Abstract
Among the many biomolecules involved in the bone mineralization processes, anionic phospholipids play an important role because of their ability to bind calcium. In particular, phosphatidylserine is a natural component of the plasmalemma and of the matrix vesicles generated from the osteoblast membrane to create nucleation centres for calcium phosphate crystal precipitation. In the present work, we demonstrate that calcium-binding phospholipids can be used as biomimetic coating materials for improving the osteointegration of metal implants. Relatively thick phosphatidylserine-based coatings were deposited on titanium coupons by dip-coating. Upon dehydration in a simulated body fluid phospholipids were quickly crosslinked by calcium and re-arranged into a three-dimensional matrix able to induce rapid formation of a calcium phosphate mineral phase. The rate of mineralization was shown to be dependent on the adopted coating formulation. In the attempt to closely mimic the cell membrane composition, heterogeneous formulations based on the mixing of anionic phospholipids (either phosphatidylserine or phosphatidylinositol) with phosphatidylcholine and cholesterol were synthesized. However, surface plasmon resonance studies as well as scanning electron microscopy and elemental analysis demonstrated that the homogeneous phosphatidylserine coating was a more effective calcification environment than the heterogeneous formulations.
Keywords: biomaterials, phospholipids, coatings, mineralization, osteointegration
1. Introduction
The nucleation of new mineral phase in embryonic bone and rapidly mineralizing skeletal tissues takes place through the presence of matrix vesicles rich in calcium-binding phospholipids (Kirsch & Pfaffle 1992). These vesicles are fragments of the cell membrane and possess calcium channels (Annexin V) able to facilitate the influx of calcium ions from the surrounding environment into the phosphate-rich interior. The calcium-binding properties of these phospholipids, as well as the saturating concentrations of calcium and phosphate in the vesicle interior, lead to the formation of an amorphous mineral phase where crystals aggregate, disrupt the vesicle, and merge with other nucleation centres (Raggio et al. 1986; Eanes 1992). Through the use of synthetic ionophores, many in vitro studies have demonstrated that liposomal system can be made permeable to calcium and lead to the formation of a mineral phase (Eanes & Hailer 1985; Eanes et al. 1992; Heywood & Eanes 1992).
Growing bone always fails to establish satisfactory bonding with the surface of titanium alloy implants commonly employed in orthopaedic and dental applications (Tanahashi & Matsuda 1997; Steflik et al. 1998). A fibrotic capsule, the consequence of an inflammatory response triggered by both the implantation procedure and the alloy surface, interposes between the bone and the implant thus leading to its mobilization. An improvement of the integration of titanium alloy implants with the bone has been obtained by coating the surface of the stem with sintered titanium beads which generate a porous surface which, upon implantation, become interpenetrated by the growing bone (Viceconti et al. 1995). However, the binding of the sintered beads is prone to detachment under the biomechanical stresses and, ultimately, to the lack of chemical bonding between the metal and the bone mineral phase (Viceconti et al. 1995; Tanahashi & Matsuda 1997; Steflik et al. 1998). Hydroxyapatite is the material most commonly used to functionalize the surface of metal implants to encourage osteointegration (Lewis 2000). The chemical nature of this material is very similar to that of the bone mineral phase and serves to provide a chemical interface between the material and the bone (Lewis 2000). However, the different degree of crystallinity between the hydroxyapatite and the bone mineral phase leads to a mismatch of the mechanical properties and, consequently, delamination of the hydroxyapatite coating from the surface of the implant may occur (Habibovic et al. 2002). Other approaches have been suggested where the alloy surface is modified with bioactive molecules able either to favour calcium phosphate crystal nucleation or to promote adhesion, spreading, and proliferation of osteoblastic cells (Nanci et al. 1998). These biomimetic approaches are intended to achieve the integration of the implant by increasing the rate of deposition of new bone on the biomaterial surface, thus preventing the formation of an interposed fibrous tissue. The performances of the biomimetic coatings have not been fully established by clinical trials and their use may be limited by several factors such as chemical bond stability of the grafted molecules and relatively high manufacturing costs at industrial level.
To increase the rate of formation of new mineral phase at an alloy surface, we envisaged the possibility of mimicking the mechanisms of bone regeneration mediated by the matrix vesicles. Different formulations of phospholipids coatings were established where the selected calcium-binding molecules were phosphatidylserine (PS) and phosphatidylinositol (PI). In some formulations, phosphatidylcholine (PC) and cholesterol (C) were also included to favour the re-arrangement of the coating to form liposomes (Eanes & Hailer 1985). In the present study, we explored the potential for a titanium alloy coated with a calcium-binding phospholipid formulation to create a micro-environment where calcium and phosphate ion concentrations could reach levels similar to those of the matrix vesicle interior.
2. Materials and methods
2.1 Coating preparation
Partial or complete coatings of different thickness were obtained by applying different volumes of natural (Sigma, UK) or synthetic (Avanti, USA) phospholipid chloroform solutions of varying concentrations (55, 111 and 222 mM) onto the surfaces of titanium alloy (Goodfellow, UK) discs (nominal diameter=13 mm). The solvent was evaporated at room temperature and the discs were stored at room temperature until use.
For visual inspection and scanning electron microscopy (SEM) analysis, a 222 mM phospholipid/chloroform solution (from 5 to 20 μl) was applied to these titanium discs to obtain either a partial (figure 2a) or a complete surface coating. For atomic force microscopy (AFM) studies, phospholipids layers were formed upon epitaxial gold coated mica (1 cm in diameter) by drop-casting 50 μl of a 111 mM phospholipid solution. Three phospholipid formulations were used for this study: PC : PS : C, PC : PI : C and pure PS. The mixed formulations had a phospholipid molar ratio of PC : X : C of 7 : 2 : 1, where X is either PS or PI. The solvent was evaporated and the coated specimens were incubated in a simulated body fluid (SBF) of different compositions.
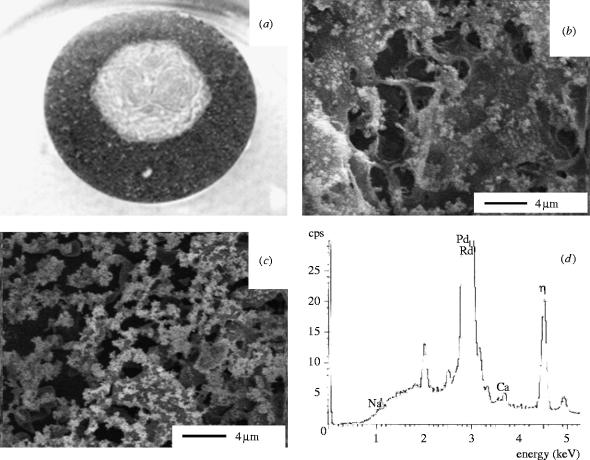
Figure 2.
PC : PS : C coating after 7 days incubation in Vogel's SBF. (a) Photograph of a partially coated titanium disk, (b) Cryo-SEM of the phospholipid exposed surface (×5000), (c) Cryo-SEM of the phospholipid exposed surface of a serum-treated sample (×5000) and (d) EDX of the mineral phase.
2.2 Mineralization studies
Uncoated and phospholipid-coated disks were incubated statically in SBF at 37 °C. SBFs with different compositions were used to study the effects of ions (Bigi et al. 1992) and serum components (Radin & Ducheyne 1996) on the mineralization process.
Two SBFs (Vogel's and Radin's) were chosen: Vogel's SBF (Vogel 1986) had a composition in which the sulphate-based buffer was the only potential hydroxyapatite inhibitor (71 mM NaCl, 5 mM KCl, 1.64 mM Na2HPO4, 2.36 mM CaCl2 in 0.05 M TES buffer, pH 7.2). Radin's SBF was included in the experimental work as buffer where the salt composition was more similar to that of body fluid (2.6 mM CaCl2, 1 mM K2HPO4, 152 mM NaCl, 1.5 mM MgCl2, 27 mM NaHCO3, 0.5 mM Na2SO4 in 0.05 M Tris, pH 7.4; Radin & Ducheyne 1993). The formation of mineral phase in these buffers was also tested after pre-treatment of the surfaces with human serum (type AB, Sigma, UK), 1 h, 37 °C. Un-coated titanium disks were used as controls. After incubation in SBF at 37 °C for various time periods, the disks were thoroughly washed with de-ionized water. For cryogenic SEM (Cryo-SEM) of the specimens were frozen in liquid nitrogen, exposed to sublimation for 15 min, sputter-coated with palladium, and analysed at 5 keV. The presence of calcium on the specimens was confirmed by elemental dispersive X-ray analysis (EDX) which was carried out at 15 keV.
Tapping mode AFM in air was carried out using a Multimode Nanoscope IIIa (Digital Instruments, Santa Barbara, CA, USA) equipped with a silicon tip (125 μm in length with ca 300 kHz resonant frequency). Topographical images were analysed for roughness and displayed as three-dimensional plots to highlight changes in the z-direction due to crystal formation. All images were obtained with a 20×20 μm scanner in tapping mode using a Si3N4 cantilever which had a resonant frequency of 278 KHz, using a scan rate of 0.1 Hz. All measurements were carried out at room temperature.
3. Results and discussion
Cryo-SEM of the discs after 1 h incubation in SBF demonstrated that thick (100 μm) PC : PS : C coatings re-arranged into a three-dimensional porous gel (figure 1a). Previous studies have demonstrated the calcium-mediated fusion of liposomes into hexagonal gels (Hui et al. 1981). The pre-treatment of the surface with human serum significantly delayed gel formation and only a partial re-arrangement of the original compact coating was visible (figure 1b). The presence of calcium was essential to the formation of a three-dimensional gel. Indeed, when hydrated in calcium-depleted SBF, the coating appeared as separated rows of liposomes (figure 1c) and no significant mineralization of the samples was detected. Over this short incubation time, the EDX showed a significant level of deposited calcium when samples were incubated in Vogel's SBF, but no detectable calcium was found when samples were pre-treated with serum (data not shown).
Figure 1.
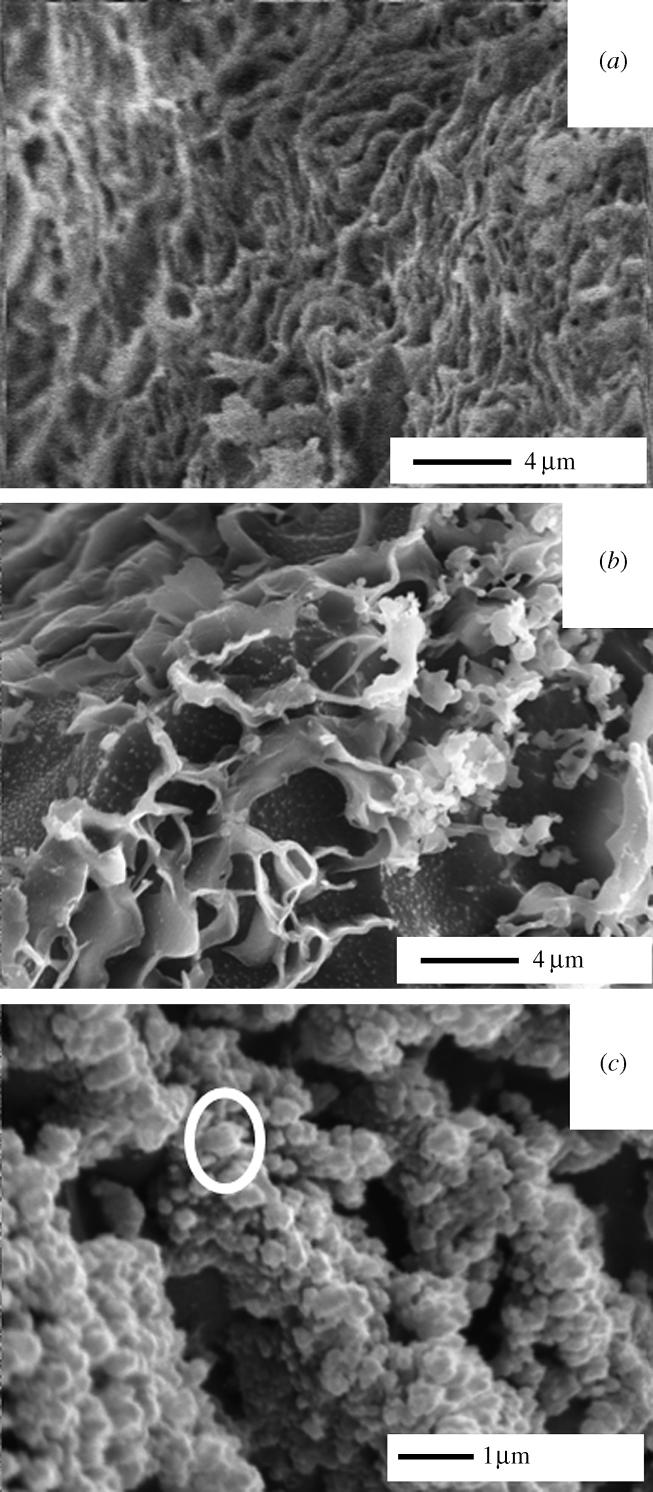
Cryo-SEM analysis of PC : PS : C coating after 1 h incubation in Vogel's SBF. (a) Complete SBF (×5000), (b) complete SBF after 1-h treatment of the sample in human serum (×5000) and (c) calcium-depleted SBF (×15 000), circle highlights a single liposome.
After 7 days incubation in Vogel's SBF, PC : PS : C coated samples, both treated and untreated with serum, showed complete mineralization of the phospholipid gel (figure 2a–c). EDX showed that the mineral phase was rich in calcium and phosphate (figure 2d). Similar results were also obtained with the PC : PI : C formulation (data not shown). Un-coated titanium surfaces did not show any mineralization (figure 2a, outer rim) and PC : C coatings failed to re-arrange into a three-dimensional gel and mineral phase (data not shown). These results suggest that the phospholipids gel formation and mineralization were driven by the calcium-binding potential of phospholipids such as PS and PI.
To enhance the mineralization process on titanium alloy surfaces, pure PS coatings were also prepared. Under dynamic conditions, needle-shaped crystals typical of the hydroxyapatite appeared on the surface after 15 and 30 min (figure 3a,b, arrows). The degree of mineralization of the pure PS coating after 7 days in Vogel's was very high, the gel being completely embedded in calcium/phosphate mineral phase with a relatively high crystallinity (figure 4a,b). Very high amounts of calcium and phosphate were detected by EDX (figure 4c). A compact mineral phase was obtained even when pure PS was incubated in Radin's SBF, a metastable solution with an ionic composition more similar to that of bone interstitial fluids (data not shown). This suggests that the mineralization process would not be significantly affected by ions which are known to inhibit hydroxyapatite formation such as those of magnesium (Bigi et al. 1992). The effect of serum components (e.g. proteins) adsorbed onto the specimen surface did not inhibit the formation of a compact mineral phase (data not shown). Therefore we speculate that the fast rate of mineral phase deposition induced by pure PS is likely to take place in vivo. Indeed, a recent in vivo study, where PS-coated titanium implants were implanted in the meta-epiphyses of rabbit femours, showed a degree of bone apposition to the coating comparable to HA-coated implants (Merolli et al. submitted). However, given the deposition method adopted and the soft nature of the phospholipids matrix, the coating does not have a binding stable to mechanical stresses and has to be regarded as a medium capable of accelerating the calcium/phosphate deposition around the implant thus reducing the risks of formation of an interposed layer of soft tissue. The in vivo study has also shown that the phospholipids coating was not completely resorbed over 6 months indicating that thinner coatings need to be applied to ensure the mechanical stability of the implant.
Figure 3.
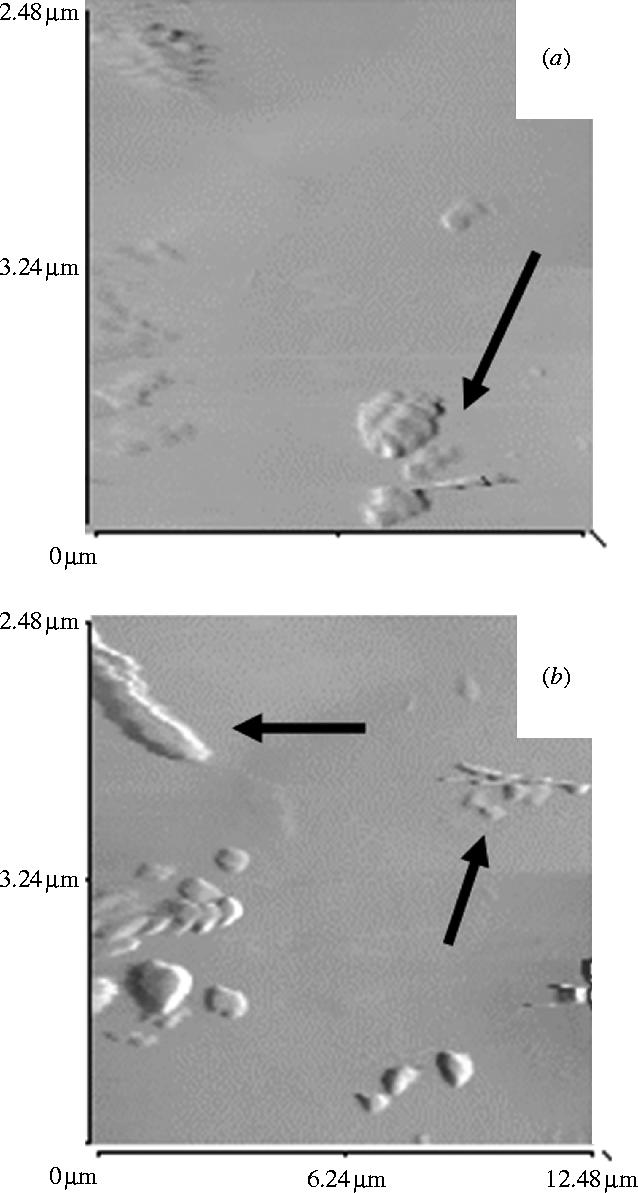
Atomic Force micrographs of pure PS coated disks in Vogel's SBF for (a) 15 min and (b) 30 min.
Figure 4.

Cryo-SEM/EDX analysis of pure PS coated disks after 7-days incubation in Vogel's SBF. (a) Phospholipid matrix exposed surface, (b) phospholipid matrix fracture surface and (c) EDX of the mineral phase.
4. Conclusions
The present work demonstrates the high mineralization potential of phospholipids, such as phosphatidylserine and phosphatidylinositol, in metastable solutions with an ionic composition similar to that of normal body fluid. The calcium-binding phospholipid functional groups appear to act as both crystal nucleation centres and phospholipid cross-linking domains. Consequently, the rapid calcium-driven formation of the calcified matrix would provide a micro-environment where calcium and phosphate ions reach supersaturating concentrations, thereby catalysing the precipitation process.
Biomimetic calcium-binding phospholipid coatings may represent materials of choice for a new generation of rapidly osteointegrating materials (Lloyd et al. 2000).
Acknowledgments
The work was supported by an European Community funded BRITE-EURAM III Programme, Industrial and Materials Technologies, Contract no. BRPR-CT97-0494.
References
- Bigi A, Foresti E, Gregorini R, Ripamonti A, Roveri N, Shah J.S. Calcif. Tissue Int. 1992;50:439–444. doi: 10.1007/BF00296775. doi:10.1007/BF00296775 [DOI] [PubMed] [Google Scholar]
- Eanes E. Bone Miner. 1992;17:269–272. doi: 10.1016/0169-6009(92)90749-4. doi:10.1016/0169-6009(92)90749-4 [DOI] [PubMed] [Google Scholar]
- Eanes E.D, Hailer A.W. Calcif. Tissue Int. 1985;37:390–394. doi: 10.1007/BF02553708. [DOI] [PubMed] [Google Scholar]
- Eanes E.D, Hailer A.W, Midura R.J, Hascall V.C. Glycobiology. 1992;2:571–578. doi: 10.1093/glycob/2.6.571. [DOI] [PubMed] [Google Scholar]
- Habibovic P, Barrere F, van Blitterswijk C.A, De Groot K, Layrolle P. J. Am. Ceram. Soc. 2002;85:517–522. [Google Scholar]
- Heywood B.R, Eanes E.D. Calcif. Tissue Int. 1992;50:149–156. doi: 10.1007/BF00298793. doi:10.1007/BF00298793 [DOI] [PubMed] [Google Scholar]
- Hui S.W, Stewart T.P, Boni L.T, Yeagle P.L. Science. 1981;212:921–922. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Pfaffle M. FEBS Lett. 1992;310:143–147. doi: 10.1016/0014-5793(92)81316-e. doi:10.1016/0014-5793(92)81316-E [DOI] [PubMed] [Google Scholar]
- Lewis G. Biomed. Mater. Eng. 2000;10:157–188. [PubMed] [Google Scholar]
- Lloyd, A. W., Santin, M., Love, W. G., Denyer, S. P. & Rhys-Williams, W. 2000 Patent PCT/GB00/03290.
- Merolli, A., Giannotta, L., Tranquilli Leali, P., Lloyd, A. W., Denyer, S. P., Rhys-Williams, W., Love, W. G., Gabbi, C., Cacchioli, A., Cannas, M. & Santin, M. Submitted. In vivo assessment of the osteointegrative potential of phospatidylserine-based coatings. [DOI] [PubMed]
- Nanci, A, McKee, M. D., Sacher, E. & Savadogo, O. 1998 US Patent 5,824,651.
- Radin S.R, Ducheyne P. J. Biomed. Mater. Res. 1993;27:35–45. doi: 10.1002/jbm.820270106. doi:10.1002/jbm.820270106 [DOI] [PubMed] [Google Scholar]
- Radin S, Ducheyne P. J. Biomed. Mater. Res. 1996;30:273–279. doi: 10.1002/(SICI)1097-4636(199603)30:3<273::AID-JBM1>3.0.CO;2-N. doi:10.1002/(SICI)1097-4636(199603)30:3<273::AID-JBM1>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Raggio C.L, Boyan B.D, Boskey A.L. J. Bone Miner. Res. 1986;1:409–415. doi: 10.1002/jbmr.5650010505. [DOI] [PubMed] [Google Scholar]
- Steflik D.E, Corpe R.S, Lake F.T, Young T.R, Sisk A.L, Parr G.R, Hanes P.J, Berkery D.J. J. Biomed. Mater. Res. 1998;39:611–620. doi: 10.1002/(sici)1097-4636(19980315)39:4<611::aid-jbm16>3.0.co;2-9. doi:10.1002/(SICI)1097-4636(19980315)39:4<611::AID-JBM16>3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- Tanahashi M, Matsuda T. J. Biomed. Mater. Res. 1997;34:305–315. doi: 10.1002/(sici)1097-4636(19970305)34:3<305::aid-jbm5>3.0.co;2-o. doi:10.1002/(SICI)1097-4636(19970305)34:3<305::AID-JBM5>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Viceconti M, Toni A, Giunti A. J. Biomed. Mater. Res. 1995;29:875–881. doi: 10.1002/jbm.820290713. doi:10.1002/jbm.820290713 [DOI] [PubMed] [Google Scholar]
- Vogel J.J. J. Colloid Interf. 1986;111:152–159. doi:10.1016/0021-9797(86)90015-9 [Google Scholar]