152/ajprenal.1991.260.1.F53. [DOI] [PubMed] [Google Scholar]
- 77.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med. 1982;72:375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 78.Hricik DE, Browning PJ, Kopelman R, Goorno WE, Madias NE, Dzau VJ. Captopril-induced functional renal insufficiency in patients with bilateral renal-artery stenoses or renal-artery stenosis in a solitary kidney. N Engl J Med. 1983;308:373–376. doi: 10.1056/NEJM198302173080706. [DOI] [PubMed] [Google Scholar]
- 79.Ichikawa I, Hoyer JR, Seiler MW, Brenner BM. Mechanism of glomerulotubular balance in the setting of heterogenous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982;69:185–198. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iversen BM, Sekse I, Ofstad J. Resetting of renal blood flow autoregulation in spontaneously hypertensive rats. Am J Physiol. 1987;252:F480–F486. doi: 10.1152/ajprenal.1987.252.3.F480. [DOI] [PubMed] [Google Scholar]
- 82.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 83.Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood pressure. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 84.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R619–R631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- 85.Just A, Arendshorst WJ. Nitric Oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol. 2005 Oct 13; doi: 10.1113/jphysiol.2005.094888. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaplan NM. Clinical Hypertension. Fifth Edition Williams & Wilkins; Baltimore, MD: 1990. Hypertensive emergencies and urgencies. [Google Scholar]
- 87.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou N-H. Dynamic autoregulation and renal injury in Dahl rats. Hypertension. 1997;30:975–983. doi: 10.1161/01.hyp.30.4.975. [DOI] [PubMed] [Google Scholar]
- 88.Karlsen FM, Leyssac PP, Holstein-Rathlou N-H. Tubuloglomerular feedback in Dahl rats. Am J Physiol. 1998;274:F1561–R1569. doi: 10.1152/ajpregu.1998.274.6.R1561. [DOI] [PubMed] [Google Scholar]
- 89.Kim GH. Long-term adaptation of renal ion transporters to chronic diuretic treatment. Am J Nephrol. 2004;24:595–605. doi: 10.1159/000082314. [DOI] [PubMed] [Google Scholar]
- 90.King AJ, Levey AS. Dietary protein and renal function. J Am Soc Nephrol. 1993;3:1723–1737. doi: 10.1681/ASN.V3111723. [DOI] [PubMed] [Google Scholar]
- 91.Kirton CA, Loutzenhiser R. Alterations in basal PKC activity modulate renal afferent arteriolar myogenic reactivity. Am J Physiol. 1998;275:H467–H475. doi: 10.1152/ajpheart.1998.275.2.H467. [DOI] [PubMed] [Google Scholar]
- 92.Kloke HJ, Branten AJ, Hyssmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal diseases: Risks or benefits of calcium channel blockers? Kidney Int. 1998;53:1559–1573. doi: 10.1046/j.1523-1755.1998.00912.x. [DOI] [PubMed] [Google Scholar]
- 93.Lassen NA. Control of cerebral circulation in health and disease. Circ Res. 1974;34:749–760. doi: 10.1161/01.res.34.6.749. [DOI] [PubMed] [Google Scholar]
- 94.Lassen NA, Agnoli A. The upper limit of autoregulation of cerebral blood flow – on the pathogenesis of hypertensive encephalopathy. Scand J Clin Lab Invest. 1972;30:113–116. doi: 10.3109/00365517209081099. [DOI] [PubMed] [Google Scholar]
- 95.Levine DZ, Burns KD, Jaffey J, Iacovitti M. Short-term modulation of distal tubule fluid nitric oxide in vivo by loop NaCl reabsorption. Kidney Int. 2004;65:184–189. doi: 10.1111/j.1523-1755.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- 96.Lorenz JN, Greenberg SG, Briggs JP. The macula densa mechanism for control of renin secretion. Semin Nephrol. 1993;13:531–542. [PubMed] [Google Scholar]
- 97.Loutzenhiser R, Bidani A, Chilton L. The renal myogenic response: kinetic attributes and physiologic role. Circ Res. 2002;90:1316–1324. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 98.Loutzenhiser R, Bidani A, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand. 2004;181:407–413. doi: 10.1111/j.1365-201X.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 99.Lincoln TM, Cornwell TL. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991;28:129–137. doi: 10.1159/000158852. [DOI] [PubMed] [Google Scholar]
- 100.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 101.Many M, Soroff HS, Birtwell WC, Wise HM, Deterling RA., Jr The physiologic role of pulsatile and nonpulsatile blood flow. 3. Effects of unilateral renal artery depulsation. Arch Surg. 1968;97:917–923. doi: 10.1001/archsurg.1968.01340060095010. [DOI] [PubMed] [Google Scholar]
- 102.Marsh DJ. Frequency response of autoregulation. Kidney Int. 1982;12(Suppl):S165–S172. [PubMed] [Google Scholar]
- 103.Marsh DJ, Osborn JL, Cowley AW., Jr 1/f fluctuations in arterial pressure and regulation of renal blood flow in dogs. Am J Physiol. 1990;258:F1394–F1400. doi: 10.1152/ajprenal.1990.258.5.F1394. [DOI] [PubMed] [Google Scholar]
- 104.Mavroudis C. To pulse or not to pulse. Ann Thorac Surg. 1978;25:259–271. doi: 10.1016/s0003-4975(10)63539-4. [DOI] [PubMed] [Google Scholar]
- 105.McDonough AA, Leong PK, Yang LE. Mechanisms of pressure natriuresis: how blood pressure regulates renal sodium transport. Ann N Y Acad Sci. 2003;986:669–677. doi: 10.1111/j.1749-6632.2003.tb07281.x. [DOI] [PubMed] [Google Scholar]
- 106.Miller PL, Rennke HG, Meyer TW. Hypertension and progressive glomerular injury caused by focal glomerular ischemia. Am J Physiol. 1990;259:F239–F245. doi: 10.1152/ajprenal.1990.259.2.F239. [DOI] [PubMed] [Google Scholar]
- 107.Moore LC, Casellas D. Tubuloglomerular feedback dependence of autoregulation in rat juxtamedullary afferent arterioles. Kidney Int. 1990;37:1402–1408. doi: 10.1038/ki.1990.129. [DOI] [PubMed] [Google Scholar]
- 108.Moore LC, Rich A, Casellas D. Ascending myogenic autoregulation: interactions between tubuloglomerular feedback and myogenic mechanisms. Bull Math Biol. 1994;56:391–410. doi: 10.1007/BF02460464. [DOI] [PubMed] [Google Scholar]
- 109.Moore LC, Schnermann J, Yarimizu S. Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am J Physiol. 1979;67:F63–F74. doi: 10.1152/ajprenal.1979.237.1.F63. [DOI] [PubMed] [Google Scholar]
- 110.Morsing P, Persson AE. Effect of prostaglandin synthesis inhibition on the tubuloglomerular feedback control in the rat kidney. Ren Physiol Biochem. 1992;15:66–72. doi: 10.1159/000173443. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura K, Harasaki H, Fukumura F, Fukamachi K, Whalen R. Comparison of pulsatile and non-pulsatile cardiopulmonary bypass on regional renal blood flow in sheep. Scand Cardiovasc J. 2004;38:59–63. doi: 10.1080/14017430410024856. [DOI] [PubMed] [Google Scholar]
- 112.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978;234:F357–F370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 113.Navar LG, Chomdej B, Bell PD. Absence of estimated glomerular pressure autoregulation during interrupted distal delivery. Am J Physiol. 1975;229:1596–1603. doi: 10.1152/ajplegacy.1975.229.6.1596. [DOI] [PubMed] [Google Scholar]
- 114.Navar LG, Mitchell KD. Contribution of the tubuloglomerular feedback mechanism to sodium homeostasis and interaction with the renin-angiotensin system. Acta Physiol. Scand. 1990;139:66–73. [PubMed] [Google Scholar]
- 115.Niederberger M, Schrier RW. Pathogenesis of sodium and water retention in liver disease. Prog Liver Dis. 1992;10:329–347. [PubMed] [Google Scholar]
- 116.Oien AH, Aukland K. A multinephron model of renal blood flow autoregulation by tubuloglomerular feedback and myogenic response. Acta Physiol Scand. 1991;143:71–92. doi: 10.1111/j.1748-1716.1991.tb09203.x. [DOI] [PubMed] [Google Scholar]
- 117.Olson JL. Hypertension: essential and secondary forms. In: Jennette JC, Olson JL, Schwartz MM, Silva FC, editors. Heptinstall's Pathology of the Kidney. ed. 5 Vol. 2. Lippincott-Raven; Philadelphia: 1998. pp. 943–1002. [Google Scholar]
- 118.Olson JL, Wilson SK, Heptinstall RH. Relation of glomerular injury to preglomerular resistance in experimental hypertension. Kidney Int. 1986;29:849–857. doi: 10.1038/ki.1986.76. [DOI] [PubMed] [Google Scholar]
- 119.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 120.Persson PB, Ehmke H, Kirchheim HR, Janssen B, Baumann JE, Just A, Nafz B. Autoregulation and non-homeostatic behaviour of renal blood flow in conscious dogs. J Physiol. 1993;462:261–273. doi: 10.1113/jphysiol.1993.sp019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pires SL, Barres C, Sassard J, Julien C. Renal blood flow dynamics and arterial pressure lability in the conscious rat. Hypertension. 2001;38:147–152. doi: 10.1161/01.hyp.38.1.147. [DOI] [PubMed] [Google Scholar]
- 123.Pires SLS, Julien C, Chapuis B, Sassard J, Barres C. Spontaneous renal blood flow autoregulation curves in conscious sinoaortic baroreceptor-denervated rats. Am J Physiol. 2002;282:F51–F58. doi: 10.1152/ajprenal.0186.2001. [DOI] [PubMed] [Google Scholar]
- 124.Ploth DW, Schnermann J, Dahlheim H, Hermle M, Schmidmeier E. Autoregulation and tubuloglomerular feedback in normotensive and hypertensive rats. Kidney Int. 1977;12:253–267. doi: 10.1038/ki.1977.110. [DOI] [PubMed] [Google Scholar]
- 125.Rein H. Vasomotorische Regulationen. Ergebn d Physiol. 1932;32:28–72. [Google Scholar]
- 126.Ritter ER. Pressure/flow relations in the kidney: Alleged effects of pulse pressure. Am J Physiol. 1952;68:480–489. doi: 10.1152/ajplegacy.1952.168.2.480. [DOI] [PubMed] [Google Scholar]
- 127.Roald AB, Ofstad J, Iversen BM. Attenuated buffering of renal perfusion pressure variation in juxtamedullary cortex in SHR. Am J Physiol Renal Physiol. 2002;282:F506–F511. doi: 10.1152/ajprenal.00199.2001. [DOI] [PubMed] [Google Scholar]
- 128.Salmond R, Seney FD., Jr Reset tubuloglomerular feedback permits and sustains glomerular hyperfunction after extensive renal ablation. Am J Physiol. 1991;260:F395–F401. doi: 10.1152/ajprenal.1991.260.3.F395. [DOI] [PubMed] [Google Scholar]
- 129.Sanchez-Ferrer CF, Roman RJ, Harder DR. Pressure-dependent contraction of rat juxtamedullary afferent arterioles. Circ Res. 1989;64:790–798. doi: 10.1161/01.res.64.4.790. [DOI] [PubMed] [Google Scholar]
- 130.Sandner P, Kornfeld M, Ruan X, Arendshorst WJ, Kurtz A. Nitric oxide/cAMP interactions in the control of rat renal vascular resistance. Circ Res. 1999;84:186–192. doi: 10.1161/01.res.84.2.186. [DOI] [PubMed] [Google Scholar]
- 131.Schnermann J, Briggs JP. Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol. 1989;256:F421–F429. doi: 10.1152/ajprenal.1989.256.3.F421. [DOI] [PubMed] [Google Scholar]
- 132.Schnermann J, Briggs JP, Weber PC. Tubuloglomerular feedback, prostaglandins, and angiotensin in the autoregulation of glomerular filtration rate. Kidney Int. 1984;25:53–64. doi: 10.1038/ki.1984.8. [DOI] [PubMed] [Google Scholar]
- 133.Schnermann J, Gokel M, Weber PC, Schubert G, Briggs JP. Tubuloglomerular feedback and glomerular morphology in Goldblatt hypertensive rats on varying protein diets. Kidney Int. 1986;29:520–529. doi: 10.1038/ki.1986.30. [DOI] [PubMed] [Google Scholar]
- 134.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 135.Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, Briggs JP. Absence of tubuloglomerular feedback responses in AT1A receptor-deficient mice. Am J Physiol. 1997;273:F315–F320. doi: 10.1152/ajprenal.1997.273.2.F315. [DOI] [PubMed] [Google Scholar]
- 136.Schnermann J, Wright FS, Davis JM, von Stackelberg W, Grill G. Regulation of superficial nephron filtration rate by tubulo-glomerular feedback. Pflugers Arch. 1970;318:147–175. doi: 10.1007/BF00586493. [DOI] [PubMed] [Google Scholar]
- 137.Schramek H, Coroneos E, Dunn MJ. Interactions of the vasoconstrictor peptides, angiotensin II and endothelin-1, with vasodilatory prostaglandins. Semin Nephrol. 1995;15:195–204. [PubMed] [Google Scholar]
- 138.Schrier RW, Fassett RG. Pathogenesis of sodium and water retention in cardiac failure. Ren Fail. 1998;20:773–781. doi: 10.3109/08860229809045175. [DOI] [PubMed] [Google Scholar]
- 139.Sealey JE, Blumenfeld JD, Bell GM, Pecker MS, Sommers SC, Laragh JH. On the renal basis for essential hypertension: nephron heterogeneity with discordant renin secretion and sodium excretion. J Hypertens. 1988;6:763–777. doi: 10.1097/00004872-198811000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Selkurt EE. Effect of pulse pressure and mean arterial pressure modification on renal hemodynamics and electrolyte and water excretion. Circulation. 1951;4:541–551. doi: 10.1161/01.cir.4.4.541. [DOI] [PubMed] [Google Scholar]
- 141.Seney FD, Jr, Wright FS. Dietary protein suppresses feedback control of glomerular filtration in rats. J Clin Invest. 1985;75:558–568. doi: 10.1172/JCI111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skarlatos S, DiPaola N, Frankel RA, Pomerantz RW, Brand PH, Metting PJ, Britton SL. Spontaneous pressure-flow relationships in renal circulation of conscious dogs. Am J Physiol. 1993;264:H1517–H1527. doi: 10.1152/ajpheart.1993.264.5.H1517. [DOI] [PubMed] [Google Scholar]
- 143.Skarlatos S, Metting PJ, Britton SL. Spontaneous pressure-flow patterns in the kidney of conscious rats. Am J Physiol. 1993;265:H2151–H2159. doi: 10.1152/ajpheart.1993.265.6.H2151. [DOI] [PubMed] [Google Scholar]
- 144.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res. 1992;71:471–480. doi: 10.1161/01.res.71.2.471. [DOI] [PubMed] [Google Scholar]
- 146.Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol. 1994;267:F879–F887. doi: 10.1152/ajprenal.1994.267.5.F879. [DOI] [PubMed] [Google Scholar]
- 147.Tang L, Loutzenhiser K, Loutzenhiser R. Biphasic actions of prostaglandin E2 on the renal afferent arteriole : role of EP3 and EP4 receptors. Circ Res. 2000;86:663–670. doi: 10.1161/01.res.86.6.663. [DOI] [PubMed] [Google Scholar]
- 148.Textor SC. Renal failure related to angiotensin-converting enzyme inhibitors. Semin Nephrol. 1997;17:67–76. [PubMed] [Google Scholar]
- 149.Thalmann M, Schima H, Wieselthaler G, Wolner E. Physiology of continuous blood flow in recipients of rotary cardiac assist devices. J Heart Lung Transplant. 2005;24:237–245. doi: 10.1016/j.healun.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 150.Thomson SC, Bachmann S, Bostanjoglo M, Ecelbarger CA, Peterson OW, Schwartz D, Bao D, Blantz RC. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J Clin Invest. 1999;104:1149–1158. doi: 10.1172/JCI5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thomson SC, Vallon V, Blantz RC. Resetting protects efficiency of tubuloglomerular feedback. Kidney Int. 1998;67(Suppl):S65–S70. doi: 10.1046/j.1523-1755.1998.06713.x. [DOI] [PubMed] [Google Scholar]
- 152.Thurau K. Proceedings of 2nd International Congress on Nephrology. Exerpta Medica; Prague, Amsterdam: 1963. Fundamentals of renal circulation; pp. 51–61. [Google Scholar]
- 153.Thurau K. Renal Hemodynamics. Am J Med. 1964;36:698–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- 154.Thurau K, Schnermann J. Die natriumkonzentration an den macula densa-zellen als regulierender facktor für das glomerulumfiltrat (mikropunktionsversuche) Klin Wochenschr. 1965;43:410–413. doi: 10.1007/BF01483845. [DOI] [PubMed] [Google Scholar]
- 155.Toda K, Tatsumi E, Taenaka Y, Masuzawa T, Takano H. Impact of systemic depulsation on tissue perfusion and sympathetic nerve activity. Ann Thorac Surg. 1996;62:1737–1742. doi: 10.1016/s0003-4975(96)00568-1. [DOI] [PubMed] [Google Scholar]
- 156.Tsukahara H, Krivenko Y, Moore LC, Goligorsky MS. Decrease in ambient [Cl−] stimulates nitric oxide release from cultured rat mesangial cells. Am J Physiol. 1994;267:F190–F195. doi: 10.1152/ajprenal.1994.267.1.F190. [DOI] [PubMed] [Google Scholar]
- 157.Tucker BJ, Blantz RC. Effects of glomerular filtration dynamics on the glomerular permeability coefficient. Am J Physiol. 1981;240:F245–F254. doi: 10.1152/ajprenal.1981.240.3.F245. [DOI] [PubMed] [Google Scholar]
- 158.Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol. 2003;14:530–537. doi: 10.1097/01.asn.0000051700.07403.27. [DOI] [PubMed] [Google Scholar]
- 159.Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflugers Arch. 2004;448:214–221. doi: 10.1007/s00424-004-1239-8. Epub 2004 Feb 6. [DOI] [PubMed] [Google Scholar]
- 160.van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol. 1999;276:R855–R863. doi: 10.1152/ajpregu.1999.276.3.R855. [DOI] [PubMed] [Google Scholar]
- 161.van Rodignen WF, van Lambalgen TA, Tangelder G-J, van Dokkum RPE, Provoost AP, ter Wee PM. Reduced reactivity of renal microvessels to pressure and angiotensin II in Fawn-Hooded rats. Hypertension. 2002;39:111–115. doi: 10.1161/hy1201.096817. [DOI] [PubMed] [Google Scholar]
- 162.Verseput GH, Braam B, Provoost AP, Koomans HA. Tubuloglomerular feedback and prolonged ACE-inhibitor treatment in the hypertensive fawn-hooded rat. Nephrol Dial Transplant. 1998;13:893–899. doi: 10.1093/ndt/13.4.893. [DOI] [PubMed] [Google Scholar]
- 163.Walker M, 3rd, Harrison-Bernard LM, Cook AK, Navar LG. Dynamic interaction between myogenic and TGF mechanisms in afferent arteriolar blood flow autoregulation. Am J Physiol Renal Physiol. 2000;279:F858–F865. doi: 10.1152/ajprenal.2000.279.5.F858. [DOI] [PubMed] [Google Scholar]
- 164.Wang X, Ajikobi DO, Salevsky FC, Cupples WA. Impaired myogenic autoregulation in kidneys of Brown Norway rats. Am J Physiol. 2000;278:F962–F969. doi: 10.1152/ajprenal.2000.278.6.F962. [DOI] [PubMed] [Google Scholar]
- 165.Wang X, Cupples WA. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can J Physiol Pharmacol. 2001;79:238–245. [PubMed] [Google Scholar]
- 166.Wang X, Salevsky FC, Cupples WA. Nitric oxide, atrial natriuretic factor, and dynamic renal autoregulation. Can J Physiol Pharmacol. 1999;77:777–786. [PubMed] [Google Scholar]
- 167.Welch WJ, Wilcox CS, Thomson SC. Nitric oxide and tubuloglomerular feedback. Semin Nephrol. 1999;19:251–262. [PubMed] [Google Scholar]
- 168.Weir MR. Renal effects of nonselective NSAIDs and coxibs. Cleve Clin J Med. 2002;69(Suppl 1):SI53–SI58. doi: 10.3949/ccjm.69.suppl_1.si53. [DOI] [PubMed] [Google Scholar]
- 169.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 170.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wilson C, Byrom FB. Renal changes in malignant hypertension. Lancet. 1939;1:136–139. [Google Scholar]
- 172.Wilson C, Byrom FB. The vicious circle in chronic Bright's disease. Experimental evidence from the hypertensive rat. Quart J Med. 1941;10:65–93. [Google Scholar]
- 173.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 174.Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP) J Am Soc Nephrol. 2002;13:2776–2782. doi: 10.1097/01.asn.0000031805.09178.37. [DOI] [PubMed] [Google Scholar]
- 175.Young DK, Marsh DJ. Pulse wave propagation in rat renal tubules: implications for GFR autoregulation. Am J Physiol. 1981;240:F446–F458. doi: 10.1152/ajprenal.1981.240.5.F446. [DOI] [PubMed] [Google Scholar]