Abstract
Context
The possible role of adiponectin, a protein uniquely produced by the adipose tissue and significantly reduced in obesity and other insulin-resistant states, in the regulation of energy expenditure (EE) is still poorly understood.
Objective
The objective of the study was to investigate the relationship between total fasting plasma adiponectin concentrations and the various components of EE measured in a metabolic chamber in Pima Indians and to test whether body fat distribution may have a role in this association.
Design
This was a cross-sectional study.
Setting
The study was an inpatient clinical research unit.
Participants
Sixty nondiabetic Pima Indians (45 males and 15 females), aged 18–45 yr, spanning a wide range of adiposity (body mass index 19.6–46.2 kg/m2) participated in the study.
Main Outcome Measures
Total fasting plasma adiponectin concentrations, EE (24-h respiratory chamber), insulin sensitivity (euglycemic-hyperisulinemic clamp), body composition (dual-energy x-ray absorptiometry), and body fat distribution (waist to thigh ratio) were the main outcome measures.
Results
Total fasting plasma adiponectin concentrations are negatively associated with sleep EE adjusted for sex, age, fat-free mass, and fat mass. This correlation is still significant, although attenuated, after inclusion of insulin-stimulated glucose disposal among the regressors and further attenuated when adjusted also for waist to thigh ratio.
Conclusions
The decrease in total fasting plasma adiponectin concentrations that accompanies fat accumulation may be a mechanism to prevent further weight gain by decreasing insulin sensitivity and increasing energy expenditure.
Abbreviations: EE, Energy expenditure; M, insulin-stimulated glucose disposal; min-max, minimum-maximum; RMR, resting metabolic rate; SLEEP, sleep EE; SPA, spontaneous physical activity; WTR, waist to thigh ratio
Plasma concentrations of adiponectin are significantly reduced in obese and diabetic mice and humans as well as patients with hypertension and cardiovascular diseases (1). Conversely, weight loss is associated with an increase in adiponectin concentrations in some (2) but not all (3) studies. Total fasting plasma adiponectin concentrations are lower in Pima Indians, a population with a high prevalence of obesity and type 2 diabetes, compared with Caucasians, although this ethnic difference is no longer significant after adjusting for insulin sensitivity (4).
The role of adiponectin in energy balance is yet to be established. Animal studies have provided contradictory results, indicating both a positive (5) and negative (6) effect of adiponectin on energy expenditure (EE). Human studies have shown a negative association between resting metabolic rate (RMR) measured by the ventilated hood technique and plasma adiponectin in both male and female Caucasians (7). A previous study of Pima Indians and Caucasians did not show a significant association between 24-h EE, as measured in a metabolic chamber, and total fasting plasma adiponectin concentrations but did not assess the various components of daily metabolic rate (8).
Central fat is a stronger determinant of plasma adiponectin concentrations than overall adiposity (4). Central fat accumulation is associated with higher EE, an observation also found in Pima Indians (9). Therefore, we hypothesized that body fat distribution may mediate the relationship between adiponectin and metabolic rates.
The aims of this study were to investigate the relationship between total fasting plasma adiponectin concentrations and the different components of daily EE in a group of Pima Indian men and women spanning a wide range of adiposity and to test whether body fat distribution may have a role in this association. EE was measured in a respiratory chamber, which provides an accurate measure of a person’s 24-h EE.
Subjects and Methods
Subjects
Sixty nondiabetic Pima Indians [45 male and 15 females, aged 28 ± 7 yr (range 18–45)] from the Gila River Indian Community were included in the study. The analysis focused on only those subjects, part of a larger study of metabolic predictors of type 2 diabetes in Pima Indians and Caucasians, for whom both insulin-stimulated glucose disposal and EE along with total fasting plasma adiponectin concentrations were available. Before participation, volunteers were fully informed of the nature and purpose of the study, and written informed consent was obtained. The experimental protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases and the Tribal Council of the Gila River Indian Community. All subjects were found to be free of disease according to physical examination, medical history, and laboratory testing. On admission to the metabolic ward, subjects were placed on a standard weight maintenance diet (20, 30, and 50% of daily calories provided as protein, fat, and carbohydrate, respectively) for 3 d before testing. Glucose tolerance was assessed by a 75-g oral glucose tolerance test according to the criteria of the World Health Organization. Only nondiabetic subjects were studied. Body composition was measured by dual-energy x-ray absorptiometry (DPX-L, Lunar Corp., Madison, WI).
EE
EE was measured in a respiratory chamber. Volunteers entered the chamber at 0745 h after an overnight fast and remained there for 23½ h. Meals were provided at 0800, 1130, 1600, and 1900 h (an evening snack). The rate of EE was measured continuously, calculated for each 15-min interval, and then extrapolated to 24 h (24-h EE). Spontaneous physical activity (SPA) was detected by radar sensors and expressed as percentage of time over the 24-h period in which activity was detected. Sleep EE (SLEEP) was defined as the average EE of all 15-min periods between 2330 and 0500 h during which SPA was less than 1.5%. SLEEP is an estimate of the basal metabolism necessary to support life. Physical activity-related EE was calculated by multiplying the mean SPA values by the slope of the regression line of 24-h EE vs. SPA.
Two-step hyperinsulinemic-euglycemic glucose clamp
Insulin-stimulated glucose disposal (M) was assessed at physiological and supraphysiological insulin concentrations using a two-step hyper-insulinemic-euglycemic glucose clamp, as previously described (10).
Analytical measurements
Blood samples were drawn after an overnight fast, at least 3 full days after subjects had consumed a weight-maintenance diet on the metabolic ward. Plasma glucose concentrations were measured using the glucose oxidase method (Beckman Instruments Inc., Fullerton, CA). Plasma insulin concentrations were measured with an automated RIA (Concept 4, ICN Biochemicals, Costa Mesa, CA). Plasma adiponectin concentrations were measured with a validated sandwich ELISA using an adiponectin-specific antibody (intra- and interassay coefficients of variation were 3.3 and 7.4%, respectively), as previously reported (4).
Data analysis
All statistical analyses were performed using software of the SAS Institute (SAS version 8.2; Cary, NC). Throughout the text, the data are expressed as means ± SD. Relationships between total fasting plasma adiponectin concentrations and the other study variables were assessed using Spearman correlation coefficients. Linear regression analysis was used to model the effects of adiponectin on the EE parameters as well as the effects of selected anthropometric and metabolic variables on total fasting plasma adiponectin concentrations.
Results
Total fasting plasma adiponectin concentrations [median (minimum-maximum [min-max]): 6.7 (3.1–15.3) μg/ml] were negatively associated with body mass index (mean ± SD: 31.4 ± 6 kg/m2; r = −0.36, P = 0.004), percent body fat (mean ± SD: 30 ± 7%; r = −0.31, P = 0.02), waist circumference (mean ± SD: 104 ± 14 cm; r = −0.33, P = 0.01), waist to thigh ratio (WTR; mean ± SD: 1.6 ± 0.1; r = −0.36, P = 0.004), fasting concentrations of glucose (mean ± SD: 84 ± 10 mg/dl; r = −0.34, P = 0.007), fasting concentrations of insulin (median [min-max]: 40 (7–108) μIU/ml; r = −0.44, P = 0.0004), and SLEEP (mean ± SD: 1634 ± 249 kcal per 24 h; r = −0.42, P = 0.0009, Fig. 1) but not 24-h EE (mean ± SD: 2338 ± 359 kcal per 24 h; r = −0.25, P = 0.06) and positively correlated with M [median (min-max): 2.2 (1.7–6.9) mg/kg estimated metabolic body size−1/min−1; r = 0.49, P < 0.0001]. M and WTR, but not percent body fat, were significant independent determinants of total fasting plasma adiponectin concentrations, explaining a total of 40% of the variance in this measure (Table 1).
Fig. 1.
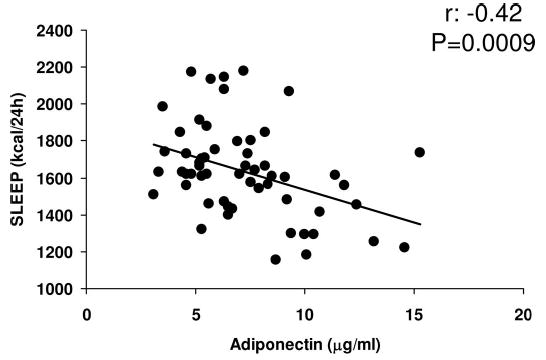
Spearman correlation between total fasting plasma adiponectin concentrations and SLEEP.
TABLE 1.
Determinants of total fasting plasma adiponectin concentrationsa
| Variable | β | P |
|---|---|---|
| Sex (male) | 0.05 | 0.35 |
| Age (yr) | 0.01 | 0.33 |
| Body fat (%) | 0.01 | 0.52 |
| WTR | −0.36 | 0.02 |
| Fasting glucose (mg/dl) | −0.01 | 0.07 |
| Fasting insulin (μIU/ml) | −0.08 | 0.47 |
| Insulin-stimulated glucose disposal | 0.39 | 0.03 |
| (mg/kg·EMBS−1·min−1) |
EMBS, Estimated metabolic body size.
Using multiple linear regression modeling.
We have previously reported in a larger sample (9) that SLEEP is determined by fat-free mass, fat mass, sex, and age (Table 2, model 1 in this study population). Total fasting plasma adiponectin concentrations were negatively associated with SLEEP after adjustment for sex, age, fat-free mass, and fat mass using general linear modeling and explained an additional 3% of the variance of SLEEP (Table 2, model 2). The correlation between total fasting plasma adiponectin concentrations and SLEEP was still significant, although attenuated, after inclusion of M among the regressors (Table 2, model 3). This association was further attenuated when adjusted also for WTR (Table 2, model 4).
TABLE 2.
Determinants of SLEEPa
| Model 1 (P < 0.0001, R2 = 0.80)
|
Model 2 (P < 0.0001, R2 = 0.83)
|
Model 3 (P < 0.0001, R2 = 0.83)
|
Model 4 (P < 0.0001, R2 = 0.83)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | β | P | β | P | β | P | β | P |
| Sex (male) | 0.01 | 0.78 | 0.01 | 0.68 | 0.01 | 0.67 | 0.01 | 0.72 |
| Age (yr) | −0.01 | 0.85 | −0.01 | 0.80 | −0.01 | 0.72 | −0.01 | 0.69 |
| Fat-free mass | 0.01 | <0.0001 | 0.01 | <0.0001 | 0.01 | <0.0001 | 0.01 | <0.0001 |
| Fat mass | 0.01 | 0.06 | 0.01 | 0.12 | 0.01 | 0.16 | 0.01 | 0.16 |
| Adiponectin | −0.01 | 0.01 | −0.01 | 0.03 | −0.07 | 0.06 | ||
| M | −0.01 | 0.44 | −0.01 | 0.45 | ||||
| WTR | 0.01 | 0.77 | ||||||
Using multiple linear regression modeling.
No independent associations were found between total fasting plasma adiponectin concentrations and 24-h EE or physical activity-related EE.
Discussion
Adiponectin is an adipocyte-specific protein that circulates in the bloodstream at concentrations 100- to 1000-fold higher than those of other hormones and cytokines, and, in contrast to all other adipocytokines, plasma concentrations of adiponectin are markedly decreased in obesity, type 2 diabetes, and coronary artery disease (1). In this study, and similar to a recent report in Caucasians using the ventilated hood technique to measure RMR (7), total fasting plasma adiponectin concentrations were negatively associated with sleep energy expenditure in Pima Indians, independent of sex, age, and body composition.
Adiponectin circulates as lower-molecular-weight hexamers and larger multimers of high molecular weight (11). The percentage of high-molecular-weight adiponectin is a better predictor of insulin sensitivity under thiazolidinedione treatment (12) and glucose intolerance than total adiponectin (13). Therefore, total plasma adiponectin concentrations may not represent as accurate an estimate of the physiological effects of this protein as the ratio between its isoforms.
This cross-sectional analysis did not allow us to determine whether energy expenditure affects adiponectin concentrations or whether adiponectin lowers metabolic rate. In their study of Caucasians, however, Ruige et al. (7) proposed that higher plasma adiponectin concentrations occur as a defense against comorbidities of obesity in subjects with lower RMR. On the other hand, adiponectin might lower EE as well. A negative association between adiponectin and other adipocytokines known to stimulate thermogenesis, such as leptin and TNFα, has been reported (14). Moreover, adiponectin inhibits the activation of signal transducer and activator of transcription-3, which is central to the effects of leptin on EE (15).
Adiponectin is a unique adipocytokine in that its plasma concentrations are markedly decreased in obesity, leading to a reduction in insulin sensitivity (4). Obesity-associated insulin resistance may represent a mechanism to counteract further expansion of body fat and therefore limit obesity, despite the drawback of an increased risk of developing type 2 diabetes. The decrease in plasma adiponectin concentrations with weight gain may also help to prevent fat accumulation by allowing for a higher EE. In fact, lower total fasting plasma adiponectin concentrations were associated with less weight gain in a prospective study of Pima Indians (16).
Eventually reduced concentrations of adiponectin may help to limit obesity but, at the same time, impair insulin sensitivity, thus leading to glucose intolerance and type 2 diabetes. The function of low plasma adiponectin as an independent predictor of type 2 diabetes has previously been established (17). Interestingly, SLEEP was higher in Pima Indians with either type 2 diabetes or impaired glucose tolerance, compared with normal glucose-tolerant subjects (18). Furthermore, a progressive increase in resting EE and insulin-induced thermogenesis paralleled the deterioration of glucose tolerance from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes in that study (18). Determinants of higher EE in glucose intolerance include hepatic glucose production, insulin-mediated glucose disposal, and fasting concentrations of insulin and free fatty acids (18). Lower total fasting plasma adiponectin concentrations are associated with higher hepatic glucose production in Pima Indians (19). Therefore, low concentration of circulating adiponectin may explain the higher EE observed in glucose intolerance.
The present study also demonstrated that total fasting plasma adiponectin concentrations in Pima Indians are more closely related to body fat distribution than to overall adiposity, as previously reported (4). The role of central fat in reducing adiponectin concentration may offer insight into the negative association between this adipocytokine and sleep EE. In fact, a positive correlation between central fat accumulation and EE has been established in previous studies of both Caucasians (20) and Pima Indians (9), and further adjustment of the association between adiponectin and adjusted sleep EE for WTR in the present study attenuated the strength of the correlation.
In conclusion, there is a negative association between total adiponectin and EE in Pima Indians that may be mediated, in part, by body fat distribution. We speculate that the decreased total adiponectin concentration associated with overweight and especially with central fat accumulation may represent a mechanism inhibiting further weight gain by affecting both insulin sensitivity (decrease) and EE (increase).
Acknowledgments
We thank the members of the Gila River Indian Community for their participation in this study. We also thank the nursing and dietary staffs, physician assistants, and lab technicians of the clinical research center for their valuable assistance.
Footnotes
All the authors have nothing to declare.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Matsuzawa Y. Adiponectin: identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler Suppl. 2005;6:7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 3.Xydakis AM, Case CC, Jones PH, Hoogeveen RC, Liu MY, Smith EO, Nelson KW, Ballantyne CM. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004;89:2697–2703. doi: 10.1210/jc.2003-031826. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 5.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 6.Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- 7.Ruige JB, Ballaux DP, Funahashi T, Mertens IL, Matsuzawa Y, Van Gaal LF. Resting metabolic rate is an important predictor of serum adiponectin concentrations: potential implications for obesity-related disorders. Am J Clin Nutr. 2005;82:21–25. doi: 10.1093/ajcn.82.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Ravussin E, Weyer C, Tataranni PA. Plasma adiponectin levels are not associated with fat oxidation in humans. Obes Res. 2002;10:1016–1020. doi: 10.1038/oby.2002.138. [DOI] [PubMed] [Google Scholar]
- 9.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord. 1999;23:715–722. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 10.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 11.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 12.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 13.Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 14.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 16.Krakoff J, Looker HE, Funahashi T, Cummings DE, Considine RV, Tataranni PA, Knowler WC, Hanson RL. Long-term weight gain in Pima Indians: a role for ghrelin and adiponectin. Obes Res. 2004;12(Suppl):A201. (Abstract 790-P) [Google Scholar]
- 17.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 18.Weyer C, Bogardus C, Pratley RE. Metabolic factors contributing to increased resting metabolic rate and decreased insulin-induced thermogenesis during the development of type 2 diabetes. Diabetes. 1999;48:1607–1614. doi: 10.2337/diabetes.48.8.1607. [DOI] [PubMed] [Google Scholar]
- 19.Stefan N, Stumvoll M, Vozarova B, Weyer C, Funahashi T, Matsuzawa Y, Bogardus C, Tataranni PA. Plasma adiponectin and endogenous glucose production in humans. Diabetes Care. 2003;26:3315–3319. doi: 10.2337/diacare.26.12.3315. [DOI] [PubMed] [Google Scholar]
- 20.Leenen R, van der Kooy K, Deurenberg P, Seidell JC, Weststrate JA, Schouten FJ, Hautvast JG. Visceral fat accumulation in obese subjects: relation to energy expenditure and response to weight loss. Am J Physiol. 1992;263(5 Pt 1):E913–E919. doi: 10.1152/ajpendo.1992.263.5.E913. [DOI] [PubMed] [Google Scholar]


