Abstract
Growing cells on surfaces bearing nanotopography signals makes many changes in cell gene expression and downstream changes in phenotype but the mechanisms for this have, so far, been obscure. We consider the question of whether the topography directly nanoimprints onto the cell as a component of the signal transduction system. Evidence we present from SEM, TEM and fluorescence detection of the arrangements of cytoskeletal components is consistent with the possibility that cells are nanoimprinted by the substrate. The nanoprinting does not interfere with integrin-mediated adhesion processes and may perhaps work through them. Time-lapse video studies of cells moving from areas bearing nanotopography to flat areas and vice versa suggests that the nanoimprinting takes 1–6 h to appear on the cell and a similar time to disappear when the cell moves from a flat surface to a nanotopographic one and back. This nanoprinting of cells would appear to be a novel type of cell signalling.
Keywords: nanoprinting, cell reactions to nanotopography, interface biological events, persistence, nanotopography
1. Introduction
Cells are known to be capable of mechanotransduction, which is the signalling into, out of and between cells by mechanical means (e.g. Wang et al. 1993; Knight et al. 2002). A wide variety of biological cells can react to growing on a nanostructured interface by changes in adhesion, cytoskeletal organization, gene expression and other cellular activities (Wojciak-Stothard et al. 1995; Dalby et al. 2002a,b, 2003a,b, 2004a–c; Riehle et al. 2002; Gadegaard et al. 2003; Arnold et al. 2004; Curtis et al. 2004). Some of these papers present evidence that mechanotransduction is involved. We have extended investigations on the mechanisms, by which cells can respond to a nanofeatured surface to the question of, whether they organize their surface structure in relation to those features. We consider, whether the cell can receive a direct imprint of the nanotopography of the substratum. The evidence on the reactions of cells to nanotopography and nanosurface chemistry suggests that this might happen (Dalby et al. 2003a,b, 2004a–c) but no direct evidence, for it appears to have been reported. Dalby and others have described many ordered and semi-ordered surface nanotopographies that induce considerable cytoskeletal reorganizations and alignments. Cells often align and adhere to nanometrically high-extended ridges (Curtis & Clark 1990) and that there is often an associated line of actin condensation very close to the edge (Wojciak-Stothard et al. 1996).
The simplest test for this idea is akin to the way in which the accuracy of printing is assessed—namely that the original pattern is transferred to the ‘paper’ as accurately as is required for easy reading. The original pattern is in the nanotopography and the replica is on or in the cell. The techniques we have used include TEM, SEM and fluorescence microscopy. Fluorescence microscopy should reveal any pattern of proteins related to the original print surface, but it would be expected that these ordered proteins would probably, only be found close to the inside of the plasmalemma. We have also looked at evidence for the possible involvement of integrin signalling and the persistence of this type of mechanotransduction signalling.
2. Experimental section
2.1 Nanofeatured surfaces
These were made by colloidal lithography or by electron-beam lithography (e-beam) followed by replication by embossing in to the polymers; polycaprolactone, polymethyl methacrylate and polycarbonate (figures 1 & 2). Details of the fabrication of such surfaces have been published by Gadegaard et al. (2003) and Hanarp et al. (2003).
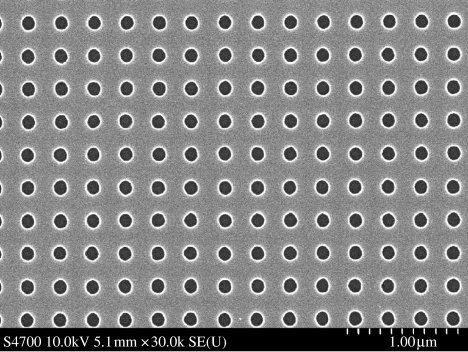
Figure 1.
Nanopitted surface prepared by electron-beam lithography. It is an array of 120 nm diameter pits on a 300 nm centre-to-centre spacing.
Figure 2.
(right) Nanocolumns produced by colloidal lithography. It is an array of columns (fabricated by, and image) courtesy of Dr Duncan Sutherland, Chalmers University, Sweden. The columns are 160 nm high and have a diameter of 100 nm.
2.2 Cell culture
Fibroblasts from skin (human, (h-tert BJ1) or endothelia (murine, LeII)) was grown on the nanofeatured substrata. Human platelets were extracted from whole blood, drawn with ethical permission by a qualified phlebotomist and with consent of the donors. The drawn blood was left for 40 min and the platelets were taken from the plasma rich supernatant. 1–2×106 cells were seeded per sample.
2.3 Preparation for EM, AFM and fluorescence microscopies
For fluorescence microscopy, epitenon cells or human platelets were cultured for 24 h (epitenon) or 2 h (platelets) on e-beam nanogrooves, fixed in 4% formaldehyde and stained with tetramethyl rhodamine–phalloidin for actin visualization. For SEM, h-tert cells were fixed in 1.5% gluteraldehyde after 4 days of culture on 160 nm high (100 nm diameter) nanocolumns fabricated by colloidal lithography figure 2 . Post fixation, they were osmium treated and dehydrated through alcohol and finally hexamethyl disilazane prior to viewing. For TEM, h-tert cells were fixed in 1.5% gluteraldehyde after 21 days of culture on 160 nm high (100 nm diameter) nanocolumns fabricated by colloidal lithography. Post fixation, they were osmium treated and dehydrated through an alcohol series prior to Araldite embedding and sectioning for TEM viewing. AFM images were acquired on a Nanoscope Dimension 3100 of SEM prepared samples.
2.4 Fast Fourier transforms of fluorescence images
These were carried out using the ImageJ program (available from http://rsb.info.nih.gov/ij/). The images used were obtained from fluorescent-antibody or rhodamine–phalloidin stained cells.
2.5 Use of anti-integrin antibody
An anti-integrin alpha 1 antibody from a mouse myeloma fusion line with immunized mouse splenocytes SE8O9 was bought from Upstate Inc (Lake Placid, NY, USA; Lot no. 24929). The reactivity of this antibody with the cells was tested using an immunocytochemical test.
Thirty-three or 66 μg of the antibody was added to a 5 ml culture of 50 000 cells and the cells allowed to settle for 2 h being observed at 60 and 120 min. The cells were then fixed and stained to determine the proportion adhering to plain or nanopatterned polycarbonate.
2.6 Time-lapse video recording
Time-lapse videos were made of the areas of transition between flat surfaces and topography. This was done using 10× phase contrast objectives and a time-lapse video recorder. Videos were shot for approximately 24 h duration at 1/80th normal speed. This was done to discover, how long it took a cell transiting from one type of substratum to another or vice versa to change morphology after making the transit. Films were analysed visually for the time for cells at the point of crossing from one topography to another, till transition and morphology change were complete or morphology change had not taken place in a 12 h period.
3. Results and discussion
Comparison of the substratum nanopattern and the SEMs of thin parts of cells showed a good correspondence between the patterns in both cell and substratum figures 3 and 4. Both these images show parts of the substratum.
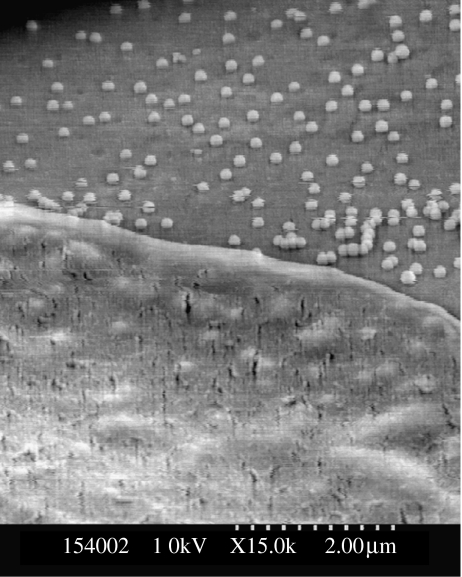
Figure 3.
SEM of fibroblast lamella on nanocolumns produced by colloidal lithography. The cell lamella is shown at the top of the micrograph and the shapes of the columns are clearly visible in the lamella.
Figure 4.
SEM of fibroblast lamellae on nanocolumns produced by colloidal lithography. The cell lamella is shown at the bottom of the micrograph and the shapes of the columns are clearly visible in the lamella.
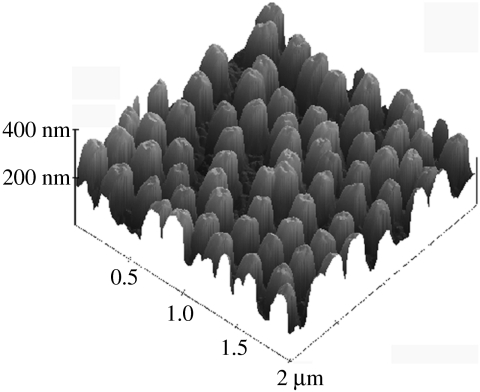
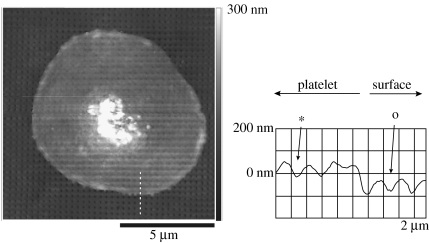
Similar evidence for printing can be seen in an AFM image of the surface of a blood platelet, figure 5.
Figure 5.
AFM micrograph of a human platelet on the same nanopitted surface as in figure 1. The underlying topography is clearly shown through the thin (80 nm) platelet membrane. This is confirmed by the cross-section where the pits clearly are seen in both the surfaces (o) and the platelet membrane. (These results demonstrate that the cell has the appropriate mechanical properties for nanoimprinting.)
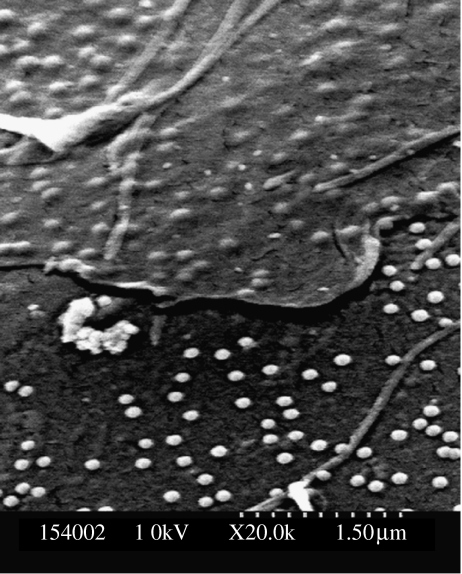
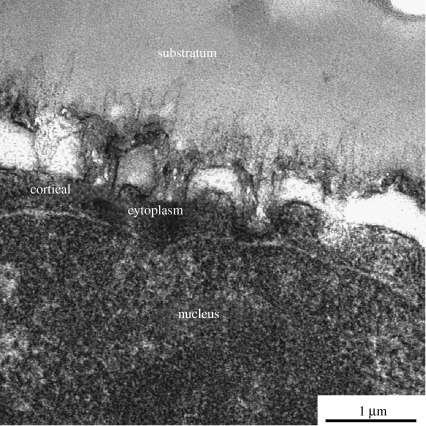
TEM examination of sections of cells growing on nanotopography shows that there is a very close apposition of the cells to the substratum though the possibility of artefacts of preparation cannot be discounted, see figure 6.
Figure 6.
(above) TEM of a fibroblast (bottom) cultured on nanocolumns (top). The nanocolumns can clearly be seen making indents on the bottom of the cell; cell is at the bottom of the picture. The proximity of the nucleus to the nanostructure should be noted.
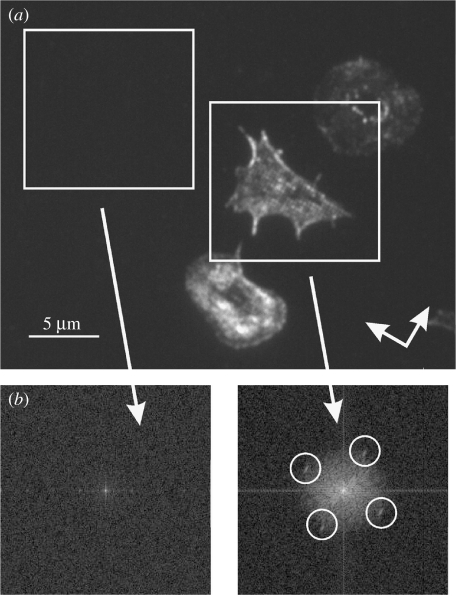
Actin organization related to the underlying topography is shown in figures 7 and 8 and a fast Fourier transform (FFT) of one of these images is also shown. It shows a periodicity of actin dots corresponding to the spacing of the nanofeatures at 300 nm. Figure 7 was acquired using an high NA, high-magnification system so that it is possible to see a distinct organization of the actin cytoskeleton corresponding to the underlying substratum. The FFT image (figure 7b) was obtained from image data in all three cells shown in figure 7a.
Figure 7.
(a) Actin labelled platelets on a nanopitted surface. The surface has a square array of 120 nm diameter pits on a 300 nm pitch with the orientation indicated by the arrows. (b) Corresponding FFT of the left-hand side image. The condensation of the actin cytoskeleton reacts to the underlying nanotopography. The position of the four peaks (white circles) corresponds to the 300 nm periodicity of the underlying nanotopography. The corresponding FFT of the flat surface image shows no characteristic features related to the underlying nanotopography.
Figure 8.
Previously published image (from Wojciak-Stothard et al. 1996) of a macrophage growing on 100 nm deep 5 μm wide grooves fabricated in silica. Fluorescent rhodamine–phalloidin stained. Note lines of actin corresponding to the grooves. Scale bar 5 μm.
3.1 Involvement of integrins
The proportion of cells adhering to nanotopography and to flat polycarbonate in the presence and absence of antibody is shown in table 1. Statistical analysis of the results (see table 1) shows that both topography and the presence of antibody depress cell adhesion significantly (p<0.01 in all comparisons in table 1.
Table 1.
Mean counts (10 repetitions) for a counting area 1/696 of total dish area.
| antibody added (μg) | mean number of cells on nanotopography | mean number of cells on flat surface | comparison nanotopog/flat t-test value | comparison Ab added/no Ab added t-test values |
|---|---|---|---|---|
| 0 | 10.5 (n=14) | 31.8 (n=10) | 7.9 | NA |
| 33 | 4.4 (n=10) | 14.4 (n=6) | 9.1 | 4.0/4.3a |
| 66 | 4.7 (n=10) | 12.2 (n=10) | 4.1 | 3.9/6.1b |
Comparison for nanotopographic surfaces.
Comparison for flat surfaces.
If there were 100% cell attachment the mean count would be 71 cells per area. All t-test results shown significant at p<1%.
The term nanoprinting implies that there will be a degree of persistence in the printed surface. Obviously, this persistence is unlikely to be maintained when the cell is separated from any surface by enzymic or other dissociation. But we would expect that the actin cytoskeleton might take some time to reorganize itself when a cell moves from one substratum to another. Persistence in direction of locomotion (Dunn 1983) can be explained as a persistence of cytoskeletal organization in one mode that takes time to be replaced by another. So, we examined, what happens as a cell moves over a nanoprinted surface or from a nanofeatured surface to a flat one or vice versa.
Results are presented in table 2 for two different types of substrata. The ‘correct’ morphology on the flat substratum is the fibroblast-like flattened and spread morphology. On the nanotopography, there are three possible morphologies, non-adherent, rounded-up but adherent and the extended hour-glass-type cells, see Gallagher et al. (2003) who first observed this. We measured the times for cells to adopt a morphology appropriate to their substratum after moving to that substratum type. Persistences up to a mean of 2 h were observed.
Table 2.
Time (in min) to respond to new type of substratum.
| time (min) | ||||
|---|---|---|---|---|
| transition | topographic pattern | number of observations | mean | (s.d.) |
| flat to topography | orthogonal | 6 | 108 | 75 |
| flat to topography | hexagonal | 3 | 44.4 | 23 |
| topography to flat | orthogonal | 6 | 129 | 97 |
| topography to flat | hexagonal | 10 | 44.4 | 21 |
The transition in morphology, on the low adhesion substratum is likely to be more rapid than the reverse transition to the flattened morphology on the flat substratum, since, usually the cell will round-up while the reverse transition requires the cell to actively spread on the substratum. This is indeed observed. A further prediction is that cells will tend to move from the nanotopographic surface to the flat one since, they are more adhesive on the latter.
3.2 Discussion
Previous results published by Dalby et al. (2002a,b, 2004a–c) and show that several cell types react to growth on nanotopographical surfaces by changes in gene expression, motility, cytoskeletal organization, tyrosine kinase activity and other features in manner suggesting that there is some type of transduction of the signal that the surface has topographic features. The type of reaction is related both to the shape, spacing and actual dimensions of the features (Curtis et al. 2001, 2004; Dalby 2004a–c, Dalby et al. 2005) so that it is clear that several different types of signal can be discriminated. Little has been reported on the actual mechanism(s) involved.
The observations, where the cells were examined by SEM or AFM (figures 3–5) do not provide evidence that the internal organelles and mechanisms of the cells were changed on nanotopography, but they do show that the cells can take the imprint of the substratum. This implies that the cells have the surface mechanical properties appropriate to accept the imprint. This is conceptually, exactly equivalent to the printing or embossing of a surface.
The observations on the actin and vinculin distributions in the cell (figures 7 and 8) show that there is a internal response of the cell to the topography organized on the print master.
This, then leads onto to further speculation about the speed with which, this reaction takes place and its persistence, if the cell moves to a different substratum. Early work suggests that the plasmalemma may have rheopectic properties, namely that it hardens on being subject to shear (Curtis 1961). Obviously, the type of test for persistence we present here, suggests that it is possible that one of the effects of exposure to a given surface structure persists but it does not directly demonstrate persistence of the nanoprinting itself. We hope to submit further evidence of this for publication in a later paper.
If the plasmalemma is imprinted, this would provide a very simple explanation of the effects of ordered nanotopography on cytoskeletal organization since, the inwards projecting features would provide an ordered array of anchor points for the actin cytoskeleton.
At certain relationships of the aspect ratio, of the inwards facing prints of substratum pillars, actin polymers would be limited in length, by falling in the ‘shadow’ of an inward facing pillar. One possibility that must be considered is that, all the cells have done is to endocytose the particles. Cursory examination of the images we present, might suggest that the cells have endocytosed the nanocolumns from the surface but Dalby et al. (2004a–c) show that cells cannot exert large enough forces to detach them.
The data we present, shows that growing cells (of several different types) on surface bearing nanotopography leads to an imprint of the topography not only on the surface of the cell but also one which is sufficiently penetrative to be seen from the other side of the cell, when the cell is relatively thin and which can be detected in interior cell components, such as the cytoskeleton and perhaps, even on the nucleus (figure 6). These novel results suggest that this nanoprinting may be an important mechanism in cell interaction and signalling. It raises the possibility that this is also an explanation of the results of raised adhesion on discontinuities and diminished adhesion on orthogonal arrays of pits (Curtis et al. 2001, 2004).
While the experiment on inhibition of cell attachment with an anti-integrin alpha 1 antibody shows that integrin-mediated adhesion can be involved in the adhesion of these cells to the polycarbonate, an experiment similar to that of Arnold et al. (2004), comparison with the effects of nanotopography table 1, shows that there is a additive effect of the topography which reduces adhesion to a still lower value.
The general relevance of our findings is that we have demonstrated the existence of an unsuspected mechanism by which a nanotopographic signal can be imparted to a cell. This mechanism might, well be part of the mechanotransduction system seen in several cells, especially the chondrocyte (Knight et al. 2002)
Though, we have obtained our evidence from the reactions of cells to nanotopography it should be borne in mind that intercellular material such as collagen fibres, biominerals and many implanted materials present nanotopography to the cells around them.
Acknowledgments
We thank Mathis Riehle for advice and encouragement. We also thank Gregor Aitchison, David McCloy, Mary Robertson and David Laughland for technical assistance. We thank Dr George Marshall for assistance and advice on platelet preparation. Parts of the work were supported by EC Contracts QLK3-CT2000-01500 NANOMED and NANOCUES NMP-STREP 2004 505868-1. M.J.D. is a BBSRC David Phillips Fellow and N.G. is a Royal Society of Edinburgh Research Fellow.
Addendum
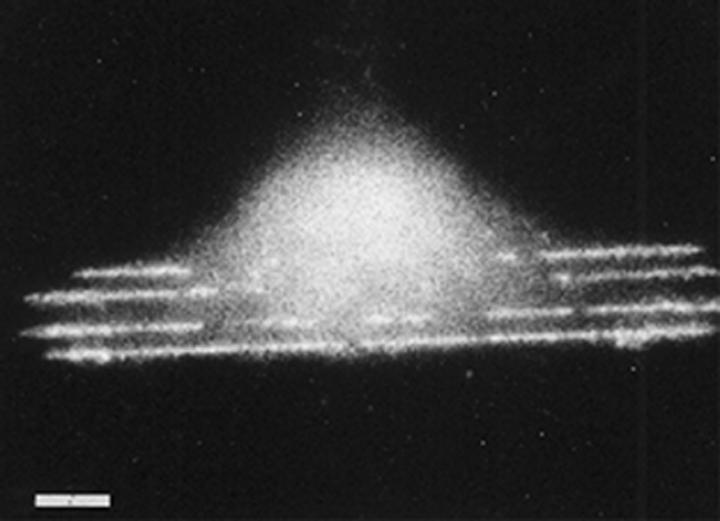
We do not think that it is possible to resolve the periodic pattern of the substrate optically. However, by using fluorescence microscopy it is possible to detect features much smaller than the diffraction limit (d0=Λ/2 NA), in fact, single molecules as well as their spacing. In our case, we can also estimate the diffraction limit and thus demonstrate that we have the required power to resolve the periodic actin condensation. The image was acquired with a 100× oil immersion objective (NA=1.4) and the actin was Rhodamine labelled (Λem>590 nm) which yields 210 nm. The pixel resolution of the images is approximately 60 nm pixel−1. It is also well known that it is possible to determine the position of an object with sub-pixel resolution by finding its centre of mass. During the FFT transformation, the latter is amplified and is thus very sensitive to the periodicity of the observed actin condensation.
The figure below is a clear demonstration of the ability to resolve the 300 nm periodic actin condensation and that it, in fact, originates from the actin cytoskeleton. An FFT transformation of the cell gives a clear twofold symmetry with peaks at 300 nm red circles. However, there is no symmetry in the FFT image of the background. Thus indicating no specific order is resolved. That includes the periodic pattern of the substrate.
References
- Arnold M, Cavalcanti-Adam E.A, Glass R, Blummel J, Eck W, Kantleher M, Kessler H, Spatzj P. Activation of integrin function by nanopatterned adhesive interfaces. Chem. Phys. Chem. 2004;4:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G. Timing mechanisms in the specific adhesion of cells. Exp. Cell Res. 1961;8(Suppl):107–122. doi: 10.1016/0014-4827(61)90343-3. doi:10.1016/0014-4827(61)90343-3 [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G, Clark P. The effects of topographic and mechanical properties of materials on cell behaviour. Crit. Rev. Biocompat. 1990;5:343–362. [Google Scholar]
- Curtis A.S.G, Casey B, Gallagher J.D, Pascui D, Wood M.A.W, Wilkinson C.D.W. Substratum nanotopography and the adhesion of biological cells. Are symmetry or regularity of nanotopography important? Biophys. Chem. 2001;94:275–283. doi: 10.1016/s0301-4622(01)00247-2. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G, Gadegaard N, Dalby M.J, Riehle M.O, Wilkinson C.D.W, Aitchison G. Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans. Nanobiosci. 2004;3:61–65. doi: 10.1109/tnb.2004.824276. [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Childs S, Riehle M.O, Johnstone H.J.H, Affrossman S, Curtis A.S.G. Fibroblast reaction to island topography: changes in cytoskeleton and morphology with time. Biomaterials. 2002a;24:927–935. doi: 10.1016/s0142-9612(02)00427-1. doi:10.1016/S0142-9612(02)00427-1 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Yarwood S.J, Johnstone H.H, Affrossman S, Riehle M. Fibroblast signaling events in response to nanotopography: a gene array study. IEEE Trans. Nanobiosci. 2002;1:12–17. doi: 10.1109/tnb.2002.806930. doi:10.1109/TNB.2002.806930 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Marshall G.E, Johnstone H.J.H, Affrossman S, Riehle M.O. Interaction of human and tissue cell types with 95 nm high nanotopography. IEEE Trans. Nanobiosci. 2003a;1:18–23. doi: 10.1109/tnb.2002.806933. doi:10.1109/TNB.2002.806933 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Riehle M.O, Yarwood S.J, Wilkinson C.D.W, Curtis A.S.G. Nucleus alignment and cell signaling in fibroblasts: response to a micro-grooved topography. Exp. Cell Res. 2003b;284:274–282. doi: 10.1016/s0014-4827(02)00053-8. doi:10.1016/S0014-4827(02)00053-8 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Berry C.C, Riehle M.O, Sutherland D.S, Agheli H, Curtis A.S.G. Attempted endocytosis of nanoenvironment produced by colloidal lithography by human fibroblasts. Exp. Cell Res. 2004a;295:387–394. doi: 10.1016/j.yexcr.2004.02.004. doi:10.1016/j.yexcr.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Giannaras D, Riehle M.O, Gadegaard N, Affrossman S, Curtis A.S.G. Rapid fibroblast adhesion to 27 nm high polymer demixed nano-topography. Biomaterials. 2004b;25:77–83. doi: 10.1016/s0142-9612(03)00475-7. doi:10.1016/S0142-9612(03)00475-7 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Riehle M.O, Sutherland D.S, Agheli H, Curtis A.S.G. Use of nanotopography to study mechanotransduction in fibroblasts—methods and perspectives. Eur. J. Cell Biol. 2004c;83:159–169. doi: 10.1078/0171-9335-00369. doi:10.1078/0171-9335-00369 [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Riehle M.O, Sutherland D.S, Agheli H, Curtis A.S.G. Morphological and microarray analysis of human fibroblasts cultured on nanocolumns produced by colloidal lithography. Euro. Cells Mater. 2005;9:1–8. doi: 10.22203/ecm.v009a01. [DOI] [PubMed] [Google Scholar]
- Dunn G.A. Characterizing a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 1983;12:14–33. doi: 10.1007/978-3-0348-9352-7_1. [DOI] [PubMed] [Google Scholar]
- Gadegaard N, Thoms S, Macintyre D.S, Mcghee K, Gallagher J, Casey B, Wilkinson C.D.W. Arrays of nano-dots for cellular engineering. Microelectron. Eng. 2003;67–68:162–168. doi:10.1016/S0167-9317(03)00067-4 [Google Scholar]
- Gallagher J.O, McGhee K.F, Wilkinson C.D.W, Riehle M.O. Interaction of animal cells with ordered nanotopography. IEEE Trans. Nanobiosci. 2003;1:14–28. doi: 10.1109/tnb.2002.806918. [DOI] [PubMed] [Google Scholar]
- Hanarp P, Sutheraland D.S, Gold J, Kasemo B. Control of nanoparticle film structure for colloidal lithography. Colloids Surf. A. 2003;214:23–36. doi:10.1016/S0927-7757(02)00367-9 [Google Scholar]
- Knight M.M, van de Breevaart Bravenboer J, Lee D.A, van Osch G.J.V.M, Weinans H, Bader D.L. Cell and nucleus deformation in compresses chondrocyte-alginate constructs : temporal changes and calculation of cell modulus. Biochem. Biophys. Acta. 2002;1570:1–8. doi: 10.1016/s0304-4165(02)00144-7. [DOI] [PubMed] [Google Scholar]
- Riehle M.O, Dalby M.J, Johnstone H, MacIntosh A, Affrossman S. Cell behaviour of rat calvaria bone cells on surfaces with random nanometric features. Mater. Sci. Eng. C. 2002;1047:1–4. [Google Scholar]
- Wang N, Butler J.P, Ingber D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Madeja Z, Korohoda W, Curtis A, Wilkinson C. Activation of macrophage-like cells by multiple grooved substrata. Topographical control of cell behaviour. Cell Biol. Int. 1995;19:485–490. doi: 10.1006/cbir.1995.1092. doi:10.1006/cbir.1995.1092 [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Curtis A, Monaghan W, Macdonald K, Wilkinson C. Guidance and activation of murine macrophages by nanometric scale topography. Exp. Cell Res. 1996;223:426–435. doi: 10.1006/excr.1996.0098. doi:10.1006/excr.1996.0098 [DOI] [PubMed] [Google Scholar]