Abstract
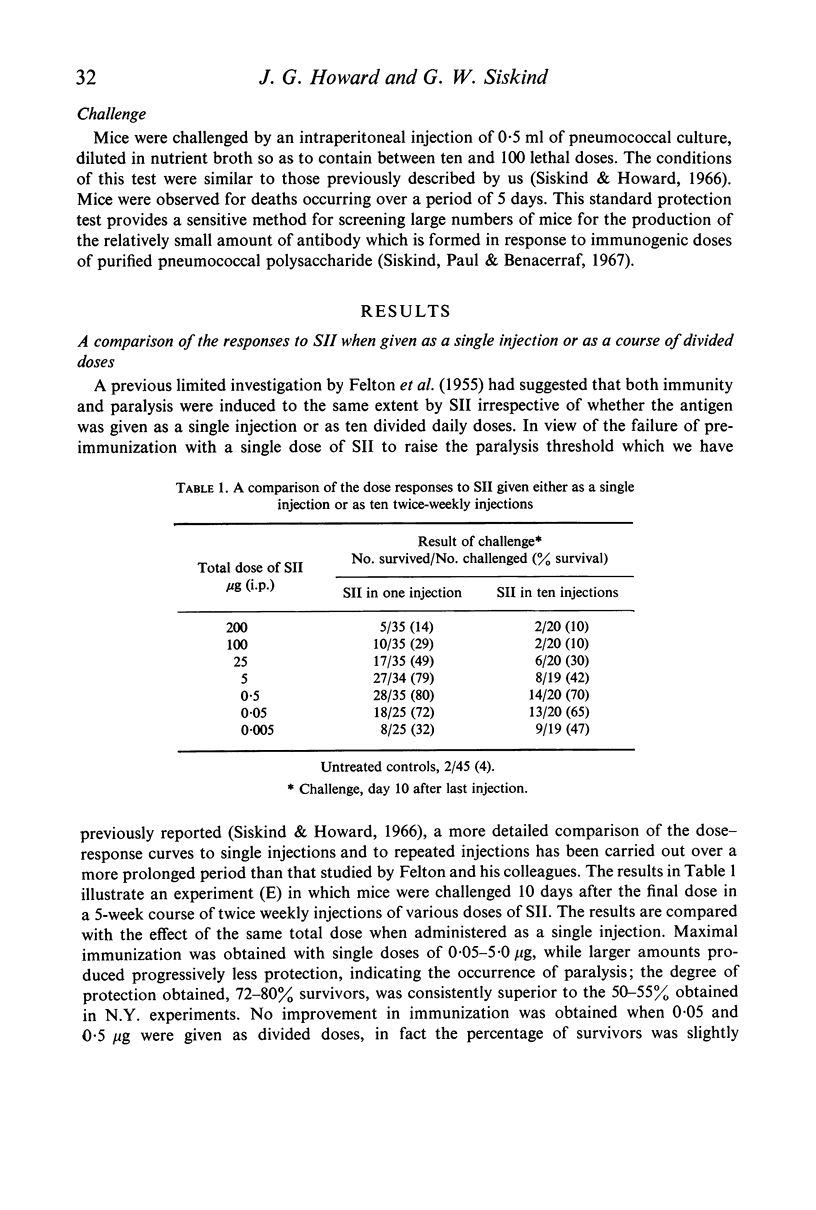
Induction of paralysis with SII in mice was slightly facilitated by administration in ten injections during 5 weeks as compared with the equivalent total dose in a single injection. No improvement was afforded by repeated injection with regard to the degree of immunization.
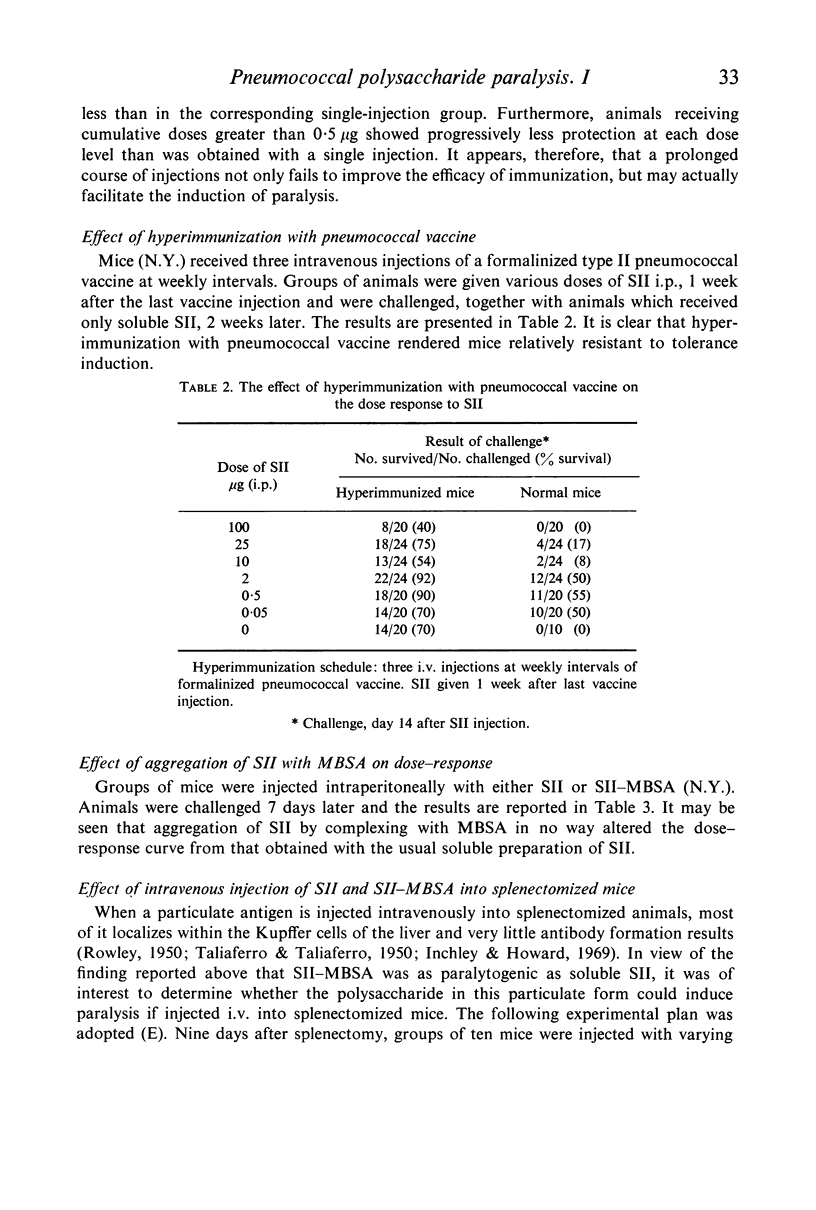
Mice which had been hyperimmunized with type II pneumococcal vaccine were relatively resistant to the induction of paralysis with SII, while animals which had been immunized with purified SII were as readily or more readily made tolerant than were normal control animals.
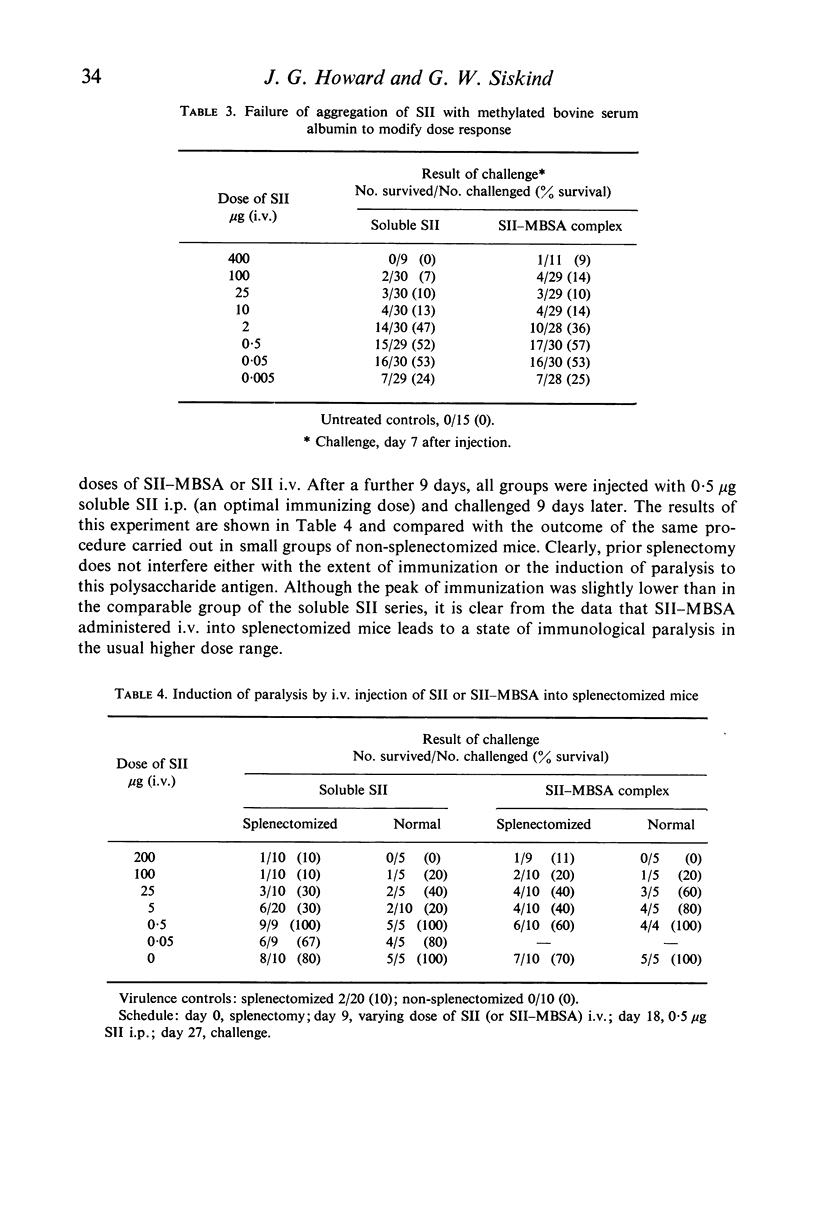
SII in particulate form (complexed with methylated bovine serum albumin) (SII–MBSA) was indistinguishable from soluble SII with regard to its immunogenicity and capacity to induce paralysis.
Paralysis could be induced by the intravenous injection of normally paralytogenic doses of either SII–MBSA or soluble SII into splenectomized mice. Such mice were no less readily paralysed than normal animals.
Polysaccharide still circulating in mice 10 days after injection of paralysing doses (100–500 μg) of SII was strongly immunogenic on transfer of serum to normal recipients.
Five hundred rad whole body irradiation 24 hr before injection of varying doses of SII failed to lower the paralysis threshold.
It is concluded that SII does not exist in distinct immunogenic (particulate) and paralytogenic (soluble) forms; that the macrophage appears to play no essential role in the initiation phase of an immune response to SII; and that the major portion of SII which is phagocytosed is ultimately involved in the induction of paralysis. The apparent anomaly of the latter feature could be explained by a continual release of phagocytosed SII from macrophages by exocytosis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTISTO J. R., MILLER J. Immunological unresponsiveness produced in adult guinea pigs by parenteral introduction of minute quantities of hapten or protein antigen. Proc Soc Exp Biol Med. 1962 Oct;111:111–115. doi: 10.3181/00379727-111-27717. [DOI] [PubMed] [Google Scholar]
- BROOKE M. S. BREAKING OF IMMUNOLOGICAL PARALYSIS BY INJECTION OF A SPECIFIC DEPOLYMERASE. Nature. 1964 Dec 26;204:1319–1320. doi: 10.1038/2041319a0. [DOI] [PubMed] [Google Scholar]
- Brooke M. S. A hemagglutination test and a specific depolymerase for studying immunologic paralysis in mice. J Immunol. 1966 Feb;96(2):358–363. [PubMed] [Google Scholar]
- CLAMAN H. N. TOLERANCE TO A PROTEIN ANTIGEN IN ADULT MICE AND THE EFFECT OF NONSPECIFIC FACTORS. J Immunol. 1963 Dec;91:833–839. [PubMed] [Google Scholar]
- Carpenter C. B., Gill T. J., 3rd, Mann L. T., Jr Synthetic polypeptide metabolism. 3. Degradation and organ localization of isomeric synthetic polypeptide antigens. J Immunol. 1967 Feb;98(2):236–250. [PubMed] [Google Scholar]
- DIXON F. J., MAURER P. H., WEIGLE W. O. Immunologic activity of pneumococcal polysaccharide fixed in the tissues of the mouse. J Immunol. 1955 Mar;74(3):188–191. [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- FELTON L. D., KAUFFMANN G., PRESCOTT B., OTTINGER B. Studies on the mechanism of the immunological paralysis induced in mice by pneumococcal polysaccharides. J Immunol. 1955 Jan;74(1):17–26. [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREI P. C., BENACERRAF B., THORBECKE G. J. PHAGOCYTOSIS OF THE ANTIGEN, A CRUCIAL STEP IN THE INDUCTION OF THE PRIMARY RESPONSE. Proc Natl Acad Sci U S A. 1965 Jan;53:20–23. doi: 10.1073/pnas.53.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Elson J., Christie G. H., Kinsky R. G. Studies on immunological paralysis. II. The detection and significance of antibod-forming cells in the spleen during immunological paralysis with type 3 pneumococcal polysaccharide. Clin Exp Immunol. 1969 Jan;4(1):41–53. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Humphrey J. H. Synthetic antigens composed exclusively of L- or D- amino acids. II. Effect of optical configuration on the metabolism and fate of synthetic polypeptide antigens in mice. Immunology. 1968 Feb;14(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Sela M. Synthetic antigens composed exclusively of L- or D-amino acids. I. Effect of optical configuration on the immunogenicity of synthetic polypeptides in mice. Immunology. 1967 Jul;13(1):29–38. [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The dosage requirements for immunological paralysis by soluble proteins. Immunology. 1968 Oct;15(4):509–530. [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- NEVEU T., BRANELLEC A., BIOZZI G. PROPRI'ET'ES ADJUVANTES DE CORYNEBACTERIUM PARVUM SUR LA PRODUCTION D'ANTICORPS ET SUR L'INDUCTION DE L'HYPERSENSIBILIT'E RETARD'EE ENVERS LES PROT'EINES CONJUGU'EES. Ann Inst Pasteur (Paris) 1964 May;106:771–777. [PubMed] [Google Scholar]
- PLESCIA O. J., BRAUN W., PALCZUK N. C. PRODUCTION OF ANTIBODIES TO DENATURED DEOXYRIBONUCLEIC ACID (DNA). Proc Natl Acad Sci U S A. 1964 Aug;52:279–285. doi: 10.1073/pnas.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckard R. N., Weir D. M., McBride W. H. Factors influencing the immune response. I. Effects of the physical state of the antigen and of lymphoreticular cell proliferation on the response to intravenous injection of bovine serum albumin in rabbits. Clin Exp Immunol. 1967 May;2(3):331–341. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- SISKIND G. W., PATERSON P. Y. A BIOASSAY FOR ESTIMATION OF PNEUMOCOCCAL POLYSACCHARIDE IN UNRESPONSIVE (PARALYZED) MICE. J Immunol. 1964 Sep;93:489–494. [PubMed] [Google Scholar]
- Siskind G. W., Howard J. G. Studies on the induction of immunological unresponsiveness to pneumococcal polysaccharide in mice. J Exp Med. 1966 Sep 1;124(3):417–429. doi: 10.1084/jem.124.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. The dynamics of hemolysin formation in intact and splenectomized rabbits. J Infect Dis. 1950 Jul-Aug;87(1):37–62. doi: 10.1093/infdis/87.1.37. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A. The immune response of mice to antigen in macrophages. Immunology. 1968 Aug;15(2):287–296. [PMC free article] [PubMed] [Google Scholar]


