Abstract
The neurotransmitter GABA mediates the majority of rapid inhibition in the CNS. Inhibition can occur via the conventional mechanism, the transient activation of subsynaptic GABAA receptors (GABAA-Rs), or via continuous activation of high-affinity receptors by low concentrations of ambient GABA, leading to “tonic” inhibition that can control levels of excitability and network activity. The GABAA-R α4 subunit is expressed at high levels in the dentate gyrus and thalamus and is suspected to contribute to extrasynaptic GABAA-R-mediated tonic inhibition. Mice were engineered to lack the α4 subunit by targeted disruption of the Gabra4 gene. α4 Subunit knockout mice are viable, breed normally, and are superficially indistinguishable from WT mice. In electrophysiological recordings, these mice show a lack of tonic inhibition in dentate granule cells and thalamic relay neurons. Behaviorally, knockout mice are insensitive to the ataxic, sedative, and analgesic effects of the novel hypnotic drug, gaboxadol. These data demonstrate that tonic inhibition in dentate granule cells and thalamic relay neurons is mediated by extrasynaptic GABAA-Rs containing the α4 subunit and that gaboxadol achieves its effects via the activation of this GABAA-R subtype.
Keywords: tonic inhibition, THIP, analgesia, sedative/hypnotic
GABA is the major inhibitory neurotransmitter in the mammalian CNS. Its primary target, GABAA receptors (GABAA-Rs), are pentameric complexes that function as ligand-gated chloride ion channels. Two types of inhibitory neurotransmission are mediated via GABAA-Rs (1, 2). Phasic inhibition results from the activation of receptors at the synapse by intermittent release of high concentrations of GABA from presynaptic terminals. Tonic inhibition, in contrast, is mediated by the continuous activation of receptors located outside the synaptic cleft by low concentrations of ambient GABA. These “extrasynaptic” GABAA-Rs have a higher affinity for GABA and have faster channel deactivation rates (3, 4) and, more importantly, slower rates of desensitization (1–5), relative to the classical “synaptic” GABAA-Rs.
There are a variety of subunit families that make up GABAA-Rs; a total of 19 distinct subunits have been cloned, α1–6, β1–3, γ1–3, δ, ε, π, θ, and ρ1–3 (6). This diversity in subunit composition results in substantial anatomical, functional, and pharmacological heterogeneity. GABAA-Rs containing the α4 subunit are highly expressed in the thalamus and dentate gyrus, with lower levels in cortex, striatum, and other brain areas (7–9). GABAA-Rs containing α4 subunits often are found with the γ2 or δ subunits, in combination with β subunits; the α4βδ subtypes are proposed to be localized to extrasynaptic sites and contribute to tonic inhibition (5, 10–13). Other extrasynaptic receptor subtypes include α5β3γ2 in hippocampal CA1 pyramidal cells (14) and α6βδ in cerebellar granule cells (15). Notably, the α4 subunit containing GABAA-Rs, especially α4βγ2, are not exclusively extrasynaptic; some are found within dentate gyrus synapses and others are located perisynaptically, where they are expected to affect both the rise time and decay of synaptic currents (12, 13). There is also evidence that a significant portion of α4 subunit-containing GABAA-Rs do not contain γ or δ subunits (16). The α4 subunit-containing receptors are insensitive to benzodiazepines but show high sensitivity to other sedative-hypnotic drugs, including ethanol (17, 18), neurosteroids (19–21), etomidate (20), and the novel hypnotic drug, gaboxadol (GBX, formerly known as THIP) (11, 20, 22).
To investigate the contribution of α4-containing GABAA-Rs to inhibition, we have created and analyzed a strain of α4 subunit knockout (KO) mice.
Results
Absence of α4 Protein in GABAA-R α4 KO Mice.
GABAA-R α4 subunit KO mice were generated by using standard gene targeting and embryonic stem cell technologies. The KO mice were viable, healthy, and superficially indistinguishable from their WT littermates. For details regarding mouse construction and general characterization, see Supporting Text, which is published as supporting information on the PNAS web site.
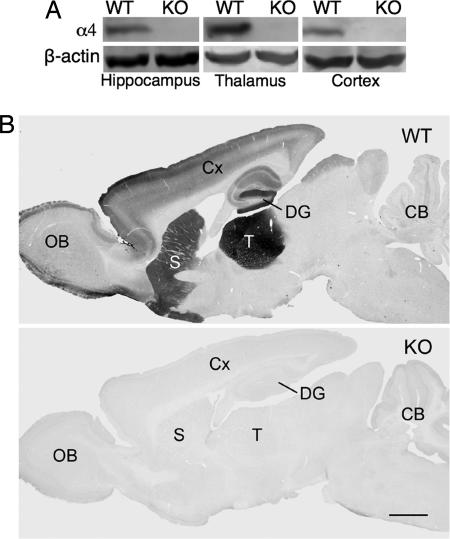
α4 Subunit protein levels in mice were examined by using Western blot analysis and immunohistochemistry. In membrane preparations of hippocampus, thalamus, and cortex from WT mice, an α4-specific antibody (16) specifically recognized a ≈67-kDa protein (Fig. 1A). This protein band was absent in membranes from KO mice. The selective pattern of α4 subunit immunolabeling in sagittal sections from WT mice showed strong α4 subunit labeling in the thalamus, and moderate α4 immunoreactivity in the striatum and molecular layer of the dentate gyrus (Fig. 1B). Lighter immunolabeling was seen in the cerebral cortex and CA1 field of the hippocampus (12, 23). No specific α4 immunolabeling was evident in brains of KO mice (Fig. 1B).
Fig. 1.
GABAA-R α4 protein is absent in KO mice. (A) Western blot analysis of hippocampal, thalamic, and cortical membranes from WT and KO mice. The ≈67-kDa immunoreactive α4 protein present in WT samples is completely absent from KO samples. Stripped blots probed for β-actin show equal loading of samples. (B) α4 Subunit immunoreactivity in sagittal sections from WT and KO mouse brain. In WT mice, α4 labeling is highest in the thalamus (T), moderate in the molecular layer of the dentate gyrus (DG) and striatum (S), and slightly lower in the outer layers of the cerebral cortex (Cx) and the external plexiform layer of the olfactory bulb (OB). α4 Labeling is essentially absent from the cerebellum (CB). No specific labeling is evident in KO mouse brain.
Dramatically Reduced Tonic Inhibition in Dentate Granule Cells (DGCs) of KO Mice.
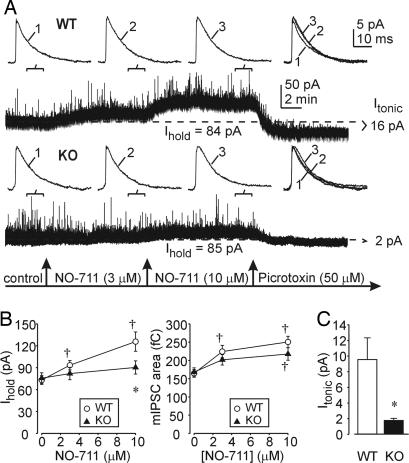
To investigate possible alterations in GABAA-R function of α4 KO mice, we recorded GABAA-R currents in DGCs in hippocampal slices. Application of the GABA uptake inhibitor, NO-711, produced significant concentration-dependent increases in the holding current (Ihold) in WT but not KO mice (Fig. 2A and B). The subsequent application of picrotoxin (50 μM) reduced Ihold in both groups of mice. The picrotoxin-sensitive current was designated as the GABAA-R-mediated tonic current (Itonic). This basal tonic current was reduced greatly in DGCs from KO mice (Fig. 2 A and C).
Fig. 2.
Decreased magnitude and potentiation of GABAAR-mediated tonic current in KO mouse DGCs. (A) The GABA uptake inhibitor, NO-711, potentiates the tonic current (Ihold) in a DGC from a WT mouse. The kinetics of mIPSCs (Upper) averaged over the indicated 100-sec periods during continuous voltage clamp (V = 0 mV throughout) recordings (Lower) are only slightly affected by NO-711 (3 and 10 μM). Picrotoxin (50 μM) application reveals a GABAAR-mediated tonic current component (Itonic). In a DGC from a KO mouse (Lower), potentiation of Ihold by NO-711 is reduced and Itonic is very small. (B) Summary graphs of Ihold and total charge transfer of averaged mIPSCs before and after NO-711 application in WT and KO mice. Each point represents a mean ± SEM value from 5–6 neurons (4–5 mice per group). ∗, (P < 0.05) between WT and KO groups; †, (P < 0.05) from pre-NO-711 value (two-way repeated measures ANOVA). (C) Summary graph of differences in the picrotoxin-sensitive tonic current between WT and KO groups. ∗, (P < 0.05, t test, n = 12–14, 3–4 mice per group). The rms of current noise was as follows: basal, 3.5 ± 0.8 pA (WT) and 3.2 ± 0.9 pA (KO); after picrotoxin, 2.6 ± 0.4 pA (WT) and 2.5 ± 0.5 pA (KO).
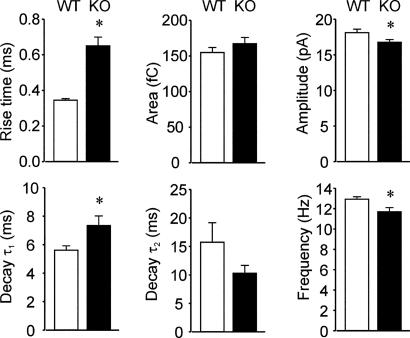
Analysis of miniature inhibitory postsynaptic currents (mIPSCs) revealed significant potentiation of mIPSC area (charge transfer) by NO-711 in DGCs from both groups of mice. This potentiation primarily was due to the prolongation of mIPSC decay and was unaltered in α4 KO mice (Figs. 2 A and B and 3). Whereas deletion of the α4 gene had little effect on the charge transferred during mIPSCs, closer examination of mIPSC kinetics recorded under basal conditions revealed marked slowing of the rise and early decay of mIPSCs from KO mice (Fig. 3).
Fig. 3.
Summary graphs of differences in mIPSC kinetics between DGCs from WT and KO mice. mIPSC amplitude and frequency were significantly reduced in neurons from KO mice compared with WT controls. Also note the slower rise and early decay of mIPSCs in DGCs from KO mice. Each bar represents a mean ± SEM value from 10–11 neurons (5 mice per group). ∗, P < 0.05, t test.
Absence of Tonic Current and Insensitivity to GBX in Ventrobasal (VB) Neurons from Thalamus.
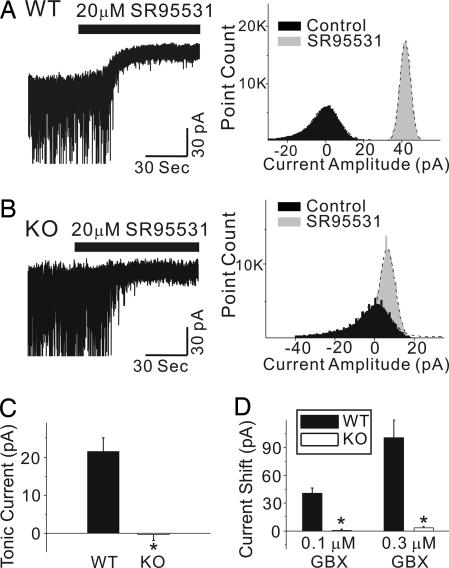
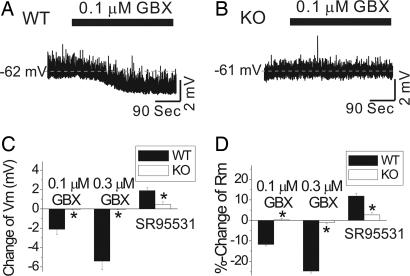
Whole-cell voltage-clamp recordings were made in VB neurons from KO and WT mice. As reported in refs. 10, 11, and 24, GABAA-R-mediated tonic currents in WT VB thalamic neurons were revealed after the addition of 20 μM SR95531 (gabazine), an antagonist of GABAA-Rs (Fig. 4A and C). In KO neurons, SR95531 had little effect on the membrane-holding current (Fig. 4 B and C). The amplitude, decay time constant, and frequency of spontaneous IPSCs were not significantly different between neurons from the two groups of mice (data not shown). We then examined the sensitivity of these neurons to GBX, which increases the amplitude of tonic currents at low concentrations (10, 11, 24). Both 0.1 and 0.3 μM GBX evoked large inward currents in WT VB neurons (Fig. 4D) but had essentially no effect in VB neurons from KO mice (Fig. 4D). These results support a requirement of α4 subunits for extrasynaptic GABAA-Rs in VB neurons.
Fig. 4.
Reduced tonic currents in KO VB thalamic neurons. (A Left) The current recorded from a thalamic VB neuron from a WT mouse before and after application of 20 μM SR95531. SR95531 abolished spontaneous IPSCs and also induced a positive shift in the holding current due to blockade of a tonic inward current. (A Right) The all-points histograms corresponding to the 30-sec traces. The black and gray histograms illustrate the holding current in the absence and presence of SR95531, respectively. The dashed lines represent best-fit curves to a single Gaussian distribution. SR95531 caused a rightward shift and reduced the width of the all-point distribution. (B Left) The current recorded from a KO mouse VB neuron before and after application of 20 μM SR95531. SR95531 abolished spontaneous IPSCs without causing a shift in the holding current. (B Right) The corresponding all-points histograms. (C) Summary data for the WT and KO mice show that thalamic VB neurons from KO mice have no tonic inhibition (n = 9 and 17 for WT and KO, respectively). (D) VB neurons from KO mice also were insensitive to currents elicited by 0.1 and 0.3 μM GBX (n = 7–12; ∗, P < 0.05). Averaged data are expressed as mean ± SE.
Current clamp recordings demonstrated that these α4 subunit-dependent tonic currents provided background inhibitory tone in VB neurons from WT mice. SR95531 (20 μM) depolarized the membrane potential and increased the membrane input resistance in WT VB neurons (Fig. 5C and D), whereas 0.1 and 0.3 μM GBX hyperpolarized neurons and reduced membrane resistance (Fig. 5 A, C, and D). All of these effects were greatly reduced or absent in VB neurons of KO mice (Fig. 5 B–D).
Fig. 5.
SR95531 and GBX modulate membrane properties of VB thalamic neurons. (A) A VB neuron from a WT mouse was hyperpolarized by nearly 2 mV after addition of 0.1 μM GBX. (B) GBX (0.1 μM) produced no significant membrane potential change in VB neuron from a KO mouse. (C) The summary data shows that VB neurons from WT mice are hyperpolarized by 0.1 and 0.3 μM GBX, whereas neurons from KO mice are insensitive to GBX. SR95531 (20 μM) also induces a depolarization in WT but not in KO neurons (n = 7–12 per genotype; ∗, P < 0.05). (D) Similar differences in modulation by GBX and SR95531 also are observed in measurements of membrane input resistance of VB thalamic neurons (n = 7–12 per genotype; ∗, P < 0.05).
Insensitivity of KO Mice to the Behavioral Effects of GBX.
We studied the effects of GBX on mice by using behavioral assays for ataxia, analgesia, and sedation. All experiments demonstrate that KO mice show greatly reduced sensitivity to the behavioral effects of GBX.
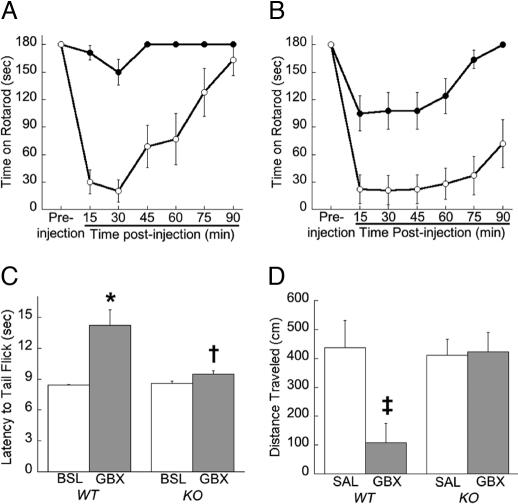
Recovery from ataxia induced by 10 or 15 mg/kg GBX was measured by using a fixed speed rotarod. KO mice were virtually insensitive to 10 mg/kg GBX when compared with WT mice (Fig. 6A; repeated measures ANOVA; F1,31 = 55, P < 0.0001). Ataxic response to 15 mg/kg GBX also was greatly reduced in KO compared with WT mice (Fig. 6B; F1,28 = 14, P < 0.001). We also tested flunitrazepam, which is a benzodiazepine that is thought to exert its effects via synaptic GABAA-Rs. We observed that KO mice did not differ significantly from WT littermates in recovery from ataxia after injection with 2 mg/kg flunitrazepam (data not shown).
Fig. 6.
KO mice are insensitive to the behavioral effects of GBX. The fixed speed rotarod measured GBX's ataxic effects at 10 mg/kg (n = 19 KO and 14 WT) (A) and 15 mg/kg (n = 18 KO and 12 WT) (B). Ataxic effects of GBX are dramatically reduced in KO mice (filled circles) compared with WT mice (open circles) at both 10 (P < 0.0001) and 15 mg/kg (P < 0.001). (C) The radiant tail-flick assay was used to measure the analgesic properties of 10 mg/kg GBX (n = 15 KO and 17 WT). Baseline latency to flick tail (BSL) or latency after GBX injection is displayed. GBX produced a marked analgesic effect in WT mice by increasing latency to flick tail (∗, P < 0.005) but only had a small but marginally significant (†, P = 0.05) effect in KO mice. (D) The open-field assay was used to measure the sedative effect of 10 mg/kg GBX (n = 7–10 mice per genotype per treatment). Total locomotor activity after injection with saline (SAL) or GBX is displayed. GBX depressed locomotor activity in WT mice (‡, P < 0.02) but had no effect in KO mice. No genotypic differences were observed between saline-treated groups.
The radiant tail-flick assay was used to measure thermal pain sensitivity and to study the analgesic effect of 10 mg/kg GBX. WT and KO mice did not differ in their basal thermal pain sensitivity, as measured by their latency to tail flick in the absence of drug (Fig. 6C). In KO mice, GBX produced only a slight but significant (P = 0.05) increase in tail-flick latency compared with baseline. In contrast, GBX markedly prolonged the latency to tail flick compared with basal responses in WT mice (P < 0.005). These data indicate that KO mice are largely insensitive to the analgesic effect of GBX as determined by the radiant tail-flick assay.
To measure the sedative effects of GBX, total locomotor activity was recorded in an open field assay in WT and KO mice 20 min after treatment with either saline or 10 mg/kg GBX. Two-way ANOVA revealed an effect of treatment (F1,28 = 6, P < 0.05), a genotype by treatment interaction (F1,28; P < 0.05), but no effect of genotype. Post hoc analysis revealed no difference in total activity between WT and KO mice treated with saline (Fig. 6D). Treatment of WT mice with GBX markedly decreased locomotor activity (P < 0.02). In contrast, treatment of KO mice with GBX had no significant effect on activity. Thus, deletion of the α4 subunit of the GABAA-R eliminated the sedative effects of GBX as determined in the open-field assay.
Discussion
The GABAA-R α4 subunit has attracted a great deal of attention despite its limited abundance in the brain, partly because of its specific localization in the thalamus, dentate gyrus, and striatum. At the cellular level, we found that α4 KO mice were highly deficient in tonic inhibition in DGCs, where picrotoxin-sensitive tonic currents were reduced by ≈80% in the KO mice. This result is consistent with a decrease in the number of functional extrasynaptic GABAA-Rs on DGCs. The biological functions of these extrasynaptic receptors largely are unknown. We also observed a small but significant decrease in the frequency of mIPSCs in the KO mice and a highly significant slowing of mIPSC rise and decay τ1 times. The change in mIPSC kinetics is suggestive of a functional reorganization of synaptic GABAA-Rs in DGCs of the KO mice. Increases in synaptic α4, but not δ subunit-containing GABAA-Rs, results in faster mIPSC kinetics of DGCs (13). Therefore, the change in mIPSC kinetics of KO mice is consistent with the loss of a small quantity of synaptically localized α4γ2 isoforms, as observed in refs. 12 and 13, even if most synaptic GABAA-Rs are other subtypes. Of course, the lack of α4 could lead to other changes in GABAA-Rs.
The results also were striking in the recordings from neurons of the VB complex in the thalamus, where tonic inhibition was completely absent in the KO mice. These results are consistent with anatomical and pharmacological evidence, suggesting a role for the α4 subunit in generating tonic inhibition in these regions (10, 11). The lack of changes in mIPSC kinetics of thalamic neurons from KO mice suggests that α4 subunits do not contribute to synaptic currents in this region. Coimmunoprecipitation data also support the idea that the assembly and functions of the extrasynaptic GABAA-Rs that generate tonic inhibition require the presence of the α4 and δ subunits (11, 25). We also studied the actions of the novel hypnotic drug GBX in thalamic relay neurons of the VB complex, which are highly sensitive to this agonist (11). As we have found previously, concentrations of GBX (100–300 nM) that are behaviorally relevant (Bjarke Ebert, personal communication) elicited a reproducible and concentration-dependent current inward response in these neurons, which was completely absent in the neurons from the KO mice. Higher concentrations of GBX (1 μM) produced inward currents in the WT and KO mice (data not shown). This result shows that the extrasynaptic receptor population, which is highly sensitive to low concentrations of GBX, is essentially absent from the VB neurons in the KO mice. This result underlines the selectivity of GBX for GABAA-Rs that contain the α4 (or α6) and δ subunits (26) over GABAA-Rs that contain the α1 and γ2 subunits (11).
We observed that KO mice were largely insensitive to the behavioral effects of GBX. In the open-field assay, WT mice showed a decrease in locomotor activity after i.p. treatment with 10 mg/kg GBX, presumably reflecting sedative and/or motor impairing effects of the drug. This inhibitory effect of GBX was eliminated in KO mice. The performance of WT mice on the rotarod also was very sensitive to impairment by GBX, but KO mice essentially were insensitive to this effect of GBX, although a modest inhibitory effect remained at the highest dose tested (15 mg/kg). These two tests demonstrate that the sedative and ataxic effects of GBX highly depend on the presence of GABAA-Rs containing the α4 subunit.
The insensitivity to the ataxic effects of GBX is interesting and, perhaps, surprising at first glance, in the sense that performance on the rotarod is very sensitive to drugs that influence cerebellar activity. GBX is known to act as a potent agonist of GABAA-Rs that contain the α4 or α6 subunits in combination with the δ subunit (26). Of these subunits, α6 is highly expressed in the cerebellum but α4 is not (9). If GABAA-Rs containing the α4 subunit are important for the ataxia produced by GBX, then these receptors must be located elsewhere, with the receptor populations in the striatum, thalamus, and motor cortex being the most obvious possibilities. Future studies using tissue-specific KO of the α4 subunit should be especially informative in this regard.
GBX is also known to possess analgesic activity (27, 28), and this effect also was seen in our experiments by using the radiant tail-flick assay. Once again, the analgesic effect of the drug was reduced significantly/eliminated in the KO mice. These experiments indicate that the analgesic properties of GBX also are mediated by GABAA-Rs containing the α4 subunit. The location of the α4 containing GABAA-Rs responsible for this effect is unknown, although the receptor population in the thalamus is one possibility. It should be pointed out that the tail-flick assay potentially could be confounded by motor effects of GBX, so this phenomenon will have to be studied further in these mice by using additional tests.
The cellular and behavioral results presented here, along with studies of GBX in δ KO mice (29, 30), indicate that α4βδ GABAA-Rs form extrasynaptic receptors that mediate a tonic current that is highly sensitive to potentiation by GBX, and potentiation of this tonic current by GBX is responsible for the behavioral effects of this drug. Potentiation of GABAA-R tonic currents in various brain regions that are mediated by distinct extrasynaptic receptor isoforms appears to be a common mechanism of action that is shared with sedative-hypnotic drugs, including the anesthetics isoflurane (31) and etomidate (10, 32).
GBX is known to shorten the latency to sleep and to enhance the quality of sleep in man (33, 34) and to produce sedation, the loss of motor coordination, and the loss of righting reflex in rodents (29, 35), but the mechanism by which these effects occur is unknown. Relay neurons within the thalamus are known to show alterations in firing patterns during the transitions between the awake and the sleeping states (36, 37), and others have shown the ability of GBX to hyperpolarize these neurons (24). Our analysis showed that the GABA antagonist SR95531 caused a small depolarization (of ≈2 mV) of VB neurons from WT mice, consistent with the blockade of the hyperpolarizing influence of ambient GABA within the slice. At the same time, SR95531 increased the input resistance of the neurons by ≈10%. These effects of SR95531 were absent in the KO animals. In contrast, GBX hyperpolarized VB neurons from WT mice by 2 mV at 100 nM and by 5 mV at 300 nM, decreasing input resistance by ≈10% and 25%, respectively. GBX (100–300 nM) had no effect on membrane potential or input resistance in the KO mice.
The data reported here in our studies of the KO mice therefore show a strong association between the sedative and motor effects of GBX and the ability of the drug to hyperpolarize thalamic relay neurons. One possibility is that GBX is able to hyperpolarize these neurons sufficiently to induce the transition between silent or tonic firing modes and the burst firing mode that is implicated in the onset of slow-wave sleep (38).
The GABAA-R α4 KO mice we have produced and characterized should be useful in a variety of experimental settings. There is substantial plasticity in the expression of the GABAA-R α4 subunit, and this plasticity has been linked to functional changes in inhibitory synaptic activity (39, 40). In this respect, the α4 subunit is especially intriguing, because it shows plasticity in a variety of experimental and pathophysiological situations. For example, α4 expression is markedly altered by electroshock (41), alcohol exposure/withdrawal (13, 42–44), steroid withdrawal (45, 46), social isolation (47), and epilepsy (48, 49).
When a specific subunit undergoes dramatic changes in vivo, the effects on its normal subunit partners are also very interesting, e.g., α4 is reduced and γ2 is increased in δ KO mice in areas where δ is normally found (23, 50–52), and the δ peptide is totally lost in the cerebellum in the α6 KO (53). The changes in subunit expression associated with these different experimental manipulations remain phenomenological, although provocative. It is not clear, for example, whether the plasticity in the α4 subunit is a cause of, or a consequence of, the neuronal hyperexcitability associated with each model syndrome. However, important clues suggesting the former have been provided by using antisense α4 mRNA (45). The GABAA-R α4 KO mice reported here should be invaluable in establishing the role of α4 plasticity in these conditions.
The α4 subunit has a unique pharmacology in that α4βγ2 subtypes are insensitive to classical benzodiazepine agonists, and receptor function is enhanced, rather than inhibited, by antagonists and inverse agonists (54). Likewise, the α4βδ subtypes are modulated by nonbenzodiazepine GABAergic drugs like steroids, anesthetics, and ethanol (18, 20, 21), for example, the δ KO is less sensitive to steroids (50). Further analysis of the behavioral and cellular pharmacology of these mice should provide additional valuable insights into the roles of α4βδ GABAA-Rs.
Materials and Methods
Generation of α4 KO Mice.
Please see Supporting Text for methods used to create α4 KO mice. All KO (homozygous for the Cre recombined allele) and WT (homozygous for the WT allele) littermates used were age-matched and experimenters were blind to genotype.
Western Blot.
Membrane preparation and Western blots were carried out as described in ref. 44. Blots were probed with antiserum to the peptide sequence 379–421 of the α4 subunit (16) (1 μg/ml final concentration), followed by HRP-conjugated anti-rabbit secondary antibody and incubation with an ECL Plus detection system (Amersham Biosciences, Piscataway, NJ). Blots also were probed with a β-actin antibody (Sigma, St. Louis, MO) to confirm equal loading of protein.
Immunohistochemistry.
The same α4-specific antiserum used for Western blots was used for immunohistochemical labeling (16). The specificity of this antiserum has been demonstrated in refs. 9, 23, 55, and 56. Methods for tissue preparation and immunohistochemical processing have been described in detail in ref. 23.
Hippocampal Slice Electrophysiology.
Transverse slices (400-μm-thick) of mouse dorsal hippocampus were obtained by using standard techniques in ref. 57. Tetrodotoxin, ionotropic glutamate, and GABAB receptor blockers were present throughout. Detailed methods for recording and analysis of GABAA-R currents have been described in refs. 13 and 57.
Thalamic Slice Electrophysiology.
Postnatal day 28–40 mice were anesthetized with isoflurane, and horizontal thalamic slices were prepared. During whole-cell voltage-clamp recordings, membrane voltage was clamped at −65 mV, and GABAA-R currents were isolated by bath application of kynurenic acid (3–5 mM). The intracellular solution for voltage-clamp recording contained 140 mM CsCl, 4 mM NaCl, 1 mM MgCl2, 10 mM Hepes, 0.05 mM EGTA, 2 mM ATP-Mg, and 0.4 mM GTP-Mg. The intracellular solution for current-clamp recordings contained 130 mM K-gluconate, 5 mM NaCl, 2 mM MgCl2, 10 mM Hepes, 0.5 mM EGTA, 2 mM ATP-K, and 0.3 mM GTP-Na. The holding current (or membrane potential) shift was measured as the difference in the holding current (or membrane potential) before and during drug application. Spontaneous IPSCs were detected and analyzed as described in ref. 11. Averaged data are expressed as mean ± SE. Data were analyzed by using Student's t test.
Rotarod.
The Ugo Basile 7650 (Varese, Italy) apparatus rotating at a fixed speed of 6 rpm was used for all ataxia experiments. Mice (8–12 weeks of age) were acclimated to the apparatus by placing them on the rotarod 1–3 times on the day before each drug-induced ataxia experiment. Mice capable of walking on the rotarod for 180 sec were used for drug-ataxia experiments. Mice were evaluated once again before drug injection. Mice were injected with 10 mg/kg GBX (i.p.; THIP hydrochloride; Sigma) and then placed on the rotarod every 15 min after injection up to 90 min. The time a mouse was able to stay on the rotarod was recorded. The experiment was repeated on the same mice the following week with 15 mg/kg GBX, and the week after that with 2 mg/kg Flunitrazepam (i.p.; Sigma). Data were analyzed by two-way ANOVA.
Tail-Flick Assay.
A radiant tail-flick assay was used as described in refs. 58 and 59. Briefly, mice were lightly restrained and tested on a tail-flick analgesia meter (IITC Life Sciences, Woodland Hills, CA). Mice were tested for basal nociception, and 1 day later, mice were injected with GBX (10 mg/kg, i.p.) and tested for latency to tail flick 30 min after injection. Data were analyzed by using a paired Student's t test.
Open-Field Assay.
The sedative effect of GBX was determined in 8- to 10-week-old mice that were injected with normal saline or 10 mg/kg GBX. Twenty minutes after injection, mice were placed into a walled arena (43.2 cm × 43.2 cm × 30.5 cm) for 6 min. Distance traveled (centimeters) was measured automatically (Med Associates, St. Albans, VT). Data were analyzed by using two-way ANOVA and Fisher's post hoc test.
Supporting Information.
For more details, see Fig. 7, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Carolyn Ferguson and Edward Mallick for expert technical assistance, Dr. Werner Sieghart (University of Vienna, Vienna, Austria) for generously donating α4 antiserum, and Dr. Robert Messing for helpful suggestions and for sharing unpublished data. This work was supported by the National Institutes of Health Grants DE14184, AA07680, NS35985, AA13646, AA13004, and GM45129.
Abbreviations
- DGCs
dentate granule cells
- GABAA-R
GABAA receptor
- GBX
gaboxadol
- KO
knockout
- mIPSC
miniature inhibitory postsynaptic current
- VB
ventrobasal.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mody I, Pearce RA. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Farrant M, Nusser Z. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 3.Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- 4.Brickley SG, Cull-Candy SG, Farrant M. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nusser Z, Mody I. J Neurophys. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 6.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 7.Wisden W, Laurie DJ, Monyer H, Seeburg PH. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan ZU, Gutierrez A, Mehta AK, Miralles CP, de Blas AL. Neuropharmacology. 1996;35:1315–1322. doi: 10.1016/s0028-3908(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 9.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 10.Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 12.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusser Z, Sieghart W, Somogyi P. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bencsits E, Ebert V, Tretter V, Sieghart W. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Faria LC, Mody I. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallner M, Hanchar HJ, Olsen RW. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohlfarth KM, Bianchi MT, Macdonald RL. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J, Cagetti E, Olsen RW, Spigelman I. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- 23.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 24.Cope DW, Hughes SW, Crunelli V. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 26.Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- 27.Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B. Biochem Pharmacol. 2004;68:1573–1580. doi: 10.1016/j.bcp.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Drasbek KR, Jensen K. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- 29.Boehm SL II, Homanics GE, Blednov YA, Harris RA. Eur J Pharmacol. 2006;541:158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 30.Maguire JL, Stell BM, Rafizadeh M, Mody I. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 31.Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HTJ, Taverna FA, Roder JC, MacDonald JF, Bhambri A, Collinson N, et al. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathias S, Zihl J, Steiger A, Lancel M. Neuropsychopharmacology. 2005;30:833–841. doi: 10.1038/sj.npp.1300641. [DOI] [PubMed] [Google Scholar]
- 34.Faulhaber J, Steiger A, Lancel M. Psychopharmacology. 1997;130:285–291. doi: 10.1007/s002130050241. [DOI] [PubMed] [Google Scholar]
- 35.Cheng SC, Brunner EA. Anesthesiology. 1985;63:147–151. doi: 10.1097/00000542-198508000-00005. [DOI] [PubMed] [Google Scholar]
- 36.McCormick DA. Int Rev Neurobiol. 2002;49:99–114. doi: 10.1016/s0074-7742(02)49009-5. [DOI] [PubMed] [Google Scholar]
- 37.Jones EG. Philos Trans R Soc London B. 2002;357:1659–1673. doi: 10.1098/rstb.2002.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llinas RR, Steriade M. J Neurophys. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 39.Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 40.Poisbeau P, Williams SR, Mody I. J Neurosci. 1997;17:3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark M. Neurosci Lett. 1998;250:17–20. doi: 10.1016/s0304-3940(98)00422-4. [DOI] [PubMed] [Google Scholar]
- 42.Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- 43.Devaud LL, Fritschy JM, Sieghart W, Morrow AL. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- 44.Cagetti E, Liang J, Spigelman I, Olsen RW. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 45.Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 46.Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. J Neurosci. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serra M, Sanna E, Mostallino MC, Biggio G. Eur Neuropsychopharm. 2006 Apr 18; doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee PK, Tillakaratne NJ, Brailowsky S, Olsen RW, Tobin AJ, Snead OC., 3rd Expt Neurol. 1998;154:213–223. doi: 10.1006/exnr.1998.6928. [DOI] [PubMed] [Google Scholar]
- 50.Mihalek RM, Banjeree PK, Korpi E, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras S, et al. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tretter V, Hauer B, Nusser Z, Mihalek RM, Hoger H, Homanics GE, Somogyi P, Sieghart W. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- 52.Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Lüddens H. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- 53.Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, et al. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korpi ER, Grunder G, Luddens H. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 55.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 57.Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. J Neurophys. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- 58.Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- 59.Gatch MB, Selvig M. Alcohol Alcohol. 2002;37:313–317. doi: 10.1093/alcalc/37.4.313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.