Abstract
Earth's biogeochemical cycle of carbon delivers both limestones and organic materials to the crust. In numerous, biologically catalysed redox reactions, hydrogen, sulphur, iron, and oxygen serve prominently as electron donors and acceptors. The progress of these reactions can be reconstructed from records of variations in the abundance of 13C in sedimentary carbonate minerals and organic materials. Because the crust is always receiving new CO2 from the mantle and a portion of it is being reduced by photoautotrophs, the carbon cycle has continuously released oxidizing power. Most of it is represented by Fe3+ that has accumulated in the crust or been returned to the mantle via subduction. Less than 3% of the estimated, integrated production of oxidizing power since 3.8 Gyr ago is represented by O2 in the atmosphere and dissolved in seawater. The balance is represented by sulphate. The accumulation of oxidizing power can be estimated from budgets summarizing inputs of mantle carbon and rates of organic-carbon burial, but levels of O2 are only weakly and indirectly coupled to those phenomena and thus to carbon-isotopic records. Elevated abundances of 13C in carbonate minerals ca 2.3 Gyr old, in particular, are here interpreted as indicating the importance of methanogenic bacteria in sediments rather than increased burial of organic carbon.
Keywords: carbon cycle, carbon isotopes, atmospheric oxygen, methanogenesis, subduction, mantle
Together, biological and geological processes—oxygenic photosynthesis and the burial of organic carbon—get credit for producing and maintaining the O2 in Earth's breathable atmosphere. This view of the carbon cycle as the engine of environmental evolution is based on sedimentary records. The disappearance of mass-independent fractionation of the isotopes of sulphur is the most reliable indicator for the accumulation and persistence of traces of O2 in the atmosphere beginning at about 2.4 Gyr ago (Ga) (PO2≥10−5 atm; Pavlov & Kasting 2002; Farquhar & Wing 2003; Bekker et al. 2004). Biomarkers derived from lipids associated with cyanobacteria first appear in sedimentary rocks with an age of 2.7 Gyr (Summons et al. 1999; Brocks et al. 2003; Eigenbrode 2004). These provide strong evidence for oxygenic photoautotrophy at that time (i.e. for production of O2 as opposed to its accumulation, persistence, and global distribution). Abundances of oxidized and reduced minerals in ancient soil profiles and sediments generally indicate a transition from weakly reducing to weakly oxidizing conditions at Earth's surface soon after 2.47 Ga (Bekker et al. 2004; Canfield 2005; Catling & Claire 2005).
But what about examining the engine itself? Records of the burial of organic carbon, which should be provided by abundances of 13C in sedimentary carbonates and organic material (Broecker 1970), could indicate the pace and the mechanism of oxidation. For events and processes during the past 500 Myr, carbon-isotopic records have been interpreted with considerable success (e.g. Garrels & Lerman 1981; Holland 1984; Berner 1991, 2004; Kump & Arthur 1999). The same approach has been extended to Precambrian records (e.g. Schidlowski et al. 1975; Hayes 1983, 1994; Knoll et al. 1986; Derry et al. 1992; Karhu & Holland 1996; Halverson et al. 2005), but conclusions have usually been qualitative rather than quantitative. The carbon-isotopic record can be described as ‘consistent with’ some postulated event or process, but understanding of the carbon cycle has not been complete enough to allow resolution of uncertainties or elaboration of details.
Two recent findings may change this. First, new evidence (Saal et al. 2002) has led to wide agreement (cf. Resing et al. 2004) on the rate at which C is delivered to the crust from the mantle at mid-ocean ridges. This significant reduction in uncertainties about the input allows a new approach to carbon budgets. Second, a previously overlooked output of carbon from the ocean has been recognized and quantified. Whereas earlier concepts limited the outputs to sedimentary carbonates and organic matter, the new view includes a very large flow of carbon that is taken up during the weathering of seafloor basalts. This changes the structure of the mass balances. The potential role of this production of ‘carbonated basalts’ in the carbon cycle was first identified by Staudigel et al. (1989). Subsequently, Alt & Teagle (1999, 2003) have examined amounts and isotopic compositions of these carbonate minerals and discussed their importance in the modern carbon cycle. Walker (1990), Sleep & Zahnle (2001), and Nakamura & Kato (2004) have called attention to carbonation of submarine basalts as an important phenomenon during the Archaean. Bjerrum & Canfield (2004) have introduced a systematic treatment of this problem.
Our purpose here, based on those developments, is to demonstrate a new approach to studies of the carbon cycle and its effects on the global environment. Among those effects, we focus on oxidation of Earth's surface over the past 4 Gyr.
1. The structure of the carbon cycle
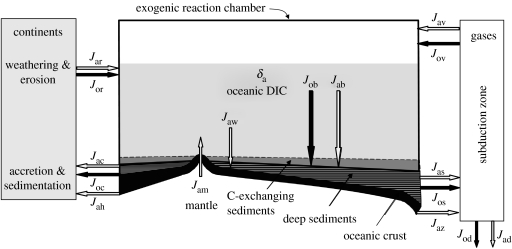
A geochemical view of the carbon cycle is shown in figure 1. The scheme chosen highlights processes that control the isotopic composition of inorganic carbon dissolved in the ocean. That carbon pool is in rough equilibrium with the atmosphere and with carbonate minerals derived from seawater. It is the senior author of our best records of how the carbon cycle has operated over the course of Earth history. To interpret those records, we must consider the fluxes indicated in figure 1. They represent processes that are linked by balances of mass and electrons. The related equations are, at present, analytical tools rather than components of an elaborate model.
Figure 1.
A schematic of the biogeochemical cycle of carbon. The arrows represent fluxes of carbon that are explained in the text.
The reaction chamber in which isotopic variations are shaped is comprised of the atmosphere, hydrosphere, and C-exchanging sediments and soils. Carbon flows into that chamber from the mantle and by recycling of carbon within the crust. It leaves through burial in sediments and during weathering of seafloor basalts. If the amount of C in the reactor is constant, the inputs will be balanced by the outputs:
| (1.1) |
The terms in this equation represent fluxes of carbon in mol per time. The subscripts ‘a’ and ‘o’ refer to inorganic and organic carbon. In detail, Jam is the total input of C from the mantle via outgassing of magmas at seafloor hydrothermal vents and at hot-spot and island-arc volcanoes; Jar and Jor are, respectively, returns of carbonate and organic C by exposure and weathering of deposits on continents and shelves; and Jav and Jov are returns, principally by arc volcanism, of carbonate and organic C remobilized during subduction. Among the outputs, Jab and Job are the net burials of carbonate-and organic-C in marine sediments, deriving mainly from processes in surface waters but often bearing strong secondary imprints; and Jaw is carbonate being taken up at the seafloor during the weathering of basalts. These, and all additional definitions pertinent to this discussion, are summarized in table 1.
Table 1.
Definitions.
| term | definition |
|---|---|
| main variables | |
| AOx | rate of accumulation of oxidants in crust (mol O2 equivalent) per time |
| J | flux of carbon (mol per time) |
| L | flux of oxidant or reductant (mol O2 equivalent) per time |
| M | molar quantity of C (no subscript: total crustal carbon, all forms) |
| f | fraction of C buried in organic form (=Job/(Jab+Jaw+Job)) |
| jx | flux of substance x (mol per time) |
| Δg | isotopic difference between DIC in surface seawater and diagenetically stabilized carbonate minerals in sedimentary rocks (‰); see equation (1.4) |
| Δm | isotopic difference between DIC in surface seawater and carbonate in weathered oceanic basalts (‰); see equation (1.6) |
| γ | fraction of crustal C recycling during τ.γ=(1−e−kτ), where k=(ln 2)/(half-mass age) |
| δ | δ13C relative to the Vienna PeeDee Belemnite standard (Zhang & Li 1990) |
| ϵ | isotopic fractionation between DIC in surface seawater and sedimentary organic carbon (‰); see equation (1.5) |
| φ | Fe3+/ΣFe |
| λ | fraction of buried carbonate accounted for by ocean-crustal carbonates (=Jaw/(Jaw+Jab)) |
| τ | time-step in numerical integrations (107 years); figures 5, 7 and 9 |
| subscripts appended to J, L and δ | |
| first or only part | |
| Ox | oxidant |
| Red | reductant |
| a | carbonate carbon |
| i | input to exogenic reaction chamber; see equation (1.3) |
| o | organic carbon |
| second part, pertaining to a directional flux | |
| b | burial, transfer from exogenic reaction chamber to deep sediments |
| c | transfer from deep sediments to continental crust |
| d | transfer from subduction zone to mantle |
| h | transfer from ocean crust to continental crust |
| m | transfer from mantle to exogenic reaction chamber |
| r | (returns) transfer from continent to exogenic reaction chamber |
| s | (subduction) transfer from deep sediments to subduction zone |
| v | (volcanism) transfer from subduction zone to exogenic reaction chamber |
| w | (weathering) transfer from exogenic reaction chamber to ocean crust |
| x | transfer from exogenic reaction chamber to space |
| z | transfer from ocean crust to subduction zone |
A second mass balance equates incoming and outgoing 13C. Its elaboration leads to a key indicator of variations in the operation of the carbon cycle. For simplicity and generality, it is often written in this form:
| (1.2) |
where Ji represents the summed inputs to the exogenic reaction chamber, δi represents their weighted-average isotopic composition and the remaining δ terms represent the isotopic compositions of the indicated fluxes. In detail, a fully correct form would be
| (1.3) |
Because values for many of the terms on the left side of this equation are inaccessible, analyses usually proceed from equation (1.2), incorporating the assumptions that Ji=Jab+Job+Jaw and that δi=δam. When historical variations are considered, the latter has two components: (i) that mixing of recycling inputs eventually yields an unbiased sample and (ii) that δam is constant. Over rock-cycle time-scales of 300 Myr or more, the first requirement is probably met. Second, the constancy and uniformity of δam are well supported. Independent of age of emplacement or location, diamonds from peridotitic xenoliths have δ=−5‰ (Pearson et al. 2004). The same value is found in carbonatites and mantle-derived basalts (Kyser 1986; Mattey 1987).
The task now is to provide a useful approach to interpreting observed variations in δab, the carbon-isotopic composition of sedimentary carbonates. As has become conventional, we define the fraction of input C buried in organic form as f≡Job/Ji. Following Bjerrum & Canfield (2004), we define the fraction of total carbonate accounted for by ocean-crustal carbonates as λ≡Jaw/(Jaw+Jab).
Our approach to the isotopic variables differs from previous expositions. As the reference point, we choose the isotopic composition of total dissolved inorganic carbon (DIC) in marine surface waters, δa. The isotopic compositions of the outputs are related to δa by the following expressions:
| (1.4) |
| (1.5) |
| (1.6) |
where Δg is the globally averaged isotopic difference between surface DIC and diagenetically stabilized sedimentary carbonates. At present, for example, comparison of pre-industrial δa (Quay et al. 2003) and average sedimentary carbonate (Shackleton 1987) indicates Δg≈2‰. Local variations in Δg can affect specific sedimentary records. The difference in isotopic composition between sedimentary organic carbon and DIC, ϵ, is principally (though not exclusively) due to isotopic discrimination during biotic carbon fixation. The sign chosen in equation (1.5), with ϵ>0 corresponding to depletion of 13C in biomass, is conventional in marine biogeochemistry. Values of Δm will be positive when ocean-crustal carbonates are depleted in 13C relative to surface waters. Reports of δaw, required to evaluate Δm, are rare. Alt & Teagle (2003) find δaw=1.7±0.4‰ for ocean-crustal carbonates that have formed during the past 160 Myr. The average value of δab during the same interval (Veizer et al. 1999) is 1.7‰. At present, therefore, Δm≈Δg≈2‰. Other reports occasionally indicate lower values of δaw and thus suggest larger values of Δm but, based on associated sedimentary features, the authors uniformly attribute the depletion of 13C to infrequent additions of C derived from oxidation of organic material.
Substitution of equations (1.4)–(1.6) in equation (1.3) and simplification of the result yields
| (1.7) |
For Δg=0, equation (1.7) is equivalent to equation (1.4) of Bjerrum & Canfield (2004). If, in addition, either or both Δm and λ are zero, equation (1.7) becomes δab−δi=fϵ, the expression found in numerous prior discussions of isotopic fractionation in the carbon cycle. Does this expression indicate that Δm and Δg can be as effective as f and ϵ in controlling δab−δi? Probably not. Their leverage is relatively small. In most circumstances, the first term, f(ϵ−Δg), will be at least four times larger than the second, λ(1−f)(Δm−Δg).
Rearrangement of equation (1.7) yields an expression for f, the fraction of carbon buried in organic form:
| (1.8) |
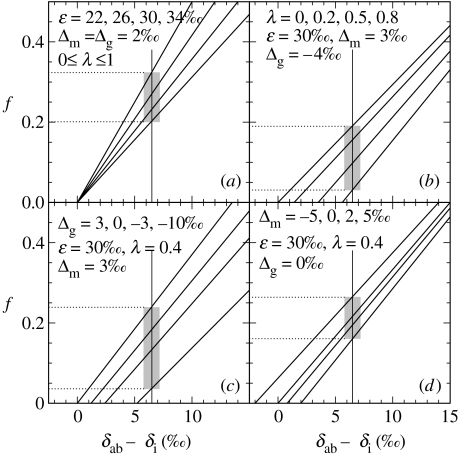
Commonly (e.g. Hayes et al. 1999), f is estimated from (δab−δi)/(δab−δob). This is precisely equivalent to (δab−δi)/(ϵ−Δg). The numerator and denominator in that fraction lack the correction terms, −λ(Δm−Δg), which are prominent in equation (1.8). If Δm≈Δg, as at present, λ(Δm−Δg)=0 and the terms are inconsequential, independent of the importance of basalt carbonation. Such cases are summarized graphically in figure 2a. The vertical line at δab−δi=6.5‰ indicates δab=1.5‰, near Earth's observed, long-term average value. The lines correspond (left to right) to ϵ=22, 26, 30 and 34‰. The shaded area indicates that, depending on the value of ϵ, δab=1.5‰ corresponds to 0.2≤f≤0.32. This range encompasses the values most frequently noted in previous discussions of the carbon cycle. In particular, δab−δob=28‰, corresponding to ϵ=30 and Δg=2‰, is representative of much of the Phanerozoic (Hayes et al. 1999).
Figure 2.
Graphs indicating relationships between f and δab−δi (1.7). Slopes and intercepts vary in response to varying values of ϵ, λ, Δm, and Δg. In each frame, one of these has been assigned four different values and the others have been held constant. The values assigned are indicated in each frame. For the parameter that varies, the sequence of values corresponds to the lines as seen from left to right.
The lower f values marked by shading in the other frames of figure 2 correspond to the same value of δab, but are based on different estimates of Δg, Δm and λ. Negative values of Δg are observed when sedimentary carbonates are strongly affected by methanogenic diagenesis (Irwin et al. 1977). In Phanerozoic strata, which have formed in the presence of relatively abundant O2 and , this phenomenon is restricted to concretions or other zones, in which supplies of sedimentary organic matter have been large enough that methanogenesis has eventually become prominent. When concentrations of O2 and in seawater were significantly lower, methanogenesis must have been more important. Accordingly, the effects of negative values of Δg, corresponding to globally important levels of methanogenic diagenesis, are explored in figure 2b,c. Figure 2d shows that variations in Δm are probably least important in affecting estimates of f. Under steady-state conditions, inversion of the oceanic 13C gradient—enrichment of 13C in bottom waters (Δm=−5‰, figure 2d)—is practically required to produce δab−δi<0.
2. Redox balances in the carbon cycle
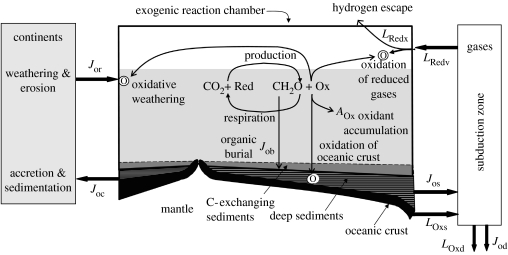
Figure 3 duplicates the plan of figure 1, but depicts flows of oxidants and reductants generated by the carbon cycle. The biological cycle of production and respiration is at its centre. Redox partners for C are generalized as Red and Ox. The focus on oxidants rather that oxygen is necessary. The carbon-burial flux provides information about the consumption of electron donors, but not about the identity of those donors. Over the course of Earth history, the electron-donating role of Red has been played by H2, Fe2+, S2− and H2O (at least). Correspondingly, the oxidized forms of these substances have accumulated in Earth's crust. The rate of accumulation will be set by the difference between the rate at which carbon-cycling produces oxidants and the rate at which those oxidants are consumed.
Figure 3.
A schematic of flows of oxidants and reductants generated by the carbon cycle. Sites of oxidation are marked by the letter O.
To quantify rates, we begin by viewing the Jo terms, introduced above as fluxes of carbon, as fluxes of reducing power. The L terms shown in figure 3 represent flows of oxidizing or reducing power carried by other elements or by mixtures of C and other elements, (mol O2 equivalent) per time. Because reduction of CO2 to organic carbon and oxidation of H2O to O2 are both four-electron processes, values of the Jo and L terms are numerically equivalent. Production of 1 mol of organic carbon could be balanced by oxidation of 2 mol of H2, 8 mol of Fe2+, 0.5 mol of S2− (to ), or 2 mol of H2O. All would be equivalent to 1 mol of O2. To emphasize that it pertains to the net effect of multiple processes, rather than to a specific flux, we use AOx to designate the rate at which oxidants accumulate in the crust and exogenic reaction chamber.
To begin, the biological carbon cycle releases oxidants at the rate at which organic carbon is buried in deep sediments (Job). The rate of accumulation is then moderated by effects of two types. Within the crust itself, a portion of the oxidants is consumed by the geological carbon cycle. Two pathways are shown in figure 3. The first is oxidative weathering of organic carbon exposed on and eroded from the continents (Jor). The second is oxidation of reduced gases produced at subduction zones. The corresponding flux is designated as LRedv. It includes not only volcanic gases (H2, CO, SO2), but also products ranging from methane to petroleum, which are delivered to the exogenic reaction chamber by thermal processes in subduction zones and deep basins.
The export of oxidants and reductants also moderates Aox. This occurs in subduction zones and at the top of the atmosphere. Within subduction zones, electrons are transferred between subducted organic materials (Jos) and oxidants (metal oxides and , LOxs) in the descending slab. Products will include not only those returned to the crust (noted above), but also unconsumed oxidants (LOxd) and reduced carbon (diamond, graphite, Jod) exported to the mantle. Subduction of carbonate decreases the mass of carbon in the crust, but, because carbon is supplied from the mantle as CO2, does not export either oxidizing or reducing power from the crust. Subduction of sulphide is similarly inconsequential. Mantle S occurs as sulphide even under oxidizing conditions (e.g. ΔFMQ∼2; Luth 2004).
At the top of the atmosphere, H2 can be lost to space, thus exporting reducing power and effectively increasing AOx. This occurs when reduced gases of either volcanic or biological origin reach high altitudes. Depending on atmospheric conditions, a portion of the reducing power carried by these gases can be lost as hydrogen escapes to space (Catling et al. 2001).
Summation provides an estimate of the rate at which oxidants will accumulate:
| (2.1) |
The second expression denotes AOx as a minimum value because the net release of oxidants by the C cycle may exceed Job−Jor−LRedv. This would occur if a portion of the organic carbon returning from continents (Jor) was not reoxidized but instead simply reburied. The efficiency of reoxidation will depend on the nature of Ox (e.g. O2 versus ) and the reactivity of Jor (e.g. graphitic kerogen versus hydrocarbons).
Equation (2.1) ignores a category of processes often considered in oxygen budgets. Sediments and oceanic crust assimilated by continents will incorporate portions of the Red and Ox from the exogenic reaction chamber. Oxidized and reduced forms of sulphur and iron are prime examples. Surging flows of these reactants to the sediments or from continents can, for example, significantly affect levels of O2 in the atmosphere and ocean. These and related phenomena, especially in the sulphur cycle, have already been elegantly treated by others (Berner 2004; Canfield 2004).
Imbalances yielding a net release or consumption of oxidizing power by the carbon cycle are of two types. The more obvious are marked by isotopic signals indicating rapid variations of f and consequent departures from steady state. At such times, the system will evolve dynamically (Rothman et al. 2003). At other times, when f varies slowly, the system will evolve quasi-statically through a succession of steady states. The persistence of small imbalances between fluxes can lead to the accumulation of crustal inventories of carbon, chiefly on the continents. In this case, the fluxes designated in equation (2.1) should be represented as time-dependent variables. Then, at any time, t,
| (2.2) |
The first term on the right-hand side is the integrated difference between burial and reoxidation of organic carbon. It quantifies the accumulation of organic carbon in continents and marine sediments. The second and third terms quantify the effects of loss of H2 to space and of subduction of oxidized and reduced materials. Notably, this summation of oxidation has no isotopic dimensions. Job is related to f and thus to the isotopic record, but it is Job−Jor−LRedv that matters, and it is further altered by effects of subduction and escape of H2 to space.
The first term in equation (2.2) is closely related to a principle dating from the nineteenth century (J. J. Ebelmen's work from 1845 to 1855, reviewed by Berner & Maasch 1996) and elaborated in modern detail most influentially by Garrels (e.g. Garrels & Perry 1974) and Berner (2004 and earlier references cited therein). Specifically, the amount of organic carbon stored in the crust should balance the oxidizing power represented by the crustal inventories of Fe3+, and O2. To this, space science and plate tectonics have added the second and third terms.
It is difficult to reconstruct the histories of the variables in equation (2.2). A boundary value for the first integral, however, can be obtained by consideration of crust–mantle carbon budgets and variations in f, the organic-carbon burial fraction.
3. Inputs of mantle carbon
Fluxes and inventories throughout the carbon cycle depend on inputs of carbon from the mantle. These occur at mid-ocean ridges, arc volcanoes, hotspots and in plume events. The present strengths of these sources will be considered sequentially. An estimate of variations over the course of Earth history will follow.
(a) Mid-ocean ridges
Each year, 21 km3 of basalt is added to the oceanic crust at spreading centres (Crisp 1984; also consistent with a plate-creation rate of 3.4 km2 yr−1 (Rowley 2002) and a plate thickness of 5–7 km (White et al. 1992; Kadko 1994)). Sampled after cooling at the seafloor, its CO2 content is dependent on the hydrostatic pressure. At the depths of mid-ocean ridges, the result is commonly about 200 p.p.m. (the routinely reported concentrations refer to weights of CO2). The question is how much CO2 was in the parent magma. The difference will have been transferred, by way of hydrothermal circulation, to the ocean.
Recently, Saal et al. (2002) have shown that, in undegassed MORB (mid-ocean ridge basalt), concentrations of CO2 vary with those of Nb. The weight ratio is CO2 : Nb=239±46 (2σ). Since Nb is not lost during degassing, the initial CO2 content of a sample of MORB can be estimated from its content of Nb. The average for normal MORBs (i.e. those in which trace-element abundances have not been affected by proximity to plumes or recently subducted continental materials) on the East Pacific Rise is 3.45 p.p.m. Nb (Su & Langmuir 2003). The estimated, average, initial content of CO2 is thus 3.45×239=825 p.p.m. If 200 p.p.m. remain in the cooled basalt, the difference transmitted to the exogenic reservoir is 625 p.p.m. Given a rock density of 2.8 g cm−3, this corresponds to 0.8 Tmol C yr−1 (Tmol=teramol=1012 mol). If the Nb average quoted for all MORBs (5.02 p.p.m.; Su & Langmuir 2003) is instead used as the basis for the calculation, the result is 1.3 Tmol C yr−1. These values bracket a third estimate, namely 0.9 Tmol C yr−1, reported in the original publication (Saal et al. 2002) and reached using a slightly different approach.
Numerous alternative estimates have been based on CO2 : 3He ratios in hydrothermal fluids and gases. These have been reviewed by Resing et al. (2004), who settle on a range of 0.5–2 Tmol C yr−1. Preferring the approach based on chemical analyses of the rocks, we adopt 1 Tmol C yr−1 as the present magnitude of the mid-ocean ridge component of Jam.
(b) Arc volcanoes
The annual magma volume is 0.5 km3 (Carmichael 2002). Carbon dioxide is abundant in the gases, but its isotopic composition often deviates from the mantle value and the CO2 is regarded as deriving from subducted sedimentary carbonates and organic carbon as well from the mantle (Sano & Marty 1995; Sano & Williams 1996; Shaw et al. 2003,2004). The total flux of CO2 from arc volcanism is approximately 1.6 Tmol yr−1 (Hilton et al. 2002). Of this, approximately 13% is from the mantle (Shaw et al. 2003), yielding an arc-volcanic component of Jam of 0.2 Tmol C yr−1. The remaining 1.4 Tmol yr−1 is recycling crustal C. Wallace (2005) obtains a similar result by a different method.
(c) Oceanic islands and plumes
In a collection of estimates, this is the most uncertain. The annual volume of magma is approximately 3 km3 (though possibly as small as 1.9 km3), combining igneous provinces and hotspot volcanoes on continents with those in the ocean (Crisp 1984). A more recent, separate tabulation of large igneous provinces by Marty & Tolstikhin (1998) finds a total of 95.5×106 km3 in the past 250 Myr, for a rate of 0.4 km3/yr. It is broadly agreed that these magmas are volatile-rich compared to MORB. Basing their estimate on 3He budgets, Marty & Tolstikhin (1998) suggest that the total output from oceanic islands and plumes ‘is at best similar to that of spreading centres’. Given our estimate above, this suggests an input of somewhat less than 1 Tmol C yr−1.
Since mid-ocean ridge magmas are roughly 10 times more voluminous, the estimated equal flux of mantle CO2 from oceanic islands and plumes calls for a 10-fold enrichment of CO2, and thus perhaps of Nb, in the parent magmas. Observed enrichments of Nb (Hofmann 2004) range from at least 13-fold (Mangaia, Pitcairn, Tahaa) to 2-fold (Mauna Loa). Moreover, Pineau et al. (2004) have suggested that CO2 : Nb ratios might range to values more than 3-fold higher than that found by Saal et al. (2002). In sum, the estimate of 1 Tmol C yr−1, equal to that at the spreading centres, is plausible but highly uncertain.
Together, mid-ocean ridges, arc volcanoes and emissions at island volcanoes and during plume events provide an annual input from the mantle of approximately 2.2 Tmol C.
4. Inventories and accumulation of carbon
Total quantities of carbonate carbon in the crust, estimated from stratigraphic inventories (Holser et al. 1988; Wedepohl 1995; Hunt 1996; Des Marais 2001; Berner 2004; Arvidson et al. in press), commonly range from 2800 to 6500 Emol (Emol=examol=1018 mol). The same reports provide estimates of the total quantity of organic carbon ranging from 675 to 1300 Emol. Only one of these (Arvidson et al. in press) includes carbonate associated with basalt. It also yields the lowest ratio of organic to total carbon, namely 0.13. The other reports yield organic fractions ranging from 0.15 to 0.20, and seem to be influenced by isotopic mass balances, which suggest higher relative quantities of organic carbon.
Wilkinson & Walker (1989) took an alternative approach and focused exclusively on sedimentary carbonates. Examining mass-age data, they found that the best fit could be provided by a constant-mass, constant-burial system with first-order recycling and including 9600 Emol carbonate carbon. An alternative fit emphasizing data from younger sequences yielded a result of 7900 Emol. If we arbitrarily adopt an organic-carbon fraction of 0.15, the corresponding inventories of organic carbon are 1700 and 1400 Emol, for total carbon inventories of 11 300 and 9300 Emol.
The most detailed inventory (Holser et al. 1988) provides total crustal C=7640 Emol. Favouring the approach using mass-age data, particularly that based on younger sequences, we weight it equally with the stratigraphic compilations and estimate total crustal C=8500 Emol.
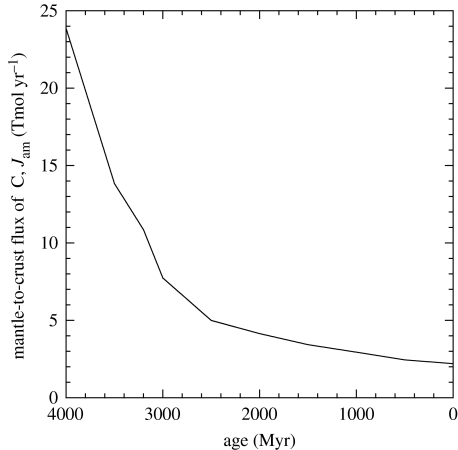
The time required to accumulate this inventory depends on assumptions about the input flux. If the total flux of 2.2 Tmol C yr−1 estimated above were constant, the time required would be 3.86 Gyr. More commonly, it is believed that, earlier in Earth history, such fluxes were higher. Here, we follow Sleep & Zahnle (2001) and Lowell & Keller (2003). These authors scale input fluxes to estimates of high-temperature heat flow, chiefly at spreading centres. For the case in which continents grew episodically from 10 to 80% of current area between 3200 and 2500 Myr ago (Ma), high-temperature heat flow is calculated to decrease from 9× the current level at 3800 Ma, to 4.9× at 3200 Ma, to 2.3× at 2500 Ma, and then to decline exponentially. The resulting scaled carbon flux is shown in figure 4.
Figure 4.
Estimated values of Jam as a function of time. The value at 0 Ma is documented in the text. The scaling relationship at earlier times derives from Sleep & Zahnle (2001) and Lowell & Keller (2003).
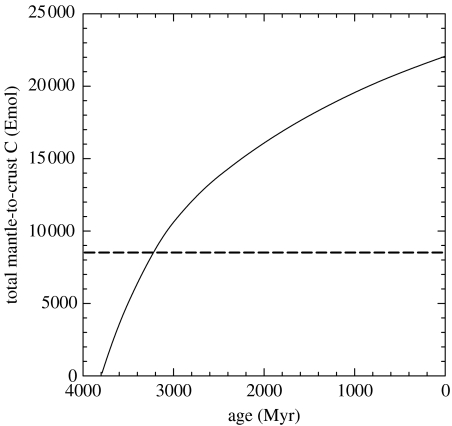
Integration of that flux beginning at 3800 Ma, the end of the late, heavy bombardment, yields the totals depicted graphically in figure 5. The sum exceeds 8500 Emol, the estimated present crustal inventory, after only 575 Myr, at 3225 Ma. In fact, with continents just beginning to form and crustal storage reservoirs thus sharply restricted, returns of carbon to the mantle would probably have become nearly equal to inputs from the mantle well before then.
Figure 5.
Total amount of mantle carbon delivered to the crust as a function of time. Specifically, , where t is the age, Myr, and τ, the time-step, is 107 years. The broken line at 8500 Emol C represents the best estimate of the present crustal inventory of C (table 2).
Together, figure 5 and equations (1.1)–(2.2) provide a new context for considering the development of the carbon cycle. Before turning to the isotopic records, however, we must first review available information regarding the fates of carbon in subduction zones.
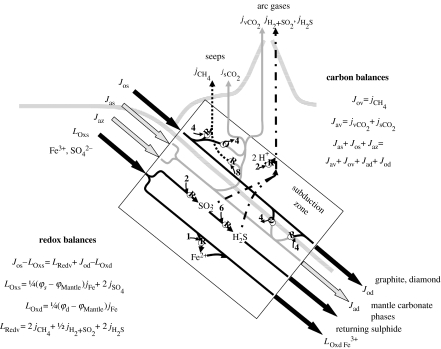
5. Carbon cycling at subduction zones
Processes in subduction zones are crucial to redox balances in the carbon cycle. Figure 6 shows flows of carbon (in oxidized and reduced form) and other products of carbon cycling into, through and out of subduction zones. Reducing power produced by the carbon cycle is carried into subduction zones by organic carbon (Jos). Oxidizing power is carried by sulphate and by ferric iron. The crossing paths and redox reactions suggest the diverse transformations involved. While many different sequences of reactions are possible, the equations in figure 6 summarize the required balances of mass and electrons. All of the carbon that enters the subduction zone must be transferred in some form to either the crust or mantle. And the balance between inputs of reductants and oxidants must be reflected by materials leaving the subduction zone. Resolution and dissection of processes within subduction zones are not currently possible, but available evidence bears on two key questions. When a slab is subducted, (i) what happens to the carbon and (ii) what happens to the reducing power carried by the organic matter?
Figure 6.
Schematic of fluxes of carbon and reducing power in a subduction zone. Terms denote Fe3+/ΣFe (φ), and fluxes of carbon (J, mol per time), specific substances (J, mol per time) and oxidizing or reducing power (L, mol O2 equivalent per time). Subscripts are defined in table 1. Chemical reductions and oxidations are marked by circled letters (R or O). Adjacent numbers indicate number of electrons gained or lost.
(a) Carbon fluxes
The downgoing flux, (Jad+Jod), might be quantified directly if we knew how much material was being incorporated by the mantle and if samples of it were returned to the surface, so that their carbon contents could be determined. Although Jod and Jad are sampled by diamond-bearing rocks and mantle xenoliths, it is not clear how representative these samples are, nor do they allow for quantification of the total downgoing C flux.
More headway can be made by considering the fluxes from arc volcanism and other volatile emissions from subduction zones and then estimating the downgoing flux from the difference between the trench input and surface output. The magnitudes of both are rather uncertain; here, we summarize the best estimates. The three inputs are the organic and carbonate components of subducted sediment (Jos and Jas) and the carbonated basalt in the subducting crust (Jaz). The global subducting-sediment budget of Plank & Langmuir (1998) puts Jas at 0.9 Tmol C yr−1. Estimates of Jos are very poorly constrained, mostly because it is small in comparison to fluxes to and from the continents and shelves. For the modern C cycle, Holser et al. (1988) estimate Joc/Jos≈40. We adopt their value, Jos=0.2 Tmol C yr−1, suggesting a mean Corg content for subducting sediment of 0.13 wt%.
The third flux, Jaz, likely delivers most of the carbon to subduction zones. It derives from Jaw, for which estimates range over more than an order of magnitude from less than 1 to more than 3 Tmol C yr−1 (Bach et al. 2003). The alternate fate for carbon buried by basalt carbonation is represented by Jah, the portion of carbonated basalt actually accreted onto continents. Since this is relatively small, it follows that carbonate can be stored in ocean crust along passive margins for hundreds of millions of years, as in much of the Atlantic basin today. The idea that Jaz approaches Jaw incorporates an assumption that, following tectonic rearrangements, such material is eventually subducted. The resulting rough estimate of the total subduction input is 2–4 Tmol C yr−1.
For the volatile outputs from arcs, the most complete budget has been compiled by Hilton et al. (2002), who considered subduction inputs to and volatile emissions from 26 arc systems worldwide. The authors calculated a global volcanic arc CO2 flux of 1.6 Tmol C yr−1 and emphasized the importance of distinguishing between sources of volatiles. For CO2, these are carbonate and organic C in the subducting slab and CO2 in the mantle wedge. Since the mantle component amounts to 0.2 Tmol C yr−1 (noted above), jvCO2=1.4 Tmol C yr−1. The portion due to subducted carbonates can be estimated from the isotopic composition of the non-mantle component. For all of the arcs, it exceeded the input of sedimentary carbonate. In all cases but one, inclusion of carbonated basalt eliminated the shortfall. Notably, these results are at odds with calculated phase equilibria that indicate carbonated basalts should undergo little devolatilization along most subduction geotherms (Kerrick & Connolly 2001). The other component of Jav is jsCO2, emission of CO2 from seeps, especially in fore-arc and back-arc regions. This flux is unconstrained, and may be as large as jvCO2 (Hilton et al. 2002). Ingebritsen & Manning (2002) have pointed to diffuse fluid flow through tectonically active crust as potentially a major flux of subduction-derived volatiles. This degassing pathway may be sufficient to reconcile the crust–mantle water balance, and could well constitute a significant return of slab-derived CO2 to the crust. Accordingly, Jav is between 1.4 and 2.8 Tmol C yr−1.
The other return flux of carbon from subduction zones to the exogenic chamber is Jov, the reduced carbon from high-and low-temperature seeps, primarily CH4. The related geological forms are diverse, and include mud volcanoes and seeps associated with gas hydrates (Milkov & Etiope 2005; Milkov 2005). Their output of CH4, estimated at 2.1 Tmol yr−1 (Milkov & Etiope 2005), derives from microbial methanogenesis and thermal processes in sediments and sedimentary rocks, as well as from subducted carbon. Since the carbon in Jov (=CH4) has oxidation number equal to −4 and the organic carbon in Jos has oxidation number equal to zero, redox balance provides the stronger constraint. If Jos is approximately 0.2 Tmol C yr−1, Jov cannot be larger than 0.1 Tmol C yr−1. Total volcanic and seep fluxes of carbon (Jav+Jov) are between 1.5 and 2.9 Tmol C yr−1.
From inputs of 2–4 Tmol C yr−1 and recycling fluxes of 1.5–2.9 Tmol C yr−1, we estimate that the fraction recycled is approximately 0.6. By difference, the flux returning to the mantle is 0.8–1.6 Tmol C yr−1.
(b) Reducing power
Any reduced C sent to the mantle—graphite, diamond, elemental C—leaves oxidant behind in the surface environment, thus contributing to AOx. It is also possible that organic C would be oxidized within the subduction zone by reaction with sulphate or an oxidized metal, such as Fe3+. When this occurs, does the reduction product (Fe2+, for example) become an organic-carbon proxy that also contributes to AOx? No, the reaction instead amounts to a last-minute reversal of processes within the carbon cycle. Within the exogenic reaction chamber, Fe3+ will have been produced within the downgoing slab by hydrothermal alteration and by oxidation of Fe2+ at the expense of O2. Or the sulphate will have been produced by processes within the carbon cycle. In either case, the oxidizing power will be represented by an equivalent quantity of organic carbon. For the carbon cycle, therefore, the oxidation of organic carbon by oxidized metals or sulphur within subduction zones is a functional equivalent of biological respiration. Details follow.
If the Fe3+ or sulphate is a product of aerobic oxidation, it carries the oxidizing power of O2 produced during photosynthesis. The same organism that produced the O2 produced an equivalent amount of organic carbon. The oxidation of that organic material within the subduction zone amounts to a reversal of the overall process.
If the Fe3+ was produced at the expense of sulphate during hydrothermal alteration, that sulphate can similarly be traced to photosynthetic O2 and carbon. The chain of chemical events has an additional link, but the oxidation of organic carbon within the subduction zone is again simply a reversal of the process.
If the sulphate was produced by anaerobic, photosynthetic bacteria, those organisms will have produced an equivalent amount of organic carbon. The oxidation of that organic material within the subduction zone amounts to a reversal of the overall process.
Finally, if the Fe3+ was produced by serpentinization, an equivalent quantity of H2 will also have been produced and used by microbiota to produce an equivalent amount of organic carbon, either directly, through chemosynthesis, or indirectly, through consumption of O2, photosynthetic production of organic matter, etc. Again, the oxidation of organic C within the subduction zone amounts simply to a reversal.
If the subducted organic carbon returned to the surface as CO2 after reducing an inorganic substance to some oxidation state lower than that of its input—if Fe3+/ΣFe in the downgoing slab were driven to values lower than Fe3+/ΣFe in unaltered MORB—that would contribute to AOx. Failing that, Jod>0 provides the only route by which processes in subduction zone can yield a net export of oxidizing power by the carbon cycle.
In magnitude, Jod is constrained to be less than Jos. Exchange of C between reduced and oxidized pools is not excluded. Some of the C in Jod might derive from Jas or Jaz. Estimation of Jod requires knowledge of Jos and LRedv. The rate of subduction of organic matter, as discussed above, is here taken as 0.2 Tmol yr−1, though better estimates are clearly warranted. LRedv has two components: the reduced gas flux from arc volcanoes, and the reduced gases from other seeps, represented by jCH4.
The reduced component of the volcanic gas flux is represented by jH2+SO2 and jH2S. In the present context, the question is how it relates to Jos, the reducing power delivered by subduction of organic carbon. The hydrogen abundance in volcanic gases is set by redox equilibrium with water, such that H2 : H2O ratios are maintained near ca 0.01 at most eruptive temperatures (Giggenbach 1996). Taking the arc magmatic-water flux at 17 Tmol yr−1 (Wallace 2005) results in a hydrogen component of 0.17 Tmol yr−1. The SO2 efflux from arcs, some of which may derive from sources other than reduction of subducted sulphate, is of similar magnitude, 0.16–0.28 Tmol yr−1 (Halmer et al. 2002; Wallace 2005). Other reduced species in arc gases are relatively minor. The ratio of H2S : SO2 is generally between 1 and 0.05 (Halmer et al. 2002), and some of the hydrogen sulphide released at volcanic arcs likely derives from volatilization of sulphide in the downgoing slab rather than reduction of sulphate by organic carbon. The reducing power carried by other volatiles, such as CO, COS and CS2, is orders of lower magnitude. Since H2 and SO2 represent two-electron reductions, the total flux is halved to convert to moles O2 equivalent, whereas H2S produced from sulphate represents eight electrons or 2 mol O2 equiv. The resulting estimate from gas chemistry is 0.2 Tmol O2 equiv. yr−1 from H2 and SO2 and 0.4–0.02 Tmol O2 equiv. yr−1 from H2S. By itself, the flux of H2 and SO2 is already equivalent to the reducing power carried by subducted organic carbon. The obvious presence of additional reduced outputs, namely volcanic H2S and hydrocarbons at seeps (jCH4), shows that better knowledge of redox budgets for subduction zones is needed. With due regard for the uncertainties imposed by the present budgets, it also suggests that most or all reducing power carried by subducted organic carbon is returned to the exogenic reaction chamber and that Jod is small at present.
6. Cycling of carbon and its redox partners over time
Over time, the crust has accumulated carbon. Integrated inputs from the mantle have exceeded integrated returns to the mantle. As a means of exploring the balance, we can accept the fluxes and reservoirs proposed thus far as hypotheses and consider how 8500 Emol C might have accumulated and what electron donors were probably associated with the production of organic carbon.
(a) The crust–mantle carbon balance
Values of (Jad+Jod)/Jam control the accumulation of crustal C. When they are less than 1, the crustal inventory will grow. Ideally, a geologic record would exist, but proxies for (Jad+Jod) are rare. The low δ13C values of some diamonds, particularly those of eclogite paragenesis, strongly suggest derivation from crustal organic carbon (Pearson et al. 2004), though this has been disputed (Deines et al. 2001). Recently, it is been suggested that organic carbon can be subducted beyond 250 km and contribute to sublithospheric diamonds (Tappert et al. 2005). The stability of carbonated eclogite at high pressures and temperatures (Dasgupta et al. 2004) indicates that eclogitization may be an important route for the subduction of carbon (both oxidized and reduced) into the mantle. This is particularly interesting in light of recent suggestions that eclogitization is a geologically recent phenomenon (Bjørnerud & Austrheim 2004). A hotter upper mantle earlier in Earth history would more efficiently devolatilize downgoing slabs at shallower depths, removing both carbon and water. Shallow decarbonation, combined with drying of the slab and consequent inhibition of the formation of eclogite, may have meant that Jod and Jad were small early in Earth history (Des Marais 1985). Much depends on the tectonic regime, and when the present style of plate tectonics began, which has been the subject of much debate (Van Kranendonk 2004; Stern 2005).
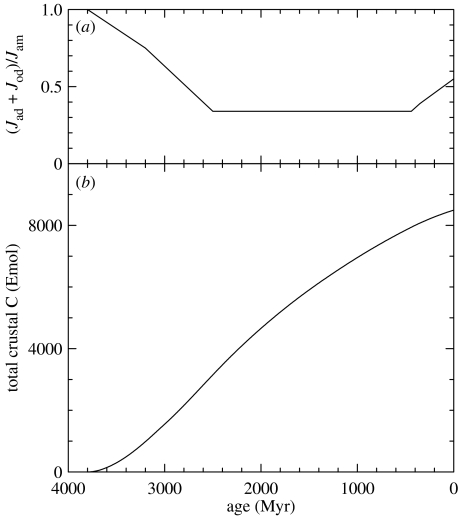
One view takes the slow growth of continents together with the steep early declines in the rate at which carbon is delivered from the mantle (figure 4) as constraining variations in (Jad+Jod)/Jam quite strongly. The scenario associated with figure 4 provides 80% of the present continental area by 2.5 Ga. By comparison, Veizer & Mackenzie (2004) point to evidence suggesting that only 25% of continental crust accumulated between 4.0 and 2.6 Ga, another 35% in the interval to 1.7 Ga, and the final 40% thereafter. Because the continents house the major reservoirs of crustal carbon, (Jad+Jod)/Jam must approach 1 (no net crustal storage) in the early Archaean and decline to lower values only as continents grow. The time course of (Jad+Jod)/Jam shown in figure 7a fits these criteria while eventually yielding a crustal inventory of 8500 Emol C (figure 7b) and a modern (Jad+Jod)/Jam=0.55. The latter value is in the middle of the range estimated above (0.36–0.73).
Figure 7.
Growth of the crustal inventory of carbon over time. (a) Outputs to the mantle relative to inputs from the mantle as a function of time. The ratio is assumed to be near unity when continents were non-existent and to have declined as they increased in size. Values have been adjusted to provide the observed total of 8500 Emol C by 0 Ma. (b) The integral obtained by applying the return fraction specified in panel (a) to the fluxes shown in figure 4. In detail, .
(b) The accumulation of organic carbon and oxidized electron donors
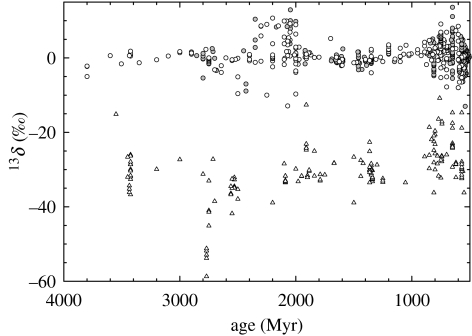
Reconstruction of redox budgets requires estimates of f. These derive from interpretation of the carbon isotopic records, with due attention to the possible importance of Δm, Δg and λ. Figure 8 summarizes observations of δab and δob. The latter represent chemically isolated samples of kerogen (Strauss & Moore 1992). The ratios of H : C in Precambrian kerogens are frequently well below 0.5 (Hayes et al. 1983). Accordingly, the kerogens have been extensively dehydrogenated by processes that frequently involve the loss of 13C-depleted hydrocarbons and thus enrichment of 13C in the residual kerogen. Because our objective is to reconstruct probable isotopic compositions of organic carbon at the time it was removed from the exogenic reaction chamber (i.e. as it flowed through the arrow representing Job in figure 1), we will base our estimates of f mainly on the lower values of δob in each time interval.
Figure 8.
Isotopic compositions of sedimentary carbonates and of sedimentary organic carbon as a function of time. Each point represents the average isotopic composition of an entire stratigraphic unit. Carbonates (Shields & Veizer 2002) are represented by circles. Filled symbols represent geologic units which are well dated. Open symbols represent units for which the dates are approximate. Samples of organic material (Strauss & Moore 1992) are represented by triangles. The ages assigned in the original compilation have been revised to agree with those assigned by Shields & Veizer (2002).
(c) 4400–3800 Ma
In parallel with the isotopic records summarized in figure 8, the integrations in figures 5 and 7 have begun at 3800 Ma. This point in time roughly marks the end of the late, heavy bombardment and the beginning of Earth's sedimentary record. Catling et al. (2001) have considered how earlier events might have set the stage for carbon cycling. In particular, they estimate that impacts of asteroids between 4400 and 3800 Ma probably delivered 1000 Emol of reduced carbon to Earth's surface. To whatever extent such material was not incorporated by the mantle, Earth's crust will have begun with an inventory of primordial organic carbon. When biological cycling of carbon began, the processes would have been
In these equations, Red and Ox are reduced and oxidized forms of a redox partner, such as Fe or S. Reactions between surviving, asteroidal organic material (termed ‘primordial’) and oxidants produced during carbon cycling might have been biologically catalysed and occurred within the exogenic reaction chamber or they might have been thermal and occurred only at depth within accumulating sediments. In either case, the resulting CO2 became part of the crustal inventory. Such reactions would have two effects: (i) an exchange of biotic organic carbon for primordial organic carbon and (ii) consumption of oxidants. Only when production acted to increase the inventory of Corg (here taken to begin at 3.8 Ga) would oxidants begin to accumulate.
(d) 3800–2800 Ma
For this interval, the isotopic record of sedimentary carbonates compiled by Shields & Veizer (2002) includes 24 stratigraphic units. With an average value of δab=−0.1‰, they are consistently enriched in 13C relative to mantle carbon. The standard deviation of the population is 1.9‰. For the same interval, figure 8 yields an estimate of δob=−36‰. Nakamura & Kato (2004) measured values of δaw in basalts from the Warrawoona Group (3425 Myr, Western Australia). Their average result, −0.3±1.2‰, does not differ significantly from δab in sedimentary carbonates in this time interval. Accordingly, Δm≈Δg, the λ(Δm−Δg) terms in equation (1.8) are small, and f=(δab−δi)/(ϵ−Δg)=(δab−δi)/(δab−δob)=0.14. Observed values of δab and δob then indicate that 14% of the carbon being delivered to the exogenic reaction chamber by outgassing of CO2 from the mantle was being buried as reduced, organic carbon.
The net production of organic carbon during any increment of time can be estimated:
| (6.1) |
The recycling carbon (i.e. Jor+Jar+Jov+Jav) will be some portion of the total crustal carbon. If a representative half-mass age is taken as 300 Myr, the fraction recycling in a 10 Myr interval will be 2.3% (see table 1). In any 10 Myr interval, therefore, 2.3% of crustal carbon will be recycled, essentially mixing with the 10 Myr input of mantle carbon. Of that total, a portion controlled by (Jad+Jod)/Jam will be returned to the mantle. Of the remainder, a fraction f will be buried in organic form. Assuming that all recycling organic carbon is oxidized, an integrable form of equation (6.1) is then
| (6.2) |
where γ is the fraction of crustal carbon recycling in a time interval of length τ (e.g. 0.023 for τ=107 years and a half-mass age of 300 Myr), and, at the beginning of the time-step, M is the total crustal inventory of carbon in all forms (figure 7b) and Mo is the total inventory of organic carbon. Stepwise summation provides an estimate of the integrated production of organic carbon, the first integral on the right-hand side of equation (2.2).
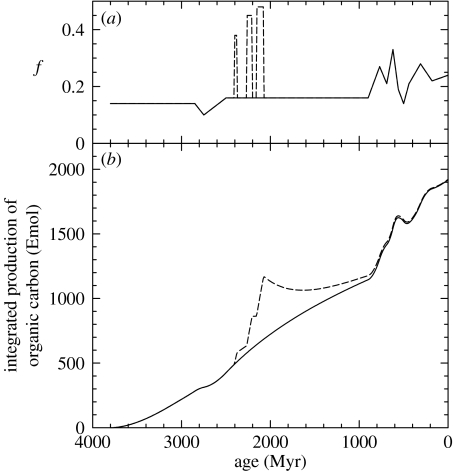
The calculation is based on (i) values of (Jad+Jod)/Jam chosen to provide the observed crustal inventory of C (figure 7a); (ii) values of Jam provided by figure 4; and (iii) values of f shown in figure 9a and estimated from the observations summarized in figure 8. Setting M=Mo=0 at 3800 Ma and summing Net Corg yields the result shown in figure 9b. Because a sharp drop in δob suggests a major change in carbon cycling at approximately 2770 Ma (Hayes 1983, 1994), we will focus first on the interval 3800–2800 Ma. By 2800 Ma, Mo=oxidant release=300 Emol O2 equiv.
Figure 9.
Integrated net production of organic carbon, equivalent to the net release of oxidizing power (Emol O2), over time. (a) Estimated values of f. For ages greater than 890 Myr, they are based on the isotopic compositions shown in figure 8. For younger ages, they are consistent with the isotopic compositions shown in figure 8 and are based in detail on records summarized by Hayes et al. (1999). The broken lines between 2080 and 2400 Myr represent isotopic excursions reviewed by Melezhik et al. (1999) and discussed in the text. (b) The integral obtained when the f values shown in figure 9a and the carbon-return ratios shown in figure 7a are substituted in equation (6.2), with γ=0.023 and M=Mo=0 at 3800 Ma.
To place this result in context, consider the inventories summarized in table 2. For all of the materials considered, a range of estimates can be found in the literature. Those included here are representative high and low values. The value of 300 Emol O2 equiv. greatly exceeds the oxidizing power represented by O2 in the modern atmosphere and ocean and approaches the low estimate of the amount of oxidizing power represented by total crustal deposits of sulphate and sulphate dissolved in seawater. These comparisons decisively eliminate two otherwise-interesting possibilities, namely that O or S could have served as the dominant redox partner for C at this stage of Earth history. In the first case, levels of O2 would have risen high enough to inhibit the mass-independent fractionation of sulphur isotopes. In the second, concentrations of dissolved sulphate would have climbed to levels that lead to large differences between the 34S contents of sedimentary sulphides and sulphates, a signal that is not observed until later (Hayes et al. 1992).
Table 2.
Crustal inventories of reduced and oxidized products of carbon cyclinga.
| product | estimated crustal total Emol O2 equivalent | reference | |
|---|---|---|---|
| high | low | ||
| O2 | 37.2 | 37.2 | Keeling et al. (1993) |
| Fe3+b | 1860 | Goldschmidt (1954) | |
| 1020 | Ronov & Yaroshevsky (1976) | ||
| 480 | Garrels & Perry (1974) | ||
| 332 | Holser et al. (1988) | ||
| total oxidants | 2377 | 1389 | |
| organic C | 1280 | Des Marais (2001) | |
| 675 | Arvidson et al. (in press) | ||
A similar compilation has been prepared by Catling et al. (2001). It includes an estimate of 1000 Emol organic C delivered to the crust by impacts of asteroids between 4400 and 3800 Ma. The entries above pertain to the present. To whatever extent primordial organic material has survived in the crustal inventory, it is included in the ‘organic C’ category.
Calculated from data summarized by Lécuyer & Rickard (1999). Represents Fe3+ in excess over mantle Fe3+/ΣFe=0.12 (Bezos & Humler 2005).
The remaining oxidized product listed in table 2 is ferric iron. Its crustal inventory is large enough to offer plenty of headroom. Iron formations are, moreover, important components of the Archaean sedimentary record. The present context, however, requires that iron served as the electron donor in primary production while the environment remained strictly anaerobic. An organism capable of utilizing Fe2+ directly has recently been isolated (Jiao et al. 2005). In principle, we need look no further, but alternatives deserve consideration. These are: (i) the true value of f was really near zero; (ii) f was 0.14 but no oxidants accumulated; or (iii) f was 0.14 and the electron donor was H2, for which the immediate oxidized product (H2O) was invisible, although iron was probably the ultimate source of the electrons.
The true value of f could be approximately zero if the basalt-carbonation correction had been wrongly excluded and the correct value of λ(Δm−Δg) was 4.9‰. If λ were 0.9, this would require that the initial average value of δaw had been −5.5‰ and that Nakamura & Kato (2004) found instead −0.3‰, because the isotopic compositions of all of their samples had been affected by post-depositional carbon-isotopic exchange. However, among 42 samples of four different types, the most negative value of δaw is −3.4‰ and there is no correlation between δ and the abundance of carbonate. Evidence for the required alteration is therefore lacking, making it likely that f truly approached 0.14.
If f approached 0.14 but no oxidants accumulated ((ii), above), (Jad+Jod)/Jam must have been 1, indicating rapid and quantitative return of all carbon to the mantle, even as 3.5 million km2 of continental crust (Lowe 1992) formed prior to 3.2 Ga. Moreover, as substantial quantities of organic carbon were forming as required by f=0.14 (and thus Job/Jam≥0.14), the putatively non-accumulating oxidant, which could not be either O2 or sulphate and which had to be present only at trace levels, was able to reoxidize all of the organic material completely even as the carbon itself returned quantitatively to the mantle. The required combination of circumstances is practically impossible.
The third alternative, in which f was approximately 0.14 and H2 served as the electron donor, fits well with multiple lines of evidence. Anaerobic organisms capable of producing organic material from CO2 and H2 are abundant and include both chemoautotrophs (methanogens, acetogens) and photoautotrophs (photosynthetic bacteria; Tice & Lowe 2004 have already postulated that such organisms were important members of near-shore microbial communities 3.4 Ga). The potential versatility of microbial ecosystems based on such organisms is great enough to provide both producer and consumer assemblages and thus to stabilize carbon cycling and yield globally consistent isotopic fractionations. Fermentative consumption would remobilize organic C as a mixture of CO2 and CH4, providing needed greenhouse warming (Kasting & Catling 2003) and setting the stage for the isotopic transient observed at 2.8–2.7 Ga (Hayes 1994).
The required net input of H2 would be 600 Emol (=300 Emol O2 equiv.). Owing to the postulated declines in Jam (figure 4) and increases in [1−(Jad+Jod)/Jam] (equation (6.2)), the required rate would rise only slowly from 0.5 Tmol yr−1 at 3500 Ma to 0.9 Tmol yr−1 at 2800 Ma. If, as is likely, conditions at the seafloor favoured serpentinization reactions (Sleep et al. 2004), a flux of 0.7 Tmol H2 yr−1 could be provided by alteration of less than 1015 g Mg-rich (komatiitic) basalt per year. The present rate of generation of ocean crust is about 6×1016 g yr−1, so the requirement poses no problem. In the overall sequence of electron transfers, it is only the immediate oxidized product of carbon fixation (H2O) which is ‘invisible’. The reducing power represented by the organic matter is balanced by Fe3+, which is accumulating or being subducted.
To summarize, from both geological and biological points of view, there are highly plausible mechanisms by which substantial amounts of organic carbon could be produced and buried in accord with the isotopic records, with complementary releases of oxidizing power, without any requirement for generation of O2 during the interval 3800–2800 Ga. The most likely redox partner for C during this interval is Fe, either directly via phototrophic oxidation of dissolved Fe2+, and/or indirectly, with H2 shuttling electrons from Fe2+ in seafloor basalts to phototrophic and chemoautotrophic producers.
(e) 2800–1800 Ma
The next billion years begin with the apparent onset of oxygenic photosynthesis (Hayes 1983, 1994; Summons et al. 1999), which includes a period during which the abundance of 13C in sedimentary carbonates was sometimes markedly enriched (Karhu & Holland 1996; Melezhik et al. 1999), and ends with the likely onset of sulphidic conditions in the deep sea (Canfield 1998).
Estimates of f derived from the isotopic records provide scant evidence that the development of oxygenic photosynthesis, for all its magnificence as a biochemical innovation, provided a significant increase in the net release of oxidizing power. If anything, Job initially declined, a feature marked by the notch visible at 2.8 Ga in figure 9a. By 2500 Ma, f appears to have risen to 0.16, a value only slightly higher than that estimated for the early Archaean. At 2450 Ma, when traces of O2 quench the mass-independent fractionation of sulphur isotopes, the estimated minimal total release of oxidizing power by the C cycle is 460 Emol O2 equiv. Although uncertainties in that total are very large, it is far short of the capacities of crustal Fe and S to supply electrons (table 2). Levels of O2 in the environment would have been sharply limited by the strength of those sinks.
What, then, happened between 2400 and 2000 Ma? Contents of 13C in carbonates are frequently elevated, occasionally exceeding +10‰ (figure 8). Accepting these compositions as representative of the global pool of oceanic DIC would imply a near-trebling of f. Writing three years after Karhu & Holland (1996) published ‘Carbon isotopes and the rise of atmospheric oxygen’, Melezhik et al. (1999) drew on additional data to define three apparently separate pulses of isotopic enrichment. That dissection of the record yields the three spikes in f that are represented by broken lines in figure 9a (alternative stratigraphic correlations yield only two spikes; Bekker et al. 2003). The isotopic signal has been associated with other lines of geochemical evidence indicating that the atmosphere became more oxidizing at about the same time. The ensemble has become known as ‘The Great Oxidation Event’ (Holland 2002).
One view of the scale of the event is provided by the broken line in figure 9b. If the isotopic signals represent pulses of carbon burial, the integrated production of organic carbon by 2000 Ma would be 600 Emol greater. As shown by table 2, that is more than enough to provide the present inventories of and O2. But there are problems with interpreting these isotopic variations in terms of carbon burial.
There is no parallel isotopic enrichment in the organic carbon, as would be expected if the carbonate represented the oceanic DIC that was the carbon source for primary producers. Karhu & Holland (1996) searched for a signal in the organic-carbon record and found its absence ‘rather odd’. If the record were not so fragmentary and noisy (preservation is a much greater problem for organic carbon than for carbonate minerals) that the problem could be set aside (Melezhik et al. 1999), this alone would be fatal to the interpretation.
Evidence is lacking for the large deposits of organic carbon that should have formed (Melezhik et al. 1999; Aharon 2005).
The sequence of isotopic signals is reversed from that expected. If the oxygenation that cut off mass-independent fractionation of sulphur isotopes was due to an organic-carbon-burial event, the disappearance of the mass-independent fractionation of sulphur isotopes should not precede the first carbon-isotopic enrichments.
It is difficult to envision supplies of nutrients great enough to sustain the levels of productivity required to supply the organic carbon. Aharon (2005) has discussed supplies of phosphate very insightfully, concluding that efficient stripping of P from organic matter prior to burial provides the only solution. To achieve the projected values of f, the C : P ratio in buried organic matter would have to be 4000 and the organic-preservation rate would have to be 6.6%, about fivefold higher than that observed in the Black Sea.
Stratigraphic correlations are not secure enough to demonstrate that the various isotopic excursions are coeval, and thus necessarily linked to variations in a global reservoir (Aharon 2005).
Most of the units displaying the isotopic enrichments are dolostones with substantial isotopic variability. In their list of 12 ‘major localities,’ Melezhik et al. (1999) report within-unit ranges of 3–13‰ and an average range of 7‰. Consistent with this, for the interval 2350–2000 Ma (inclusive), Shields & Veizer (2002) report 589 δ-values between −2.5 and +2.5‰ and 424 between +5 and +13‰. That is, ‘normal’ isotopic abundances are more common than elevated abundances.
When complete chemical analyses of the carbonates are reported, they often include substantial concentrations of SiO2 (to 40%; Melezhik et al. 1999). Stratigraphic columns indicate that some of the isotopically enriched carbonates are closely interbedded with shales (Buick et al. 1998), others are described as ‘nodular’ (Bekker et al. 2003).
The latter features (vi) and (vii) are more characteristic of diagenetic carbonates than of marine limestones faithfully carrying records of oceanic DIC. When it is recalled that values of 13δ to +13‰ are common in much younger dolomites that have been affected by methanogenic diagenesis (Klein et al. 1999; Mazzullo 2000), that alternative interpretation demands attention. In fact, attribution of the isotopic signals to methanogenic diagenesis has already been favoured by several sets of authors (Yudovich et al. 1991; Dix et al. 1995).
In all likelihood, the diagenetic alternative has failed to win popularity because an alternative does not appear to be needed. Multiple, convergent lines of evidence—independent of carbon-isotopic signals—indicate that thresholds of environmental oxidation were crossed during this time interval (Bekker et al. 2004). When the carbon-isotopic record fits into this picture at least roughly, why not include it? Even more to the point, given the apparent reality of the oxidation, how else should the carbon-isotopic enrichments be explained? They are temporally associated, dramatic and numerous (Bekker et al. 2003), globally distributed, and have the right polarity (enrichment rather than depletion).
(f) Mechanisms of oxygenation
The problem lies not in accepting the isotopic signals as markers of the oxidation but in assuming that they represent the cause. This is a key distinction. Either the steady functioning of the carbon cycle catalysed biological and ecological developments that shifted the relative strengths of oxygen sources and sinks (Holland 1978) or dramatic changes in carbon fluxes were required. If the former, the carbon-isotopic enrichments are environmental reporters comparable to the mass-independent fractionations of sulphur isotopes. If the latter, they point to increased burial of organic carbon but not necessarily to permanent changes in oxidation.
Flux-driven changes are subject to reversal. Buried organic material will be recycled by erosion and volcanism. As indicated by the downward trend of the broken line in figure 9b after 2000 Ma, oxidants will be consumed. Even if a rise in O2 were driven by increases in Job, some additional change would be required to stabilize the transition and make it permanent. Geophysical phenomena such as rifting and increased sedimentation can increase Job, but are inherently cyclical. What they accomplish in one epoch will be undone in another. Increased burial of organic carbon is a half answer. It can push O2 levels higher, but maintaining them will require some further change, a second half to the answer.
Unidirectional change, a permanent strengthening of the source of oxidants, is in the realm of evolutionary biology. Physiological and ecological changes could stabilize higher levels of O2. Do they serve as the second half of the answer? If so, they are the biological results of geophysical stimuli and we need an explanation for the linkage. Or are physiological and ecological changes answers in themselves? If so, advances in oxygenation have largely biological origins and combinations of disparate phenomena are not required.
Kinetic factors are also pertinent. In discussing the carbon cycle, it is common to distinguish between the fast, biological cycle of photosynthesis and respiration and the slow, geological cycle in which erosion, weathering and volcanism are balanced by carbon burial. In global chemical terms, O2 is a highly reactive, transient intermediate that is produced and consumed within the exogenic reaction chamber. Its steady-state abundance must depend on the relative strengths of sources and sinks in that system. In contrast, sedimentary carbonates and organic materials are outputs from that system. Their relative abundances are controlled by geophysical factors that vary over relatively long time-scales.
(g) Causes of the carbon-isotopic transient
We can suggest a sequence of biologically driven environmental changes, associated with a rise in levels of O2, that would produce the observed isotopic signals without requiring enhanced burial of organic carbon. They would also account for the absence of congruent variations in the organic-carbon isotopic record.
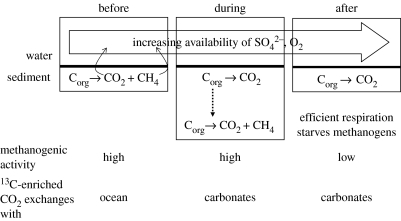
Especially prior to 2.3 Ga, methanogenic diagenesis must have been common. If electrons for biosynthesis were supplied mainly by Fe2+, the insolubility of the oxidized product, Fe3+, meant that electron acceptors were rare in most surface environments (Walker 1987). When respiring heterotrophs are excluded, the recycling of organic carbon in microbial communities will be managed by fermenters, with methanogens playing a vital role. This will have occurred in the same zones that are now occupied by aerobes. These methanogenic communities will have exchanged CO2 freely and directly with the oceanic water column, and, as a result, the isotopically enriched pools of CO2 that are characteristic of methanogenic diagenesis in modern environments will not have developed. This situation is summarized in the ‘before’ segment of figure 10.
Figure 10.
Summary of a sequence of conditions that could account for the abundance of 13C-enriched sedimentary carbonates 2400–2080 Myr ago.
The development of oxygenic photosynthesis by at least 2700 Ma began to change that situation, however slowly. Particularly near oxygen-producing communities (many of the isotopically enriched dolostones are associated with stromatolites; Melezhik et al. 1999), methanogens will eventually have been pushed to deeper levels in the sediment. When this occurred, apparently beginning at about 2400 Ma, isotopically enriched pools of porewater DIC will have developed. As carbon was exchanged with the carbonate minerals in the sediment (a process catalysed by the organic acids produced during fermentation), the isotopic signal characteristic of methanogenesis will have been recorded for the first time. Within about 400 Myr, a second event shut the signal off. Fundamentally, it must have been some weakening of the supply of organic material to methanogenic communities. As suggested in the ‘after’ segment of figure 10, it could have been caused by a further rise in the availability of electron acceptors. Alternatively, the tight association between stromatolitic producers and methanogenic consumers, indicated by many of the 13C-enriched sequences, might have been destabilized by environmental changes.
As in the Neoproterozoic, the isotopic excursions are associated with apparently extreme glaciations (Young et al. 2001; Tajika 2003). The effects of those glaciations on global redox geochemistry, if any, are unclear. It has conversely been proposed that the glaciations were caused by global oxidation which destroyed a methane greenhouse and that this occurred promptly after the development of oxygenic photosynthesis (Kopp et al. 2005). The second part of this does not fit with biomarker records that place the advent of oxygenic photosynthesis at or before 2.7 Ga (Summons et al. 1999; Brocks et al. 2003; Eigenbrode 2004). It also does not fit with the inescapably slow effects of carbon cycling. Releases of oxidizing power have been continuous (figure 9b). But the oxidized products accumulated by 2.5 Ga cannot have accounted for more than a fraction of the inventory of ferric iron and almost none of the sulphate (cf. table 2). In those circumstances, by what mechanism did the development of oxygenic photosynthesis promptly sustain steady-state concentrations of O2 high enough to destroy the methane greenhouse? Until that question can be answered, the evidence for (i) a 400 Ma delay between the evolutionary event and its environmental impact and (ii) the continuing capacity of Fe and S as redox buffers should overrule speculation.
Oxidation of S by O2 would have produced sulphate in surface environments. However, by 1800 Ma, the estimated integrated release of oxidizing power (figure 9b) was only 820 Emol O2 equiv. This is still well below the low estimate of the amount required to build the crustal inventory of Fe3+ (table 2). As suggested by Canfield (1998), therefore, it is likely that production of sulphate mainly provided a route, via sulphate-reducing bacteria, to delivery of excess sulphide to deep ocean waters and the consequent cessation of deposition of banded iron formations.
(h) 1800 Ma–present
Carbon-isotopic records from the Neoproterozoic onward are good enough that values of f can be estimated with some confidence (Hayes et al. 1999). The results are crudely summarized in figure 9a. When these values of f are applied to the carbon fluxes estimated here, the projected final total release of oxidizing power is 1920 Emol O2 equiv. This is close to the average estimate of the total release represented by the sum of crustal Fe3+, and O2.
The agreement is notable, but discrepancies within the redox accounts are more impressive. Estimates of crustal inventories of oxidants vary by a factor of 1.7. Similarly, the estimated crustal inventories of organic carbon fall short (perhaps by two times) of the summed inventories of oxidized products and of the estimated total burial of organic carbon (figure 9b). At face value, this requires that reduced products have been exported from the crust more efficiently than oxidized products. Unless organic carbon could be exported to the mantle more efficiently than Fe3+, we are practically required to attribute such an imbalance to loss of H2 from the top of the atmosphere. The redox imbalance and the likely importance of H2 loss have been incisively examined and elaborated by Catling et al. (2001) and by Catling & Claire (2005). They associate loss of H2 mainly with atmospheric CH4 and thus with the Archaean. By contrast, Tian et al. (2005) have noted the possible importance of the low exobase temperature of an anoxic atmosphere in limiting the rate of H2 escape from the early Earth. They contend that higher levels of O2 in the atmosphere after 2.4 Ga would have increased exobase temperatures and promoted escape of hydrogen. Resolution of the history of hydrogen escape will constrain the second integral on the right-hand side of equation (2.2), and be a key step in reconstructing the time course of AOx.
The dearth of reduced products becomes particularly notable when the likely integrated effects of subduction, represented by the third integral in equation (2.2), are considered. Lécuyer & Rickard (1999) contend that the net rate at which subduction is transferring Fe3+ from the crust to the mantle is presently 7 Tmol yr−1, or 1.8 Tmol O2 equiv. yr−1. If the continents are at steady state with respect to organic carbon, so that, in equation (6.2), fγM+γMo=0, the rate at which the carbon cycle is releasing oxidizing power is given by (cf. equation (6.2))
| (6.3) |
where the values substituted are centre points of the ranges estimated in earlier sections of this paper. Since the net rate of subduction of Fe3+ proposed by Lécuyer & Rickard (1999) greatly exceeds this value, it is either incorrect or represents a temporary situation. Attention to subduction of Fe3+ is, however, a very good idea. The continuing release of oxidizing power, coupled with the minimal variations in atmospheric levels of O2 during the Phanerozoic (Berner 2004) and balanced exchange of oxidizing power by the cycles of carbon and sulphur during the same interval (Canfield 2004), requires a continuing sink. It seems inescapable that this is supplied by oxidation of Fe2+ at the seafloor.
Bounds can be placed on the extent of this transfer over Earth history, since iron returning to the mantle with a higher Fe3+/ΣFe than it emerged with will result in oxidation of the upper mantle. There is a growing consensus that the redox state of the upper mantle, as measured by its oxygen fugacity, has not changed greatly over Earth history. The recent constraint of Li & Lee (2004), from the V/Sc systematics of MORBs, indicates that the oxygen fugacity of the mantle source has increased by at most 0.3 log unit since 3.5 Ga. Using the relation of Kress & Carmichael (1991), this translates into a maximal 6% increase in the ferric iron content of the mantle, if all of that increase were attributable to addition of Fe3+. Taking the mass of the upper mantle to be 1.34×1027 g (Ballentine et al. 2002), the mantle Fe content to be 6.3 wt% (Palme & O'Neill 2003) and the mantle Fe3+/ΣFe to be 0.12 (Bezos & Humler 2005), this yields a maximal input of 1.1×1022 mol of ferric iron since 3.5 Ga, or a maximum average input rate of 3 Tmol Fe3+ yr−1 (0.75 Tmol O2 equiv.). This is comfortably within potential loads imposed by AOx.
The agreement between the projected release of oxidizing power and the average estimate of oxidized products is far from comforting. Because considerable amounts of Fe3+ must have been exported to the mantle, the total release of oxidizing power by the carbon cycle should exceed considerably the inventory of Fe3+ remaining in the crust. Part of any shortfall can be accommodated by recalling that the present estimates are minima. Finite values of Jod and a failure to reoxidize organic carbon in Jor will increase AOx.
The reburial of organic carbon can be treated quantitatively. If a portion, x, of Jor is simply reburied, two things will happen: (i) since it does not re-enter the exogenic system as CO2, that C will be unavailable for reduction. The organic burial flux and thus the overall production of oxidizing power will be decreased by an amount fxJor; (ii) the oxidizing power consumed by oxidation of Jor will be reduced by an amount xJor. The first term is a loss of oxidizing power, the second is a gain. The difference is (1−f)xJor. Unfortunately, this term cannot simply be added to equation (6.2). Although it would account for the carbon and redox balances in a single increment of time, the proper treatment of subsequent increments would require that the reburied material be followed, with appropriate adjustments being made to sedimentary and continental inventories. Even during the Phanerozoic, the presence of detrital coal and kerogen in marine sediments (Sackett et al. 1974) shows that x is not zero. On the other hand, it is known that micro-organisms in weathering profiles incorporate carbon even from refractory kerogens (Petsch et al. 2001) and that the great bulk of organic carbon in marine sediments is not recycled, detrital organic material. Under anaerobic conditions, however, reburial of organic carbon is likely to have been more important.
Can the carbon cycle operate at a point of redox neutrality, neither releasing nor consuming oxidants, even when isotopic compositions indicate significant rates of burial of organic carbon? The answer is in equation (6.3). Even if burial and recycling are balanced, redox neutrality still requires Jam=Jad+Jod. In other words, inputs from the mantle are equal to outputs to the mantle. This is not a likely coincidence. Carbon flows into subduction zones not just from Jam but from multiple sources. If the portion of Jas+Jos+Jaz equal to Jam is to be returned to the mantle while the balance flows to Jav and Jov, a very clever demon will be required to operate the carbon valve in the subduction zone. Notably, f (and thus δab−δi) is not a measure of Jam−(Jad+Jod). No particular value of δab can be recognized as indicating redox neutrality. This yields two fundamental insights. First, caution is required when interpreting the isotopic record in terms of redox variations. Second, the conditions required for redox neutrality are precise and improbable.
7. Concluding remarks
We have taken a new approach to mass and redox balances involving the C cycle. Its principal strength is a realistic acknowledgement of the importance of inputs from and outputs to the mantle. This broader context has invited consideration of processes that may otherwise be overlooked or misconstrued. Its weakness is that our knowledge of some of the key processes is presently imprecise. We need to learn more about inputs of carbon, hydrogen escape and processes in subduction zones to better evaluate each component of AOx. Even now, however, the resulting uncertainties do not cripple the approach. The key finding is dramatic: the persistence of δab at values near zero rather than −5‰ can be interpreted only in terms of continuous and substantial releases of oxidizing power by the carbon cycle. Two points follow.
The oxidation of the crust has been more continuous than episodic. Increases in steady-state levels of oxidants derive either from combinations of geological and biological changes or from biological changes alone. The scientific problem of reconstructing Earth's oxidation is more biological than geochemical.
Continuous sinks for oxidizing power are required, at least since atmospheric levels of O2 stabilized at current levels approximately 550 Ma. Since exchanges of oxidizing power between the carbon and sulphur cycles have been balanced during that same time interval, this can have been provided only by the oxidation of iron.
Together, these affect our view of the carbon cycle. It should not be seen as the competent and versatile manager of an electron-transfer market, effortlessly balancing offers and bids. Instead, it is a wrong-way passenger on a downgoing escalator. It continuously receives new CO2 from the mantle, relentless supplies of solar energy and nutrients leached from deposits that have retained their organic carbon. To hold its place, it must constantly produce new organic matter. While doing so, it releases oxidants that threaten to reverse its accomplishments. The stability of δab is an indicator of the success of its evolutionary strategies. The stability of PO2 is evidence of an equally competent sink.
Acknowledgments
The seeds from which the ideas discussed have grown were planted when J.M.H. looked out of Alvin's viewports and observed the H2-rich waters of the Lost City hydrothermal vent system. The generosity of Deborah Kelley and Jeffrey Karson, the co-chief scientists of the Lost City cruise, is greatly appreciated. Since then, we have benefited from helpful and stimulating exchanges with Stan Hart, Don Anderson, David Rowley and Alberto Saal. We are very grateful for critical reviews of this manuscript provided by David Catling and Jennifer Eigenbrode. J.M.H. receives support as a member of the Astrobiology team led by S. D'Hondt, University of Rhode Island. J.R.W. is supported by an NDSEG Graduate Fellowship from the Office of Naval Research.
Footnotes
One contribution of 14 to a Discussion Meeting Issue ‘Major steps in cell evolution’.
References
- Aharon P. Redox stratification and anoxia of the early Precambrian oceans: implications for carbon isotope excursions and oxidation events. Precambrian Res. 2005;137:207–222. [Google Scholar]
- Alt J.C, Teagle D.A.H. The uptake of carbon during alteration of ocean crust. Geochim. Cosmochim. Acta. 1999;63:1527–1535. 10.1016/S0016-7037(99)00123-4 [Google Scholar]
- Alt J.C, Teagle D.A.H. Hydrothermal alteration of upper oceanic crust formed at a fast-spreading ridge: mineral, chemical, and isotopic evidence from ODP Site 801. Chem. Geol. 2003;201:191–211. 10.1016/S0009-2541(03)00201-8 [Google Scholar]
- Arvidson, R. S., Mackenzie, F. T. & Guidry, M. In press. MAGic: a Phanerozoic model for the geochemical cycling of major rock-forming components. Am. J. Sci
- Bach W, Peucker-Ehrenbrink B, Hart S.R, Blusztajn J.S. Geochemistry of hydrothermally altered oceanic crust: DSDP/ODP Hole 504B—implications for seawater-crust exchange budgets and Sr- and Pb-isotopic evolution of the mantle. Geochem. Geophys. Geosyst. G3. 2003;4:1–29. [Google Scholar]
- Ballentine C.J, van Keken P.E, Porcelli D, Houri E.H. Numerical models, geochemistry and the zero-paradox noble-gas mantle. Phil. Trans. R. Soc. A. 2002;360:2611–2631. doi: 10.1098/rsta.2002.1083. 10.1098/rsta.2002.1083 [DOI] [PubMed] [Google Scholar]
- Bekker A, Karhu J.A, Eriksson K.A, Kaufman A.J. Chemostratigraphy of Paleoproterozoic carbonate successions of the Wyoming Craton: tectonic forcing of biogeochemical change? Precambrian Res. 2003;120:279–325. 10.1016/S0301-9268(02)00164-X [Google Scholar]
- Bekker A, Holland H.D, Wang P.-L, Rumble D, III, Stein H.J, Hannah J.L, Coetzee L.L, Beukes N.J. Dating the rise of atmospheric oxygen. Nature. 2004;427:117–120. doi: 10.1038/nature02260. 10.1038/nature02260 [DOI] [PubMed] [Google Scholar]
- Berner R.A. A model for atmospheric CO2 over Phanerozoic time. Am. J. Sci. 1991;291:339–376. doi: 10.2475/ajs.289.4.333. [DOI] [PubMed] [Google Scholar]
- Berner R.A. Oxford University Press; New York, NY: 2004. The Phanerozoic carbon cycle. [Google Scholar]
- Berner R.A, Maasch K.A. Chemical weathering and controls on atmospheric O2 and CO2: fundamental principles were enunciated by J. J. Ebelmen in 1845. Geochim. Cosmochim. Acta. 1996;60:1633–1637. 10.1016/0016-7037(96)00104-4 [Google Scholar]
- Bezos A, Humler E. The Fe3+/ΣFe ratios of MORB glasses and their implications for mantle melting. Geochim. Cosmochim. Acta. 2005;69:711–725. 10.1016/j.gca.2004.07.026 [Google Scholar]
- Bjerrum C.J, Canfield D.E. New insights into the burial history of organic carbon on the early Earth. Geochem. Geophys. Geosyst. 2004;5:1–9. 10.1029/2004GC000713 [Google Scholar]
- Bjørnerud M.G, Austrheim H. Inhibited eclogite formation: the key to the rapid growth of strong and Buoyant Archean continental crust. Geol. Soc. Am. 2004;32:765–768. 10.1130/G20590.1 [Google Scholar]
- Brocks J.J, Buick R, Logan G.A, Summons R.E. Composition and syngeneity of molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Pilbara Craton, Western Australia. Geochim. Cosmochim. Acta. 2003;67:4289–4319. 10.1016/S0016-7037(03)00208-4 [Google Scholar]
- Broecker W.S. A boundary condition on the evolution of atmospheric oxygen. J. Geophys. Res. 1970;75:3553–3557. [Google Scholar]
- Buick I.S, Uken R, Gibson R.L, Wallmach T. High δ13C Paleoproterozoic carbonates from the transvaal Supergroup, South Africa. Geology. 1998;26:875–878. 10.1130/0091-7613(1998)026%3C0875:HCPCFT%3E2.3.CO;2 [Google Scholar]
- Canfield D.E. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–453. 10.1038/24839 [Google Scholar]
- Canfield D.E. The evolution of the earth surface sulfur reservoir. Am. J. Sci. 2004;304:839–861. [Google Scholar]
- Canfield D.E. The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 2005;33:1–36. 10.1146/annurev.earth.33.092203.122711 [Google Scholar]
- Carmichael I.S.E. The ‘andesite aqueduct’ perspectives on the evolution of intermediate magmatism in west-central (105–99°W) Mexico. Contrib. Mineral. Petrol. 2002;143:641–663. [Google Scholar]
- Catling D.C, Claire M.W. How Earth's atmosphere evolved to an oxic state: a status report. Earth Planet. Sci. Lett. 2005;237:1–20. 10.1016/j.epsl.2005.06.013 [Google Scholar]
- Catling D.C, Zahnle K.J, McKay C.P. Biogenic methane, hydrogen escape, and the irreversible oxidation of the early earth. Science. 2001;293:839–843. doi: 10.1126/science.1061976. 10.1126/science.1061976 [DOI] [PubMed] [Google Scholar]
- Crisp J.A. Rates of magma emplacement and volcanic output. J. Volcanol. Geotherm. Res. 1984;20:177–211. 10.1016/0377-0273(84)90039-8 [Google Scholar]
- Dasgupta R, Hirschmann M, Withers A.C. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 2004;227:73–85. 10.1016/j.epsl.2004.08.004 [Google Scholar]
- Deines P, Viljoen F, Harris J.W. Implications of the carbon isotope and mineral inclusion record for the formation of diamonds in the mantle underlying a mobile belt: Venetia, South Africa. Geochim. Cosmochim. Acta. 2001;65:813–838. 10.1016/S0016-7037(00)00569-X [Google Scholar]
- Derry L.A, Kaufman A.J, Jacobsen S.B. Sedimentary cycling and environmental change in the Late Proterozoic: evidence from stable and radiogenic isotopes. Geochim. Cosmochim. Acta. 1992;56:1317–1329. 10.1016/0016-7037(92)90064-P [Google Scholar]
- Des Marais D.J. Carbon exchange between the mantle and the crust, and its effect upon the atmosphere: today compared to Archean time. In: Sundquist E.T, Broecker W.S, editors. The carbon cycle and atmospheric CO2: natural variations Archean to present. American Geophysical Union; Washington, DC: 1985. pp. 602–611. Geophysical Monograph 32. [Google Scholar]
- Des Marais D.J. Isotopic evolution of the biogeochemical carbon cycle during the Precambrian. Rev. Mineral. Geochem. 2001;43:555–578. [Google Scholar]
- Dix G.R, Thomson M.L, Longstaffe F.J, McNutt R.H. Systematic decrease of high delta13C values with burial in late Archaean (2.8 Ga) diagenetic dolomite: evidence for methanogenesis from the Crixas Greenstone Belt, Brazil. Precambrian Res. 1995;70:253–268. 10.1016/0301-9268(94)00044-R [Google Scholar]
- Eigenbrode, J. 2004 Late archean microbial ecology: an integration of molecular, isotopic, and lithologic studies. Ph.D. thesis, Earth Sciences, the Pennsylvania State University.
- Farquhar J, Wing B.A. Multiple sulfur isotopes and the evolution of the atmosphere. Earth Planet. Sci. Lett. 2003;213:1–13. 10.1016/S0012-821X(03)00296-6 [Google Scholar]
- Garrels R.M, Lerman A. Phanerozoic cycles of sedimentary carbon and sulfur. Geology. 1981;78:4652–4655. doi: 10.1073/pnas.78.8.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels R.M, Perry E.A., Jr Cycling of carbon, sulfur, and oxygen through geologic time. Sea. 1974;5:303–336. [Google Scholar]
- Giggenbach W.F. Chemical composition of volcanic gases. In: Scarpa S, Tilling R.I, editors. Monitoring and mitigation of volcano hazards. Springer; Berlin: 1996. pp. 221–256. [Google Scholar]
- Goldschmidt V.M. Clarendon Press; Oxford: 1954. Geochemistry. [Google Scholar]
- Halmer M.M, Schmincke H.-U, Graf H.-F. The annual volcanic gas input into the atmosphere, in particular into the stratosphere: a global data set for the past 100 years. J. Volcanol. Geotherm. Res. 2002;115:511–528. 10.1016/S0377-0273(01)00318-3 [Google Scholar]
- Halverson G.P, Hoffman P.F, Schrag D.P, Maloof A.C, Rice A.H.H. Toward a Neoproterozoic composite carbon-isotope record. Geol. Soc. Am. 2005;117:1181–1207. 10.1130/B25630.1 [Google Scholar]
- Hayes J.M. Geochemical evidence bearing on the origin of aerobiosis, a speculative interpretation. In: Schopf J.W, editor. The Earth's earliest biosphere: its origin and evolution. Princeton University Press; Princeton, NJ: 1983. pp. 291–301. [Google Scholar]
- Hayes J.M. Global methanotrophy at the Archean–Proterozoic transition. In: Bengtson S, editor. Early life on Earth. Nobel Symposium. Columbia University Press; New York, NY: 1994. pp. 220–236. [Google Scholar]
- Hayes J.M, Kaplan I.R, Wedeking K.W. Precambrian organic geochemistry, preservation of the record. In: Schopf J.W, editor. The Earth's earliest biosphere: its origin and evolution. Princeton University Press; Princeton, NJ: 1983. pp. 93–134. [Google Scholar]
- Hayes J.M, Lambert I.B, Strauss H. The sulfur-isotopic record. In: Schopf J.W, Klein C, editors. The Proterozoic biosphere, a multidisciplinary study. Cambridge University Press; Cambridge, UK: 1992. pp. 129–132. [Google Scholar]
- Hayes J.M, Strauss H, Kaufman A.J. The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem. Geol. 1999;161:103–125. 10.1016/S0009-2541(99)00083-2 [Google Scholar]
- Hilton D.R, Fischer T, Marty B. Noble gases and volatile recycling at subduction zones. MSA Special Volume: Noble Gasses. 2002;47:319–370. [Google Scholar]
- Hofmann A.W. Sampling mantle heterogeneity through oceanic basalts: isotopes and trace elements. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry. vol. 2. Elsevier; Oxford: 2004. pp. 61–101. [Google Scholar]
- Holland H.D. Wiley; New York, NY: 1978. The chemistry of the atmosphere and oceans. [Google Scholar]
- Holland H.D. Princeton University Press; Princeton, NJ: 1984. The chemical evolution of the atmosphere and oceans. [Google Scholar]
- Holland H.D. Volcanic gases, black smokers, and the great oxidation event. Geochim. Cosmochim. Acta. 2002;66:3811–3826. 10.1016/S0016-7037(02)00950-X [Google Scholar]
- Holser W.T, Schidlowski M, MacKenzie F.T, Maynard J.B. Geochemical cycles of carbon and sulfur. In: Gregor C.B, Garrels R.M, Mackenzie F.T, Maynard J.B, editors. Chemical cycles in the evolution of the Earth. Wiley; New York, NY: 1988. pp. 105–173. [Google Scholar]
- Hunt J.M. Freeman; New York, NY: 1996. Petroleum geochemistry and geology. [Google Scholar]
- Ingebritsen S.E, Manning C.E. Diffuse fluid flux through orogenic belts: implications for the world ocean. Proc. Natl Acad. Sci. USA. 2002;99:9113–9116. doi: 10.1073/pnas.132275699. 10.1073/pnas.132275699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin H, Curtis C.D, Coleman M.L. Isotopic evidence for source of diagenetic carbonates formed during burial of organic rich sediments. Nature. 1977;269:209–213. 10.1038/269209a0 [Google Scholar]
- Jiao Y, Kappler A, Croal L.R, Newman D.K. Isolation and characterization of a genetically tractable photoautotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl. Environ. Microbiol. 2005;71:4487–4496. doi: 10.1128/AEM.71.8.4487-4496.2005. 10.1128/AEM.71.8.4487-4496.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadko D. An assessment of the effect of chemical scavenging within hydrothermal plumes upon ocean geochemistry. Earth Planet. Sci. Lett. 1994;120:361–374. 10.1016/0012-821X(93)90250-D [Google Scholar]
- Karhu J.A, Holland H.D. Carbon isotopes and the rise of atmospheric oxygen. Geology. 1996;24:867–870. 10.1130/0091-7613(1996)024%3C0867:CIATRO%3E2.3.CO;2 [Google Scholar]
- Kasting J.F, Catling D. Evolution of a habitable planet. Annu. Rev. Astron. Astrophys. 2003;41:429–463. 10.1146/annurev.astro.41.071601.170049 [Google Scholar]
- Keeling R.F, Najjar R.P, Bender M.L, Tans P.P. What atmospheric oxygen measurements can tell us about the global carbon cycle. Global Biogeochemical Cycles. 1993;7:36–67. [Google Scholar]
- Kerrick D.M, Connolly J.A.D. Metamorphic devolatilization of subducted oceanic metabasalts: implications for seismicity, arc magmatism and volatile recycling. Earth Planet. Sci. Lett. 2001;189:19–29. 10.1016/S0012-821X(01)00347-8 [Google Scholar]
- Klein J.S, Mozley P, Campbell A, Cole R. Spatial distribution of carbon and oxygen isotopes in laterally extensive carbonate-cemented layers: implications for mode of growth and subsurface identification. J. Sediment. Res. 1999;69:184–191. [Google Scholar]
- Knoll A.H, Hayes J.M, Kaufman A.J, Swett K, Lambert I.B. Secular variation in carbon isotope ratios from Upper Proterozoic successions of Svalbard and East Greenland. Nature. 1986;321:832–838. doi: 10.1038/321832a0. 10.1038/321832a0 [DOI] [PubMed] [Google Scholar]
- Kopp R.E, Kirschvink J.L, Hilburn I.A, Nash C.Z. The Paleoproterozoic snowball Earth: a climate disaster triggered by the evolution of oxygenic photosynthesis. Proc. Natl Acad. Sci. USA. 2005;102:11 131–11 136. doi: 10.1073/pnas.0504878102. 10.1073/pnas.0504878102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress V.C, Carmichael I.S.E. The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contrib. Mineral. Petrol. 1991;108:82–92. 10.1007/BF00307328 [Google Scholar]
- Kump L.R, Arthur M.A. Interpreting carbon-isotope excursions: carbonates and organic matter. Chem. Geol. 1999;161:181–198. 10.1016/S0009-2541(99)00086-8 [Google Scholar]
- Kyser T.K. Stable isotope variations in the mantle. Stable isotopes in high temperature geological processes. Rev. Mineral. 1986;16:141–164. [Google Scholar]
- Lécuyer C, Ricard Y. Long-term fluxes and budget of ferric iron: implication for the redox states of the Earth's mantle and atmosphere. Earth Planet. Sci. Lett. 1999;165:197–211. 10.1016/S0012-821X(98)00267-2 [Google Scholar]
- Li Z.-X.A, Lee C.-T.A. The constancy of upper mantle fO2 through time inferred from V/Sc ratios in basalts. Earth Planet. Sci. Lett. 2004;228:483–493. 10.1016/j.epsl.2004.10.006 [Google Scholar]
- Lowe D.R. Major events in the geological development of the Precambrian Earth. In: Schopf J.W, Klein C, editors. The Proterozoic biosphere. Cambridge University Press; Cambridge, UK: 1992. pp. 67–76. [Google Scholar]
- Lowell R.P, Keller S.M. High-temperature seafloor hydrothermal circulation over geologic time and Archean banded iron formations. Geophys. Res. Lett. 2003;30:1–44. 10.1029/2002GL016536 [Google Scholar]
- Luth R.W. Mantle volatiles—distribution and consequences. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry, vol. 2. Elsevier; Oxford: 2004. pp. 319–361. [Google Scholar]
- Marty B, Tolstikhin I.N. CO2 fluxes from mid-ocean ridges, arcs and plumes. Chem. Geol. 1998;145:233–248. 10.1016/S0009-2541(97)00145-9 [Google Scholar]
- Mattey D.P. Carbon isotopes in the mantle. Terra Cognita. 1987;7:31–37. [Google Scholar]
- Mazzullo S.J. Organogenic dolomitization in peritidal to deep-sea sediments. J. Sediment. Res. 2000;70:10–23. [Google Scholar]
- Melezhik V.A, Fallick A.E, Medvedev P.V, Makarikhin V.V. Extreme 13Ccarb enrichment in ca. 2.0 Ga magnesite–stromatolite–dolomite–‘red beds’ association in a global context: a case for the world-wide signal enhanced by a local environment. Earth Sci. Rev. 1999;48:71–120. 10.1016/S0012-8252(99)00044-6 [Google Scholar]
- Milkov A.V. Molecular and stable isotope compositions of natural gas hydrates: a revised global dataset and basic interpretations in the context of geological settings. Org. Geochem. 2005;36:681–702. 10.1016/j.orggeochem.2005.01.010 [Google Scholar]
- Milkov A.V, Etiope G. Global methane emission through mud volcanoes and its past and present impact on the Earth's climate—a comment. Int. J. Earth Sci. (Geol. Rundsch.) 2005;94:490–492. 10.1007/s00531-005-0480-5 [Google Scholar]
- Nakamura K, Kato Y. Carbonatization of oceanic crust by the seafloor hydrothermal activity and its significance as a CO2 sink in the Early Archean. Geochim. Cosmochim. Acta. 2004;68:4595–4618. 10.1016/j.gca.2004.05.023 [Google Scholar]
- Palme H, O'Neill H.S.C. Cosmochemical estimates of mantle composition. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry, vol. 2. Elsevier; Oxford: 2003. pp. 1–38. [Google Scholar]
- Pavlov A.A, Kasting J.F. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2:27–41. doi: 10.1089/153110702753621321. 10.1089/153110702753621321 [DOI] [PubMed] [Google Scholar]
- Pearson D.G, Canil D, Shirey S.B. Mantle samples included in volcanic rocks: xenoliths and diamonds. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry, vol. 2. Elsevier; Oxford: 2004. pp. 172–221. [Google Scholar]
- Petsch S.T, Eglinton T.I, Edwards K.J. 14C-Dead living biomass: evidence for microbial assimilation of ancient organic carbon during shale weathering. Science. 2001;292:1127–1131. doi: 10.1126/science.1058332. 10.1126/science.1058332 [DOI] [PubMed] [Google Scholar]
- Pineau, F., Cartigny, P. & Javoy, M. 2004 Carbon flux and C/Nb ratios in the mantle in ridge context, Eos Trans. AGU, vol. 85, Fall Meet. Suppl., Abstract V31C-1453P.
- Plank T, Langmuir C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998;145:325–394. 10.1016/S0009-2541(97)00150-2 [Google Scholar]
- Quay P, Sonnerup P, Westby T, Stutsman J, McNichol A. Changes in the 13C/12C of dissolved inorganic carbon in the ocean as a tracer of anthropogenic CO2 uptake. Global Biogeochem. Cycles. 2003;17:1–4. 10.1029/2001GB001817 [Google Scholar]
- Resing J.A, Lupton J.E, Feely R.A, Lilley M.D. CO2 and 3He in hydrothermal plumes: implications for mid-ocean ridge CO2 flux. Earth Planet. Sci. Lett. 2004;226:449–464. 10.1016/j.epsl.2004.07.028 [Google Scholar]
- Ronov A.B, Yaroshevsky A.A. A new model for the chemical structure of Earth's crust. Geochem. Int. 1976;13:89–121. [Google Scholar]
- Rothman D.H, Hayes J.M, Summons R.E. Dynamics of the Neoproterozoic carbon cycle. Proc. Natl Acad. Sci. USA. 2003;100:8124–8129. doi: 10.1073/pnas.0832439100. 10.1073/pnas.0832439100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D.B. Rate of plate creation and destruction: 180 Ma to present. Geol. Soc. Am. Bull. 2002;114:927–933. 10.1130/0016-7606(2002)114%3C0927:ROPCAD%3E2.0.CO;2 [Google Scholar]
- Saal A.E, Hauri E.H, Langmuir C.H, Perfit M.R. Vapour undersaturation in primitive mid-ocean-ridge basalt and the volatile content of Earth's upper mantle. Nature. 2002;419:451–446. doi: 10.1038/nature01073. 10.1038/nature01073 [DOI] [PubMed] [Google Scholar]
- Sackett W.M, Poag C.W, Eadie B.J. Kerogen recycling in the Ross Sea, Antarctica. Science. 1974;185:1045–1047. doi: 10.1126/science.185.4156.1045. [DOI] [PubMed] [Google Scholar]
- Sano Y, Marty B. Origin of carbon in fumarolic gas from island arcs. Chem. Geol. 1995;119:265–274. 10.1016/0009-2541(94)00097-R [Google Scholar]
- Sano Y, Williams S.N. Fluxes of mantle and subducted carbon along convergent plate boundaries. Geophys. Res. Lett. 1996;23:2749–2752. 10.1029/96GL02260 [Google Scholar]
- Schidlowski M, Eichmann R, Junge C.E. Precambrian sedimentary carbonates: carbon and oxygen isotope geochemistry and implications for the terrestrial oxygen budget. Precambrian Res. 1975;2:1–69. 10.1016/0301-9268(75)90018-2 [Google Scholar]
- Shackleton N.J. The carbon isotope record of the Cenozoic: history of organic carbon burial and of oxygen in the ocean and atmosphere. In: Brooks J, Fleet A.J, editors. Marine petroleum source rocks. Geological Society Special Publication No. 26. Blackwell; Oxford: 1987. pp. 423–434. [Google Scholar]
- Shaw A.M, Hilton D.R, Fischer T.P, Walker J.A, Alvarado G.E. Contrasting He–C relationships in Nicaragua and Costa Rica: insights into C cycling through subduction zones. Earth Planet. Sci. Lett. 2003;214:499–513. 10.1016/S0012-821X(03)00401-1 [Google Scholar]
- Shaw A.M, Hilton D.R, Macpherson C.G, Sinton J.M. The CO2–He–Ar–H2O systematics of the Manus back-arc basin: resolving source composition from degassing and contamination effects. Geochim. Cosmochim. Acta. 2004;68:1837–1856. 10.1016/j.gca.2003.10.015 [Google Scholar]
- Shields G, Veizer J. Precambrian marine carbonate isotope database: version 1.1. Geochem. Geophys. Geosyst. G3. 2002;3:1–12. [Google Scholar]
- Sleep N.H, Zahnle K. Carbon dioxide cycling and implications for climate. J. Geophys. Res. 2001;106:1373–1399. 10.1029/2000JE001247 [Google Scholar]
- Sleep N.H, Meibom A, Fridriksson T, Coleman R.G, Bird D.K. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl Acad. Sci USA. 2004;101:12 818–12 823. doi: 10.1073/pnas.0405289101. 10.1073/pnas.0405289101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudigel H, Hart S.R, Schmincke H.-U, Smith B.M. Cretaceous ocean crust at DSDP Sites 417 and 418: carbon uptake from weathering versus loss by magmatic outgassing. Geochim. Cosmochim. Acta. 1989;53:3091–3094. 10.1016/0016-7037(89)90189-0 [Google Scholar]
- Stern R.J. Evidence from ophiolites, blueschists, and ultrahigh-pressure metamorphic terranes that the modern episode of subduction tectonics began in Neoproterozoic time. Geol. Soc. Am. 2005;33:557–560. 10.1130/G21365.1 [Google Scholar]
- Strauss H, Moore T.B. Abundances and isotopic compositions of carbon and sulfur species in whole rock and kerogen samples. In: Schopf J.W, Klein C, editors. The Proterozoic biosphere. Cambridge University Press; Cambridge, UK: 1992. pp. 709–798. [Google Scholar]
- Su, Y., & Langmuir, C.H. 2003 Global MORB chemistry compilation at the segment scale. MS thesis, Department of Earth and Environmental Sciences, Columbia University, Database available at http://www.petdb.org
- Summons R.E, Jahnke L.L, Hope J.M, Logan G.A. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature. 1999;400:554–557. doi: 10.1038/23005. 10.1038/23005 [DOI] [PubMed] [Google Scholar]
- Tajika E. Faint young sun and the carbon cycle: implications for the Proterozoic global glaciations. Earth Planet. Sci. Lett. 2003;214:443–453. 10.1016/S0012-821X(03)00396-0 [Google Scholar]
- Tappert R, Stachel T, Harris J.W, Muehlenbachs K, Lugwig T, Grey G.P. Subducting oceanic crust: the source of deep diamonds. Geol. Soc. Am. Bull. 2005;33:565–568. [Google Scholar]
- Tian F, Toon O.B, Pavlov A.A, De Sterck H. A hydrogen-rich early earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. 10.1126/science.1106983 [DOI] [PubMed] [Google Scholar]
- Tice M.M, Lowe D.R. Photosynthetic microbial mats in the 3416-Myr-old ocean. Nature. 2004;431:549–552. doi: 10.1038/nature02888. 10.1038/nature02888 [DOI] [PubMed] [Google Scholar]
- Van Kranendonk M. Archean tectonics 2004: a review. Precambrian Res. 2004;131:143–151. 10.1016/j.precamres.2003.12.008 [Google Scholar]
- Veizer J, Mackenzie F.T. Evolution of sedimentary rocks. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry. vol. 7. Elsevier; Oxford: 2004. pp. 369–407. [Google Scholar]
- Veizer J, et al. 87Sr/86Sr, and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999;161:59–88. 10.1016/S0009-2541(99)00081-9 [Google Scholar]
- Walker J.C.G. Was the Archean biosphere upside down? Nature. 1987;329:710–712. doi: 10.1038/329710a0. 10.1038/329710a0 [DOI] [PubMed] [Google Scholar]
- Walker J.C.G. Precambrian evolution of the climate system. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990;82:261–289. [Google Scholar]
- Wallace P.J. Volatiles in subduction zone magmas: concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geotherm. Res. 2005;140:217–240. 10.1016/j.jvolgeores.2004.07.023 [Google Scholar]
- Wedepohl K.H. The composition of the continental crust. Geochim. Cosmochim. Acta. 1995;59:1217–1232. 10.1016/0016-7037(95)00038-2 [Google Scholar]
- White R.S, McKenzie D.P, O'Nions R.K. Oceanic crustal thickness from seismic measurements and rare earth element inversions. J. Geophys. Res. 1992;97:683–714. [Google Scholar]
- Wilkinson B.H, Walker J.C.G. Phanerozoic cycling of sedimentary carbonate. Am. J. Sci. 1989;289:525–548. [Google Scholar]
- Young G.M, Long D.G.F, Fedo C.M, Nesbitt H.W. Paleoproterozoic Huronian basin: product of a Wilson cycle punctuated by glaciations and a meteorite impact. Sediment. Geol. 2001;141-142:233–254. 10.1016/S0037-0738(01)00076-8 [Google Scholar]
- Yudovich Y.E, Makarikhin V.V, Medvedev P.V, Sukhanov N.V. Carbon isotope anomalies in carbonates of the Karelian Complex. Geochem. Int. 1991;28:56–62. [Google Scholar]
- Zhang Q.-L, Li W.-J. A calibrated measurement of the atomic weight of carbon. Chinese Sci. Bull. 1990;35:290–296. [Google Scholar]