Abstract
Apoptosis is a cell-suicide process that appears to play a central role not only during normal neuronal development but also in several neuropathological disease states. An important component of this process is a proteolytic cascade involving a family of cysteine proteases called caspases. Caspase inhibitors have been demonstrated to be effective in inhibiting neuronal cell death in various apoptotic paradigms. We have created transgenic mice that neuronally express the baculoviral caspase inhibitor p35. Neuronal expression of the p35 protein was found to confer functional caspase inhibitory activity and prevent apoptosis in isolated cerebellar granular cultures induced to undergo apoptosis either via staurosporine treatment or through withdrawal of extracellular potassium. Neuronal expression of p35 was also found to attenuate neurodegeneration associated with the excitotoxic glutamate analogue kainic acid (KA) in vitro and in vivo. Organotypic hippocampal cultures isolated from p35 transgenics demonstrated lowered caspase activity and decreased apoptosis compared with wild type when exposed to KA. In vivo injection of KA also produced decreased caspase activity and cell death in p35 transgenics vs. wild type. These results suggest that the presence of p35 in neurons in vivo is protective against various types of apoptosis, including seizure-related neurodegeneration, and that caspases may be attractive potential targets for preventing neuronal injury associated with diseases such as epilepsy. These mice also provide a valuable tool for exploring the role of caspases in other neuropathological conditions in which apoptosis has been implicated.
Apoptosis is a highly ordered, morphologically distinct process of cell death involving the activation of a family of cysteine proteases called caspases (1). Caspases were first implicated in apoptosis by the discovery of the Caenorhabditis elegans ced-3 gene (2). Since then, a large family of these caspases has been described in a wide variety of organisms. Caspases are expressed as proenzymes and are activated during apoptosis either by autocatalytic cleavage or via other caspases (3).
Much interest in the process of neuronal apoptosis has been generated recently because of a growing body of evidence suggesting that inappropriate apoptosis may contribute to the pathology associated with several neurological disorders (4–6). In several instances, inhibition of caspases has been shown to functionally rescue neurons from death. After permanent focal ischemia, for example, transgenic mice expressing a dominant-negative form of caspase-1 display significantly reduced brain injury and behavioral deficits (7). The presence of this transgene also delays the appearance of symptoms and increases survival rates in mouse models of both amyotrophic lateral sclerosis and Huntington's disease (8, 9).
To further explore the role of caspases in various neuropathological processes, we have created transgenic mice that neuronally express the baculoviral caspase inhibitor protein, p35. Expression of p35 prevents blindness in Drosophila mutants that undergo retinal degeneration (10). Recent crystallographic analysis of the p35 protein has confirmed that it acts as an irreversible or slowly reversible suicide inhibitor of activated caspases (11). p35 has been shown to block apoptosis in several different species (12, 13). We report here that p35 expression in neurons prevents apoptosis induced by various agents in different neuronal populations, including that in a toxin-induced model of epilepsy.
Materials and Methods
Creation of Transgenic Mice.
A 1.2-kb XbaI fragment containing the p35 gene was inserted into plasmid containing upstream rat neuron-specific enolase (NSE) promoter sequence (14). A 3.5-kb DNA fragment containing the p35 gene, along with upstream NSE promoter and downstream simian virus 40 splice and polyadenylation sequences, was released by BamHI digestion and microinjected into fertilized oocytes from B6C3F1 mice to create p35 transgenic founder animals. Mice were fed ad libitum, kept on a 12-h light/dark cycle, and maintained in a pathogen-free environment. Genotyping was done by Southern blot analysis of genomic tail DNA by using an XbaI p35 DNA fragment as probe. Animals found to be positive for the presence of the transgene were bred out to create transgenic lines. Northern blot analysis was performed on these lines by using RNA isolated from the whole brain of adult mice. Nontransgenic littermates were used as controls.
In Situ Hybridization.
Brains dissected from adult mice (3–5 months) were cryoprotected in 20% sucrose followed by freezing on dry ice. Cryostat sections (20 μm) were mounted on polylysine-coated slides and hybridized with 35S-labeled single-stranded RNA antisense probe prepared from plasmids containing p35 DNA by using the Riboprobe system-T7 according to the manufacturer's directions (Promega). Slides were coated with Kodak NTB-2 emulsion, exposed at 4°C for 5 weeks, developed in Kodak D-19, and counterstained with cresyl violet.
Immunohistochemistry.
Adult mice were anesthetized with Avertin and perfused with 4% paraformaldehyde, and the brains were dissected out and frozen on dry ice. Sagittal sections (20 μm) were used to perform immunocytochemistry with polyclonal antibody against p35 (a gift of Lois Miller, University of Georgia, Athens) at a dilution of 1:2000. Signal was amplified by using a Vectastain kit according to the manufacturer's directions (Vector Laboratories).
Preparation and Treatment of Cerebellar Granular Cultures (CGCs).
Primary neuronal cultures of CGCs were prepared from 5–7-day-old pups (15, 16). After trypsin digestion and mechanical dissociation, cells were plated in standard medium (Eagle's basal medium/10% FCS/25 mM KCl/2 mM glutamine/penicillin/streptomycin; GIBCO) on 12-well plates (Corning) coated with poly-l-lysine. After 24 h at 37°C in 5% CO2, 10 μM cytosine-β-d-arabinofuranoside was added and incubation continued for 6 more days. For potassium-deprivation experiments, after 6–8 days, CGCs were washed and switched to serum-free Eagle's basal medium containing 5 mM KCl. For staurosporine treatment, a final concentration of 0.5 μM staurosporine was added directly to cultures maintained in serum-free medium containing 25 mM KCl.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Staining.
Twelve hours after treatments, CGCs were fixed with 4% paraformaldehyde, and treated with 20 μg/ml proteinase K for 10 min, and then 1× terminal deoxynucleotidyl transferase buffer followed by 20 μg/ml terminal deoxynucleotidyl transferase enzyme (GIBCO) in the presence of peroxidase-conjugated dUTP at 37°C. The cells were washed and DNA fragmentation visualized according to the manufacturer's directions by staining with 0.25% diaminobenzidine/0.075% H2O2.
Measurement of Caspase Activities.
Caspase-3 activity was measured with a Flourace apopain assay kit (Bio-Rad) by using either the caspase-3-specific substrate acetylated DEVD aminofluororoumarin (AFC) or IETD-AFC for caspase-8 (Bachem). Cells or tissues were lysed in 100 μl of lysis buffer (10 mM Hepes⋅KOH (pH 7.2)/2.0 mM EDTA/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/5 mM DTT/1 mM phenylmethylsulfonyl fluoride/10 μg/ml pepstatin A/10 μg/ml aprotinin/20 μg/ml leupeptin) and then vortexed gently and freeze-thawed four to five times. Lysates were centrifuged at 13,000 × g for 30 min and the supernatants collected. Protein concentrations were estimated by using the Bradford reagent (Bio-Rad). Supernatant aliquots were incubated with the fluorescent substrates at 37°C for 1 h. Free AFC accumulation resulting from cleavage of the aspartate-AFC bond was measured by using a cytfluor II fluorometer at 360 nm excitation and 515 nm emission wavelengths. Serial dilutions of AFC were used as standards.
Kainic Acid (KA) Treatment of Hippocampal Slice Cultures and Propidium Iodide (PI) Staining.
Hippocampal slice cultures were prepared from 8- to 12-day-old mouse pups (17). Slices (400 μm) were placed on 25-mm millicell filters (Millipore) in 6-well tissue culture plates in 1 ml of growth medium (50% MEM/25% Hanks' balanced salt solution/25% horse serum; GIBCO). Cultures were maintained for 8–12 days at 36°C and 5% CO2 in a humidified incubator before KA treatment. After KA was added to cultures, slices were incubated for 3 h, washed with sera-free media, and incubated with 0.5 μg/ml PI overnight. Cultures were observed by using a Zeiss fluorescence microscope.
Lactate Dehydrogenase (LDH) Assay.
Slice cultures were treated with KA for various times. LDH was measured in sera-free medium from the culture plates (16, 18). Samples of media (0.5–0.8 ml) were added to 2.3 μM sodium pyruvate and 0.2 mg of NADH in 0.1 M KPO4 (pH 7.5) at 25°C; the total volume was 3 ml. The absorbance of this reaction was measured at 340 nm at 20-s intervals. Concentrations of LDH were expressed in units/ml where one unit is the amount of LDH that will decrease the absorbance by 0.001/min.
In Vivo Treatment With KA and TUNEL Staining.
KA dissolved in PBS was injected i.p. into 8- to 12-week-old mice at a dosage of 38 mg/kg body weight. This dose was sufficient to produce seizures in a majority of the animals. Each mouse was observed continuously for 3 h after KA administration, and the occurrence and duration of limb and generalized seizures were recorded. Seizure severity was rated according to an arbitrary scale: mild, very light seizures of forepaws only; medium, whole body seizures for a short time (3–6 min); and severe, prolonged whole-body seizures (8–20 min). Brains were obtained for histological analysis 24–72 h after KA treatment. Coronal sections through the hippocampus were taken from fresh frozen brains and then fixed for 30 min in 4% paraformaldehyde in PBS (pH 7.4). Sections were stained by TUNEL (19).
Statistical Analysis.
Results expressed as means ± SD difference between wild type and p35 transgenics were assessed by using ANOVA. Values of P < 0.01 were taken as being statistically significant.
Results
Creation and Characterization of p35 Transgenic Mice.
To determine whether neuronal expression of baculoviral p35 in vivo could inhibit apoptosis in various neuropathological paradigms, transgenic mice were produced by using a construct containing the p35 gene under the transcriptional control of the NSE promoter. Transgenic mice expressing lac-Z or bcl-2 genes under the control of this promoter have been well characterized; transgene expression in these animals was detected in all postmigratory postmitotic neurons (20, 21).
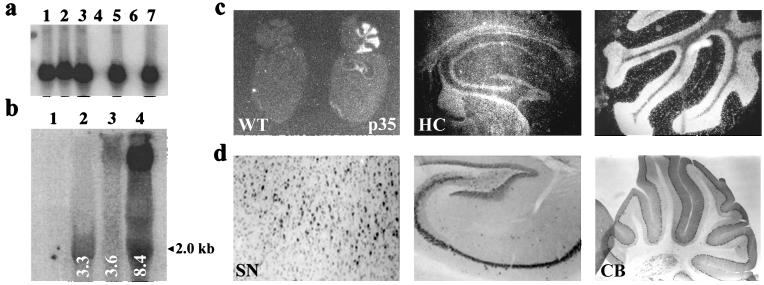
Southern blotting was used to genotype the NSE–p35 transgenic founder animals (Fig. 1a). Analysis by Northern blotting showed that three of the resulting transgenic lines expressed the expected 2.0-kb p35 transcript in the brain at varying levels with line 8.4 showing the highest levels of RNA expression (Fig. 1b). An additional band of unknown origin was also observed in lines 3.6 and 8.4, but this band was not present in line 3.3 or the wild type. Cellular distribution of p35 mRNA was examined via in situ hybridization in adult mouse brains (Fig. 1c). Expression of p35 mRNA was readily detectable throughout the brains of the transgenic mice with particularly high levels of expression in the hippocampus (including pyramidal neurons in the CA1–CA3 sectors and granular cells in the dentate gyrus) and in the cerebellum. The cellular distribution of the p35 protein as assessed by immunocytochemistry with a polyconal p35 antibody was found to mimic that of the p35 mRNA (Fig. 1d).
Figure 1.
(a) Southern blot analysis of genomic tail DNA from p35 transgenic (p35) vs. wild-type (WT) mice. DNA was digested with XbaI and hybridized to a 32P-labeled full-length p35 DNA probe. Lanes 1, 2, 3, 5, and 7, p35 transgenics; lanes 4 and 6, nontransgenics. (b) Northern blot of whole brain RNA from three lines of transgenics (3.3, 3.6, and 8.4) vs. wild-type mice; blots were hybridized with 35S-labeled full-length p35 riboprobe. Lanes 2–4, p35 transgenic lines; lane 1, wild-type. (Expected p35 transcript size = 2.0 kb). (c) Analysis of mRNA distribution in brains of transgenic mice by in situ hybridization of sagittal sections with a 35S-labeled riboprobe specific for p35. (d) Immunocytochemistry with polyclonal p35 antibody on sagittal sections of brains from p35 transgenics. HC, hippocampus; SN, substantia nigra; and CB, cerebellum.
pNSE–p35 mice display no overt phenotype and reproduce normally, and no gross alterations in histological features were noted in fixed brain tissue (data not shown). Brain weights at 10 weeks showed no significant difference between p35 transgenics and wild-type animals [wild type (n = 6) 450 mg ± 15 mg; p35 transgenics (n = 6) 435 mg ± 20 mg].
Inhibition of Apoptosis in CGCs Isolated From p35 Transgenic Mice.
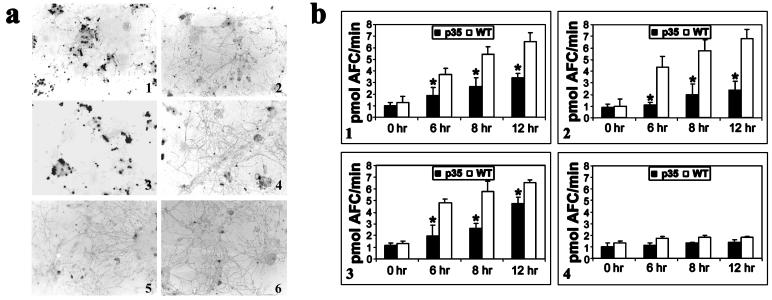
Dissociated CGCs from early postnatal mice undergo apoptosis after K+ deprivation by an unknown mechanism that seems to involve caspase-3 activation (15). These cultures can also be induced to undergo apoptosis when exposed to the nonselective protein kinase inhibitor staurosporine (16). Lowering extracellular K+ concentrations in the culture medium of 1-week-old CGCs isolated from wild-type animals resulted in a marked increase in apoptosis as visualized by TUNEL staining which was attenuated in cultures from the p35 transgenics (Fig. 2 a1 and a2). Treatment with staurosporine also induced a rapid apoptotic death in wild-type CGCs (Fig. 2a3). Morphological observation indicated that, particularly during staurosporine-induced apoptosis, neuronal cell bodies in wild-type CGCs were rounded up and the neurites appeared degenerated, taking on a beaded appearance. Apoptosis after staurosporine treatment was also attenuated in the p35 cultures (Fig. 2a4). TUNEL-positive cells were negligible in untreated cultures from both wild-type and p35 transgenic animals (Fig. 2 a5 and a6).
Figure 2.
(a) TUNEL staining of cells 12 h after treatment with staurosporine or growth in lowered extracellular K+. (1 and 3) CGCs isolated from wild-type animals 12 h after growth in low K+-containing medium (5 mM) or 0.5 μM staurosporine, respectively. (2 and 4) CGCs isolated from p35 transgenics after growth in either lowered extracellular K+ or staurosporine, respectively. (5 and 6) CGCs from untreated wild-type and p35 transgenics, respectively. (b) Time course of caspase-3 and -8 activities in cytosolic protein extracts isolated from wild-type (WT) vs. p35 transgenic (p35) CGCs after growth in either low K+ or staurosporine. (1 and 3) Caspase-3 activity assayed fluorometrically by the cleavage of acetylated DEVD-AFC substrate in extracts from CGCs induced to undergo apoptosis by lowered K+ or staurosporine, respectively. (2 and 4) Caspase-8 activity as measured by cleavage of fluorescent IETD-AFC substrate in extracts from CGCs treated with either lowered K+ or staurosporine, respectively. Values represent the means ± SD from three experiments. (*, P < 0.01.)
Downstream caspase-3 family (effector) caspases appear to be an essential and conserved component of the apoptotic machinery in many cell types (3) and have been suggested to be a key component in many types of neuronal apoptosis (15). We therefore analyzed the ability of staurosporine and lowered extracellular K+ concentration to trigger the activation of caspase-3-like activity in CGCs isolated from p35 transgenic vs. wild-type littermates. CGCs either treated with 0.5 μM staurosporine or grown in 5 mM K+ showed an induction in caspase-3-specific substrate cleavage by 6 h which peaked by 12 h (Fig. 2 b1 and b3). No further increases were observed even up to 24 h after treatment (data not shown). Neuronal expression of p35 was found to significantly lower caspase-3-like activity when apoptosis was induced by either of the two agents.
We also analyzed the activation of upstream (activator) caspase-8-like activity in both apoptotic paradigms. Caspase-8 activity was seen to greatly increase when apoptosis was induced by lowered K+ and its activity was inhibited in the presence of p35 (Fig. 2b2). When apoptosis was induced by staurosporine, caspase-8-like activity was not significantly induced (Fig. 2b4) (22).
Cultured Hippocampal Neurons From p35 Transgenic Mice Are Less Susceptible to KA-Mediated Excitotoxicity.
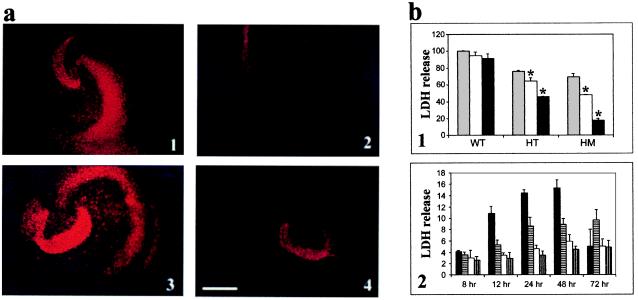
Systemic administration of the excitotoxic glutamate analogue KA produces recurrent epileptiform discharge and neuronal damage in distinct regions of the brain including the hippocampus reminiscent of that associated with human temporal lobe epilepsy (23, 24). Apoptosis seems to be involved in the damage elicited by KA (25); however, the distinct mechanism(s) by which this occurs are not fully understood. To examine the effects of caspase inhibition on KA-induced apoptosis, hippocampal slice cultures were prepared from both transgenic and wild-type littermates. Organotypic slice cultures were used because they provide a physiologically relevant model of the mature central nervous system as nerve cells continue to differentiate and develop a tissue organization that closely resembles that observed in situ (17). Exposure of cultures to 10 or 25 μM KA led to extensive cell death in cultures from wild-type mice as visualized by PI staining which was found to be attenuated in transgenic cultures (Fig. 3a). PI is a fluorescent probe that enters cells when the membrane is disrupted and therefore only labels the nuclei of dead or disintegrating cells (26). The effect of KA appears to be concentration dependent as more damage was observed at the higher dosage, i.e., 25 μM, in the wild-type cultures (Fig. 3 a3 vs. a1). The transgenic cultures displayed significantly less cell death than cultures from wild-type littermates at both the dosages examined (Fig. 3 a2 and a4).
Figure 3.
(a) PI-stained cultures 24 h after treatment with KA. Cultures from wild type (1 and 3) and p35 transgenics (2 and 4) after treatment with either 10 or 25 μM KA, respectively. (Bar = 500 μm.) (b) Cell viability of p35 transgenic vs. wild-type control cultures after treatment with KA as measured by LDH release. Values represent the means ± SD from three experiments. (*, P < 0.01.) (1) LDH efflux 24 h after KA treatment; values are reported as % wild type treated with 40 μM KA. WT, wild type; HT, p35 heterozygote; and HM, p35 homozygote. Shaded bars, 40 μM KA; open bars, 20 μM KA; filled bars, 10 μM KA. (2) Time course of LDH release after treatment with 25 μM KA; LDH is reported as units/min. Filled bars, KA-treated wild type; horizontal striped bars, KA-treated p35 transgenic; open bars, untreated wild type; and vertical striped bars, untreated transgenic.
The decrease in KA susceptibility in the p35 hippocampal slice cultures vs. controls as visualized by PI staining was supported by LDH cell viability assay data. In the setting of a neuronal-specific injury paradigm such as kainate-induced toxicity, LDH efflux has been shown to serve as a reliable in vitro indicator of cell lysis and death (18). Cultures from transgenic mice demonstrated markedly increased viability after KA treatment as indicated by decreases in levels of LDH efflux (Fig. 3b). Cultures from homozygous p35 transgenics were more resistant to KA toxicity when compared with cultures from heterozygous p35 mice, suggesting a protective gene dosage effect (Fig. 3b1). In addition, after an initial increase, there was no significant increase in LDH efflux in culture medium isolated from the p35 cultures between 24 and 72 h after treatment with KA (Fig. 3b2). In the control cultures, however, the increase in LDH efflux increased sharply up to 48 h after KA treatment. After this time, visual evidence of extensive cell loss was observed in the wild-type cultures, and by 72 h, the amount of LDH efflux (from the few remaining cells) dramatically decreased. This demonstrates that inhibition of caspases in the p35 cultures provided protection even up to 72 h after KA treatment, at a time when cells in the control cultures were undergoing extensive cell death. Because control cultures did not survive longer than 72 h in serum-free medium, experiments could not be performed for longer periods of time.
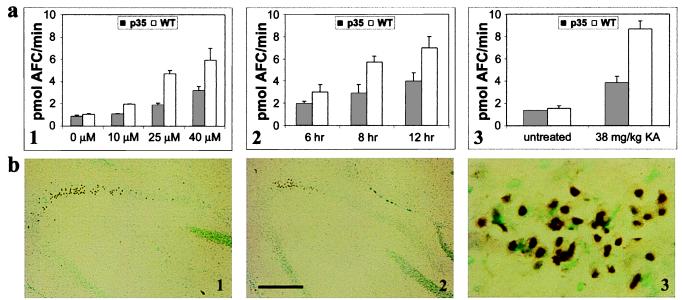
Caspase-3-like activity was analyzed in slice cultures isolated from transgenic vs. wild-type animals after treatment with KA (Fig. 4a). Caspase-3 activation was found to be dose dependent in the wild-type cultures and inhibited in the p35 transgenic cultures at all dosages examined (Fig. 4a1). In wild-type cultures, activity was found to peak by 12 h after treatment with KA and to be attenuated in the p35 cultures (Fig. 4a2; 24-h data not shown). Activation of caspase-3 preceded the increase in LDH efflux because its activity was significantly induced by 6 h, whereas the release of LDH was not observed until at least 12 h following KA treatment.
Figure 4.
(a) Caspase-3 activity of cytosolic protein extracts from hippocampal slice cultures or dissected hippocampi after treatment with KA. Cleavage of acetylated DEVD-AFC substrate was assayed fluorometrically by measuring the accumulation of free AFC. (1) Caspase-3 activity in slice cultures isolated from p35 transgenic (p35) vs. wild type (WT) 12 h after treatment with 0, 10, 25, and 40 μM KA. (2) Caspase-3-like activity in slice cultures from p35 vs. WT at 6, 8, and 12 h after treatment with 25 μM KA. (3) Caspase-3-like activity in hippocampi isolated from p35 vs. WT 8 h after i.p. injection of KA. (b) TUNEL staining in the hippocampal CA3 region 72 h after i.p. injection of 38 mg/kg KA. TUNEL-positive cells in the hippocampal CA3 region of WT (1 and 3) and p35 animals (2). (Bar = 250 μm.) (3) Magnification (×10) of 1.
Damage Caused by Systemic Administration of KA Was Decreased in p35 Transgenics.
To test whether p35 is capable of protecting against systemic administration of KA, we examined the effects of i.p. injection of the toxin in p35 transgenics vs. controls. Seizures began within 60 min after KA administration and increased in severity over the next 2 h as the animals entered status epilepticus. At the dosage of KA used, both the wild-type and p35 transgenic mice had a similar degree and pattern of seizure (Table 1). There were no significant differences in the seizure grade or the rate of mortality observed in the p35 transgenics vs. controls.
Table 1.
Seizure severity after i.p. injection of KA in p35 transgenic and wild-type mice
| Seizure grade
|
|||
|---|---|---|---|
| Mild | Medium | Severe | |
| p35 (n = 26) | 9 (34.6%) | 5 (19.2%) | 12 (46.1%) |
| Wild type (n = 21) | 7 (33.3%) | 5 (23.8%) | 9 (42.8%) |
A significant degree of toxin-induced hippocampal neuronal cell damage was observed after KA administration in the nontransgenic mice which was greatly reduced in the brains of the p35 transgenics. TUNEL-positive cells were first observed in pyramidal neurons of the hippocampal CA3 sector 24 h after KA treatment and by 72 h; staining was maximal in these cells in the wild-type animals (Fig. 4b1). TUNEL-positive neurons in the CA3 had densely labeled nuclei and displayed the presence of nearby small particles resembling apoptotic bodies (Fig. 4b3). TUNEL labeling was also seen in the amygdala, thalamus, and hypothalamus (data not shown). In contrast, the brains of the p35 transgenic mice showed significantly fewer TUNEL-positive cells (Fig. 4b2). Consistent with the TUNEL results, neuronal expression of the p35 gene also resulted in an inhibition of caspase-3-like activity in the hippocampus after treatment with KA (Fig. 4a3).
Discussion
Our report describes the construction of a genetic mouse model for use in exploring the effects of general caspase inhibition in neurons in various apoptotic paradigms via the neuronal expression of the baculoviral caspase inhibitor protein p35. Although the genetic elimination of specific caspases in various mouse lines has been reported to result in embryonic phenotypes (27, 28), the p35 transgenics appear to develop normally. The apparent lack of an embryonic phenotype may imply that p35 does not block developmental neuronal apoptosis. Alternatively, it may be that because the NSE promoter is active at low levels during embryogenesis, the expression of the p35 transgene may not reach a threshold sufficient to block developmental cell death (20). CGCs in vitro develop characteristics of mature cerebellar granule cells in vivo, including an extensive neurite network and expression of excitatory amino acid receptors (29). K+ deprivation-induced apoptosis is thought to mimic the developmental cell death which naturally occurs in 20–30% of all granule cells between postnatal weeks 3 and 5 (30). We found that both upstream (caspase-8) and downstream (caspase-3) caspases were activated in wild-type CGCs induced to undergo apoptosis by withdrawal of high concentrations of extracellular K+ and that this activation was reduced in cultures isolated from the p35 transgenics. These results suggest that the presence of p35 in neurons may be capable of attenuating developmental apoptosis to some degree in the cerebellum. A more systematic analysis of neuronal cell numbers during development in vivo is currently being performed on these animals to assess this issue.
When apoptosis in the CGCs was induced by staurosporine, downstream caspase-3 activity was induced in wild-type cultures whereas upstream caspase-8 activities were not. This result is in keeping with previous work showing that in staurosporine-induced apoptosis, caspase-8 does not appear to be activated whereas caspase-9 is (22). Caspase-3 activity was reduced in cerebellar granule cells isolated from p35 transgenics.
The difference in upstream vs. downstream caspase activation patterns in CGCs induced to undergo apoptosis by either staurosporine or lowered K+ levels suggests that these two processes occur by different mechanisms. p35 has been shown to act as a general caspase inhibitor (31). Our results demonstrate that the p35 transgenic model can be utilized in combination with various caspase-specific substrates to delineate the specific profiles of caspases involved in various neuronal apoptotic events. Such a model is superior to the application of pharmacological inhibitors that may not be easily useable in vivo, particularly in the brain. In addition, the gross embryonic phenotypes manifested in available caspase knockouts prohibit their use as models to explore the role of caspases in adult-onset neurological conditions (27, 28). In the dominant-negative caspase-1 transgenic line, the activities of other caspases have not been explored. Furthermore, under normal biological conditions, caspase-1 is generally considered to be more involved in cytokine processing rather than in apoptotic-related events (32).
Interestingly, neuronal expression of p35 also appeared to decrease susceptibility of neurons to KA-induced cell death both in vitro and in vivo. Previous studies have shown that KA can induce cell death via apoptosis as demonstrated by biochemical evidence of DNA laddering and in situ nick translation in the mouse hippocampus and neocortex 24 and 48 h after i.p. injection at concentrations well known to provoke seizures (33). However, the molecular mechanisms involved are not well understood. An increase in caspase-3 mRNA levels in hippocampal CA3 neurons has been reported to occur in animals exposed to KA; however, neither caspase-3 protein nor activity levels were measured (34). In our studies, not only was the CA3 region of the hippocampus of wild-type animals the most vulnerable to KA-induced damage, but caspase-3 activity was significantly induced after KA administration. The vulnerability of the CA3 region to KA may be related to induction of caspase-3 levels or could also be caused by the high levels of expression of both high- and low-affinity KA receptors in this region (34, 35).
Although the degree of seizure did not vary between transgenic and wild-type animals, KA-induced neuronal damage was attenuated in p35 transgenic mice. Seizures after KA administration appear to occur as a consequence of N-methyl-d-aspartate receptor activation which leads to downstream events resulting in neuronal cell death (25), suggesting that caspase inhibition occurs downstream of N-methyl-d-aspartate receptor activation, or alternatively, is a parallel event not required for seizure activity. Hippocampal slice cultures from the p35 animals showed less damage than those from wild type even after 72 h after KA administration in addition to having lowered caspase-3-like activity, indicating that caspase inhibition may protect cells against KA-induced damage for prolonged periods of time.
The clear-cut gradient of vulnerability of neurons to KA mimics the pattern of cell death associated with human adult temporal lobe epilepsy (23, 24). Epilepsy is a chronic brain disorder characterized by recurrent seizures and cell loss predominantly in hippocampal pyramidal CA3 neurons. Additional damage is also seen in granules of fascia dentata, amygdala, and thalamic structures. Evidence suggesting a role for apoptosis in the neuronal damage associated with epilepsy has been reported (36). The kainate model has been used extensively to study the pathological consequences of epileptiform discharge associated with epilepsy because its pattern of seizure, cell damage, and response to anticonvulsants is similar to that seen in this condition (23, 24). Because the etiology and mechanism of selective cellular damage is not well understood in epilepsy, the use of animal models such as the p35 transgenics in combination with KA treatment are likely to be very useful in clarifying the role of caspases in this process. The fact that the p35 transgenic mice exhibit a resistance to kainate toxicity suggests that inhibition of caspases can attenuate cell death caused by the convulsant. This observation may have important therapeutic implications for the treatment of epilepsy. Caspase inhibition has also been demonstrated to ameliorate damage associated with ischemic models in which excitotoxicity has also been implicated to play a role such as during middle cerebral artery occlusion (37–39).
In conclusion, our studies using a novel p35 transgenic model suggest that caspase inhibitors may be beneficial in the treatment of seizure-related neuronal injury. In addition, this model may be useful for exploring the role of caspases in other neurodegenerative disorders in which apoptosis has been suggested to play a role as well as for determining whether caspase inhibition may be an effective therapeutic treatment for these diseases.
Acknowledgments
We thank Dale Bredesen (Buck Center for Research in Aging, Novato, CA) for the gift of the p35 clone and Lois Miller (University of Georgia, Athens) for providing us with p35 antibody. National Institutes of Health Grants AG12141 and AG09793 and a Brookdale National Fellowship and Grant (to J.K.A.) supported this work.
Abbreviations
- NSE
neuron-specific enolase
- CGC
cerebellar granular culture
- KA
kainic acid
- PI
propidium iodide
- LDH
lactate dehydrogenase
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- AFC
aminofluorocoumarin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030365297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030365297
References
- 1.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 2.Ellis H M, Horvitz H R. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 4.Su J H, Anderson A J, Cummings B J, Cotman C W. NeuroReport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Tompkins M M, Basgall E J, Zamrini E, Hill W D. Am J Pathol. 1997;150:119–131. [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez I, Chi-Jie X, Kakizak A, Blenis J, Yuan J. Neuron. 1999;22:623–633. doi: 10.1016/s0896-6273(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 7.Friedlander R M, Gagliardini V, Hara H, Fink K B, Li W, MacDonald G, Fishman M C, Greenberg A H, Moskowitz M A, Yuan J. J Exp Med. 1997;185:933–940. doi: 10.1084/jem.185.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ona V O, Li M, Vonsattel J P, Andrews L J, Khan S Q, Chung W M, Frey A S, Menon A S, Li X J, Stieg P E, et al. Nature (London) 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander R M, Brown R H, Gagliardini V, Wang J, Yuan J. Nature (London) 1997;388:31. doi: 10.1038/40299. [DOI] [PubMed] [Google Scholar]
- 10.Davidson F F, Steller H. Nature (London) 1998;391:587–591. doi: 10.1038/35385. [DOI] [PubMed] [Google Scholar]
- 11.Fisher A J, Cruz W D, Zoog J S, Scheneider L C, Friesen D P. EMBO J. 1999;18:2031–2039. doi: 10.1093/emboj/18.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay B A, Wolff T, Rubin G M. Development (Cambridge, UK) 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 13.Rabizadeh S, LaCount D J, Friesen P D, Bredesen D E. J Neurochem. 1993;61:2318–2321. doi: 10.1111/j.1471-4159.1993.tb07477.x. [DOI] [PubMed] [Google Scholar]
- 14.Andersen J K, Frim D M, Isacson O, Breakefield X O. Neurodegeneration. 1994;3:97–109. [Google Scholar]
- 15.Eldadah B A, Yakovlev A G, Faden A I. J Neurosci. 1997;17:6105–6113. doi: 10.1523/JNEUROSCI.17-16-06105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor J, Gatchalian C L, Keen G, Rubin L L. J Neurochem. 1997;68:1598–1605. doi: 10.1046/j.1471-4159.1997.68041598.x. [DOI] [PubMed] [Google Scholar]
- 17.Stoppini L, Buchs P A, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 18.Koh J Y, Choi D J. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 19.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forss-Petter S, Danielson P E, Catsicas S, Battebberg E, Price J, Nerenberg M, Sutcliff G J. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 21.Bernard R, Farlie P, Bernard O. Dev Neurosci. 1997;19:79–85. doi: 10.1159/000111188. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, MacFarlane M, Zhuang J, Wolf B B, Green D R, Cohen G M. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 23.Lothman E W, Collins R C. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Ari Y, Tremblay E, Otterson O P. Neuroscience (Basel) 1980;5:515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- 25.Simonian N A, Getz R L, Leveque J C, Konradi C, Coyle J T. Neuroscience. 1996;75:1047–1055. doi: 10.1016/0306-4522(96)00326-0. [DOI] [PubMed] [Google Scholar]
- 26.Malouf A T. Neurobiol Aging. 1992;13:543–551. doi: 10.1016/0197-4580(92)90054-2. [DOI] [PubMed] [Google Scholar]
- 27.Kuida K, Zheng S T, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R A. Nature (London) 1996;384:368–371. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 28.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 29.Miller T M, Johnson E M., Jr J Neurosci. 1996;16:7487–7495. doi: 10.1523/JNEUROSCI.16-23-07487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams R W, Herrup K. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Krebs J F, Snipas S J, Price A, Alnemri E S, Tomaselli K J, Salvesen S. Biochemistry. 1998;37:10757–10765. doi: 10.1021/bi980893w. [DOI] [PubMed] [Google Scholar]
- 32.Wolf B B, Green D R. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 33.Filipowski R K, Hetman M, Kaminska B, Kaczmarek L. NeuroReport. 1994;5:1538–1540. doi: 10.1097/00001756-199407000-00032. [DOI] [PubMed] [Google Scholar]
- 34.Becker A J, Gillardon F, Blumcke I, Langendorfer D, Beck H, Westler O D. Mol Brain Res. 1999;67:172–176. doi: 10.1016/s0169-328x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 35.Young A B, Fagg G E. Trends Pharmacol Sci. 1990;11:126–133. doi: 10.1016/0165-6147(90)90199-i. [DOI] [PubMed] [Google Scholar]
- 36.Charriaut-Marlangue C, Aggoun-Zouaoui D, Represa A, Ben-Ari Y. Trends Neurosci. 1996;19:109–114. doi: 10.1016/s0166-2236(96)80039-7. [DOI] [PubMed] [Google Scholar]
- 37.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli K J, Yuan J, Moskowitz M A. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara H, Fink K, Endres M, Friedlander R M, Gagliardini V, Yuan J, Moskowitz M A. J Cereb Blood Flow Metab. 1998;17:370–375. doi: 10.1097/00004647-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Hara H, Friedlander R M, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz M A. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]