Abstract
Songbirds (Oscines) learn their songs from a tutor. It is not known where in the brain the memories of these learned sounds are stored. Recent evidence suggests that song perception in songbirds involves neuronal activation in brain regions that have not traditionally been implicated in the control of song production or song learning, notably the caudal part of the neostriatum (NCM) and of the hyperstriatum ventrale. Zebra finch males (Taeniopygia guttata castanotis) were reared without their father and exposed to a tape-recorded song during the sensitive period for song learning. When, as adults, they were reexposed to the tutor song, the males showed increased expression of the protein products of the immediate early genes egr-1 (ZENK) and c-fos in the NCM and caudal hyperstriatum ventrale, but not in the conventional “song-control nuclei.” The strength of the immediate early gene response (which is a reflection of neuronal activation) in the NCM correlated significantly and positively with the number of song elements that the birds had copied from the tutor song. These results show localized neural activation in response to tutor song exposure that correlates with the strength of song learning.
Of all avian species, approximately half are songbirds (Passeriformes: Oscines). Bird song has an important function in communication between conspecifics (1). Songbirds learn their song from an adult tutor when they are young (2, 3). The study of the neural substrates of bird song learning has become a prominent model system for the investigation of the neural mechanisms of learning and memory (3–7). Until recently it was thought that two forebrain pathways connecting a number of “song-control nuclei” (Fig. 1a) comprise the neural substrate for bird song (3–6). The caudal pathway, including the high vocal center (HVC) and the robust nucleus of the archistriatum (RA), was considered to be involved in the production of song. Lesions to nuclei in this pathway, or to any of its connections result in immediate, profound, and irreversible deficits in song in adult birds (8). The rostral pathway, including HVC, the lateral part of the magnocellular nucleus of the neostriatum (lMAN), and Area X, was thought to play a role in song learning. This suggestion was supported by the finding that bilateral lesions to lMAN or Area X disrupt song acquisition, but have little effect on crystallized song in adults (9–11). However, despite the obvious involvement of these forebrain pathways in song, it is still not known where in the songbird brain learned sounds are stored (4).
Figure 1.
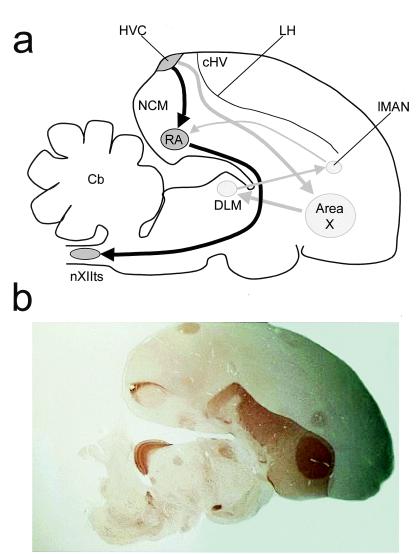
(a) A schematic diagram of a composite of parasagittal sections of the zebra finch brain. Drawing gives approximate positions of nuclei and brain regions. RA, robust nucleus of the archistriatum; LH, lamina hyperstriatica; Cb, cerebellum; DLM, medial part of the dorsolateral thalamic nucleus; nXIIts, tracheosyringeal portion of the nucleus hypoglossus. Dark arrows, caudal pathway; gray arrows, rostral pathway. (b) Photomicrograph of a parasaggital section of a zebra finch brain, stained for acetylcholinesterase. The section clearly shows HVC, Area X, lMAN, and RA.
Recent studies have investigated the expression of immediate early genes (IEGs) (12, 13) in adult zebra finch males after exposure to unfamiliar conspecific song (14–17). Expression of IEGs is a measure of neuronal activation (12, 13). The results of these studies suggest that song perception in songbirds involves neuronal activation in brain regions that have not traditionally been implicated in the control of song production or song learning. Exposure of adult zebra finch males to conspecific song led to significantly increased expression of the IEG known as ZENK in the caudal part of the neostriatum (NCM) and of the hyperstriatum ventrale (cHV), but not in HVC, Area X, lMAN or RA, nor in Field L, a primary auditory projection area (14–20). The increase in ZENK expression did not occur after exposure to heterospecific song or to tone busts (14, 15). Subsequent studies suggest a dissociation between the neural substrates of production and perception of song (20). When an adult zebra finch male sings, there is neuronal activation in the song-control nuclei (20, 21). However, when the bird hears a conspecific song without singing itself, there is no increased activation in these nuclei, but there is in other regions, including NCM and cHV (14–20). These findings suggest that NCM and cHV are important for song perception, but until now there is no evidence that indicates the role (if any) of either of these forebrain regions in the storage of auditory memories during song learning. The present experiment was designed to address this issue explicitly.
Methods
Subjects.
A total of 19 zebra finch males was used, 15 of whom were trained in a previous experiment (22). The results of two birds were discarded because of a failed perfusion. Eventually there were nine birds in the experimental group and eight in the control group. They were reared and maintained as described in a behavioral study in which they participated (22). Permission to perform this experiment was obtained from the Animal Experiments Committee of Leiden University (UDEC 98075).
Song Learning and Reexposure Procedure.
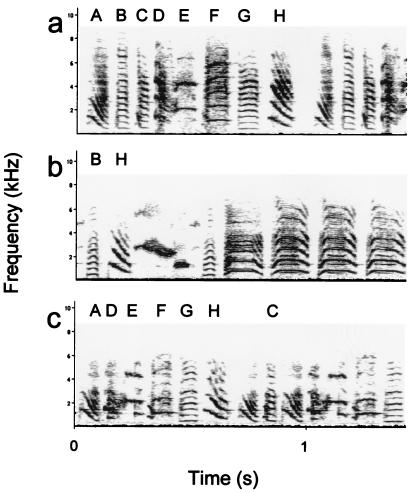
The males were reared without their father from day 7 after hatching onwards. When they were 38 days old, they were placed individually in cages (75 × 35 × 40 cm) in a soundproof chamber. From days 38 to 76 after hatching (in the sensitive period for song learning), the birds were exposed to a taped song of an adult male zebra finch (22). All birds were exposed to a song playback of one of four possible tutor songs, played through a speaker in the back wall of the cage. The average length of the songs was 5.6 s (± 0.3, SD). In addition, with every exposure to the song, the birds received exposure to a stuffed zebra finch male (6 s) either simultaneously with presentation of the song or after song presentation. There was no difference in song copying between these two training procedures (22), and they were counterbalanced over the two groups in the present study. The song was presented 20 times per h according to a random presentation schedule, at 70 dB (A) measured at a distance of 30 cm from the center of the speaker. At the end of the tutoring period, when they were 76 days old, the birds were placed individually in soundproof isolation cages (50 × 53 × 40 cm) until their songs were recorded, at a mean age of 142.7 days (± 3.20 SEM). Recording took place in a soundproof chamber. After recording, a sonogram was made of a representative song for each bird, using canary software on an Apple MacIntosh computer, and compared with sonograms of tutor songs, which were produced in the same manner (see Fig. 2). Three independent observers, who did not know the identity of the experimental animals or the tutors, compared the sonograms of each of the experimental males with that of its tutor song and with another, unfamiliar, song from the pool of four tutor songs that were used for this experiment. For each element in the sonograms of the experimental animals, the observers rated the degree of similarity with the best matching element in the sonograms of each of the two tutor songs. For each experimental bird, the fraction of elements shared with the tutor song and with the unfamiliar song was calculated in relation to the total number of elements in the tutor or unfamiliar song. Kendall's coefficient of concordance for the three observers was 0.88. The means of the results of the three observers were used for analysis.
Figure 2.
Sonograms of a tutor song (a), of an experimental bird that shared the smallest number of elements with the tutor song (b), and that shared the greatest number of elements with the tutor song (c).
When the birds were 247.12 (± 10.38, SEM) days old, they were placed in a soundproof chamber and exposed to the taped tutor song for 30 min or not exposed to a song (controls), in darkness. The birds remained in darkness until they were sacrificed. Tape recordings revealed that none of the birds sang while they were placed in darkness.
Immunocytochemistry and Image Analysis.
One hour after the end of exposure to the tutor song (or no exposure, in the control group), the birds were given an overdose of Nembutal (i.m.) and subsequently perfused with saline and a fixative (4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M PBS, pH 7.4). Free-floating sections (40 μm) were prepared by using standard immunocytochemical techniques (17, 19, 21, 23). The sections were placed in 0.1 M PBS (pH 7.4) and subsequently incubated in 0.3% H2O2 in PBS for 15 min. The sections were then washed in PBS and incubated for 30 min in normal goat serum, washed again in PBS, and incubated for 20 h with the primary antibody (at 4°C). We used polyclonal antibodies against egr-1 (C-19, SC-189, dilution 1:20,000) and Fos (K-25, SC-253, dilution 1:30,000), respectively, raised in rabbits (Santa Cruz Biotechnology) (17). Staining involved incubation for 1 h in ABC (avidin/biotinylated enzyme complex; Vector Laboratories), incubation in a diaminobenzidine medium for 10 min, followed by the addition of H2O2 for 15 min. Control sections were subjected to the same procedure, with the exception of incubation in the primary antibody. Alternate sections were stained for acetylcholinesterase to enable identification of song-control nuclei (see Fig. 1b).
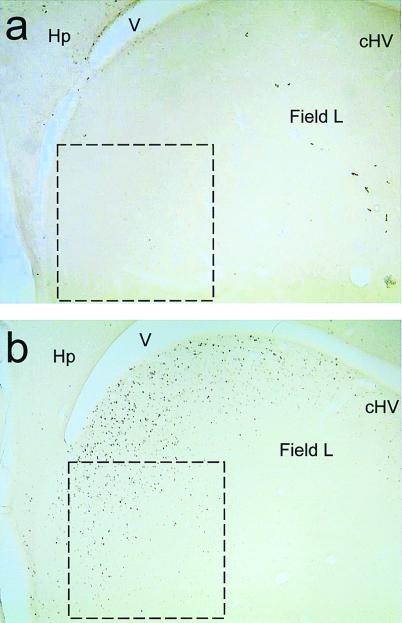
At ×100 magnification, the number of immunopositive cell nuclei in a standard size frame (0.5 × 0.5 mm for the NCM and Area X (see Fig. 3); 0.25 × 0.25 mm for the HVC and the cHV) were counted blind by using NIH image analysis software. For NCM, the counting frame was placed at the extreme caudal pole of the neostriatum, immediately adjacent to the hippocampus (see Fig. 3). For cHV, the counting frame was positioned adjacent to the ventricle and to the lamina hyperstriatica (LH; see Fig. 1a). For HVC and Area X, the counting frame was positioned in the center of the nuclei. Thresholds were similar between brains and brain regions, at ≈130 (range 120–145). For each region, the mean was calculated from two sections, for the NCM and cHV at ≈0.5 mm and 1.0 mm from the midline, and for the HVC and Area X at ≈1.3 and 1.5 mm from the midline, using the atlas of Vates et al. (24).
Figure 3.
Photomicrographs of parasagittal sections of the zebra finch brain at the level of the NCM, showing egr-1 (ZENK)-like immunostaining. Overlay is the counting frame used (0.5 × 0.5 mm). The sections are from a bird in the control (a) and of a bird in the experimental group (b) that both showed a high degree of song learning. V, ventricle; Hp, hippocampus.
Results
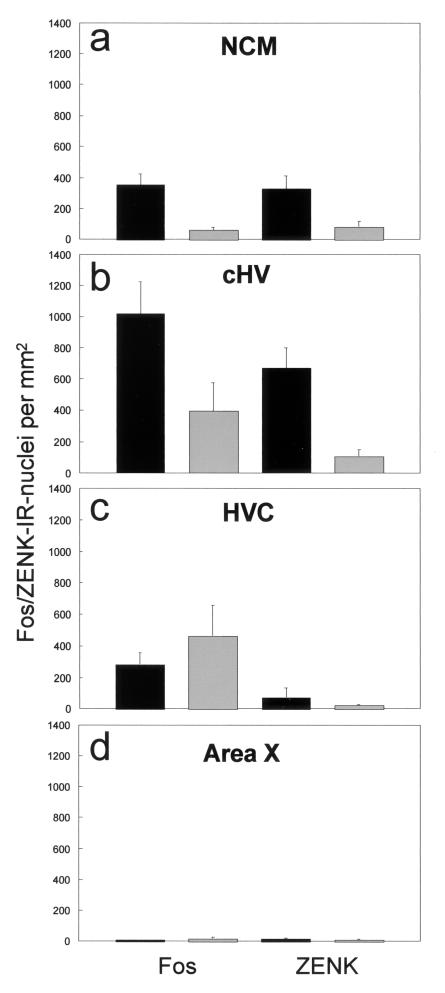
On average, there was significant song learning from the tutor songs: the mean fraction of song elements shared with the tutor song was 0.30 (± 0.06, SEM), whereas the fraction of song elements shared with an unfamiliar song (indicating random similarities) was 0.037 (± 0.017). Visual inspection of the sections revealed considerable staining in the neostriatum (including the NCM), medial hyperstriatum ventrale (including the cHV), and hippocampus, but very little staining in HVC, lMAN, RA, and Area X. Fig. 3 shows two representative photomicrographs of parasagittal sections of the caudal forebrain, including area NCM. ANOVA revealed that there was a significant increase in expression of both Fos and Zenk protein in the experimental birds compared with the control birds in the NCM and in the cHV but not in the other two regions that were sampled (Fig. 4). The results of the ANOVA were, for the NCM: experimental vs. control: F1,15 = 13.56, P < 0.01; Fos vs. ZENK protein: F1,15 = 0.008, not significant; For the cHV: experimental vs. control: F1,15 = 10.34, P < 0.01; Fos vs. ZENK protein: F1,15 = 7.20, P < 0.05; there was no significant interaction between these two factors: F1,15 = 0.060, not significant.
Figure 4.
Mean number of immunoreactive (IR) cell nuclei per mm2 for the experimental birds (filled bars) and controls (open bars) in the NCM (a), cHV (b), HVC (c), and Area X (d), for Fos (Left) and ZENK protein (Right). Only in the NCM and the cHV was there a significant increase in expression of both Fos and ZENK protein in the experimentals compared with the controls.
In addition, we investigated the relationship between the degree of song learning (expressed as the number of song elements shared between the experimental birds and their tutor songs, relative to the number of elements in the tutor song) and the expression of IEG proteins (as reflected in the number of immunopositive cells per mm2) in the NCM and cHV. In the experimental birds, but not in the controls, there was a significant correlation between the number of elements copied and the expression of both Fos and ZENK protein in the NCM (Fig. 5). There were no significant correlations between these two factors in the cHV.
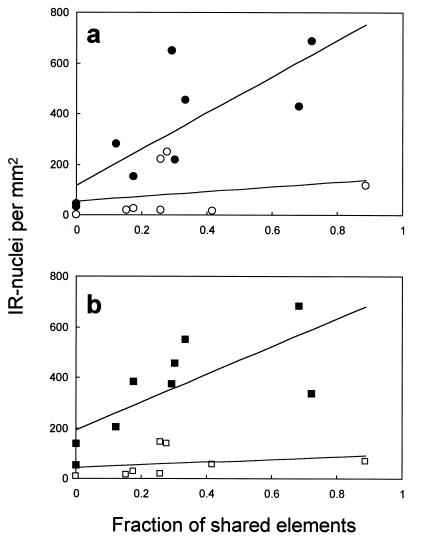
Figure 5.
Scattergrams of the number of immunoreactive cell nuclei per mm2 in the NCM, related to the fraction of the number of song elements that were shared with the tutor song. (a) Zenk protein. (b) Fos. In the experimental birds (filled symbols), there was a significant positive correlation for both Fos (r = 0.722, P < 0.03) and ZENK protein (r = 0.775, P < 0.02). There was no significant correlation in the control birds (open symbols) in these two conditions (r = 0.264 and r = 0.250, respectively). Lines are linear regression lines for the respective groups.
Discussion
These results suggest that the neuronal activation in the NCM on exposure to song is related to the song learning experience. The increase in IEG expression is unlikely to be nonspecific, because (i) it only occurred in two of the sampled regions, which have both been associated with song perception previously (14, 15); (ii) both proteins showed the same pattern of increased expression; and (iii) the IEG response in the NCM was related to the strength of song learning. It seems likely, therefore, that the NCM is (part of) the neural substrate for the representation of the learned tutor song, which is activated when the animal is reexposed to that song. This suggestion is consistent with previous electrophysiological recordings in the NCM of zebra finches, showing some degree of specificity in the response to conspecific songs (25). In the present study, increased IEG expression in the cHV did not correlate significantly with song copying, suggesting that the cHV may have a different role than the NCM. However, the absolute levels of IEG expression were greater in the cHV than in the NCM. It may be that neurons in the cHV have a low threshold for responding to a limited number of familiar song elements. Electrophysiological evidence suggests that in the zebra finch, neurons in the cHV are responsive to conspecific songs, but that these responses are not specific to the bird's own song (26). However, in this study electrophysiological responses to the tutor song were not measured. In the short term, repeated exposure to a song leads to a waning of the IEG response in the NCM (27), consistent with a role for this region in the detection of stimulus familiarity, which is an important aspect of recognition memory (28).
One prediction arising from the present findings is that early lesions to the NCM should impair song learning. Furthermore, the electrophysiological responses of neurons in the NCM when a trained bird is exposed to a song are predicted to reflect the similarity of the song to the bird's tutor song. Our findings have important implications for the study of the neural mechanisms of bird song learning and of recognition memory in general. They have a parallel in the analysis of the neural mechanisms of recognition memory in filial imprinting (23, 29–31). As in imprinting (30, 32, 33), the localization of function found in the present study should stimulate future research into the neuronal and synaptic events (28, 30, 34) that accompany the recognition memory of song learning.
Acknowledgments
We are grateful to Carel ten Cate, Peter Sharp, Brian McCabe, and Dennis van Mil for advice. We thank M. de Bakker, E. Feuth, and G. Lamers for technical assistance.
Abbreviations
- HVC
high vocal center
- NCM
caudal medial neostriatum
- cHV
caudal medial hyperstriatum ventrale
- IEGs
immediate early genes
- lMAN
lateral part of the magnocellular nucleus of the neostriatum
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030539097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030539097
References
- 1.Kroodsma D E, Miller E H, editors. Ecology and Evolution of Acoustic Communication in Birds. Ithaca, NY: Cornell Univ. Press; 1996. [Google Scholar]
- 2.Marler P. Trends Neurosci. 1991;14:199–206. doi: 10.1016/0166-2236(91)90106-5. [DOI] [PubMed] [Google Scholar]
- 3.Konishi M. Annu Rev Neurosci. 1985;8:125–170. doi: 10.1146/annurev.ne.08.030185.001013. [DOI] [PubMed] [Google Scholar]
- 4.Nottebohm F, Alvarez-Buylla A, Cynx J, Kirn J, Ling C-Y, Nottebohm M, Suter R, Tolles A, Williams H. Philos Trans R Soc London B. 1990;329:115–124. doi: 10.1098/rstb.1990.0156. [DOI] [PubMed] [Google Scholar]
- 5.Nottebohm F. Trends Neurosci. 1991;14:206–211. doi: 10.1016/0166-2236(91)90107-6. [DOI] [PubMed] [Google Scholar]
- 6.DeVoogd T J. In: Causal Mechanisms of Behavioural Development. Hogan J A, Bolhuis J J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 49–81. [Google Scholar]
- 7.Bottjer S W. BioEssays. 1997;19:1109–1115. [Google Scholar]
- 8.Nottebohm F, Stokes T, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 9.Bottjer S W, Miesner E A, Arnold A P. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 10.Sohrabji F, Nordeen E J, Nordeen K W. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 11.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt S P, Pini A, Evans G. Nature (London) 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 13.Sagar S M, Sharp F R, Curran T. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 14.Mello C V, Vicario D S, Clayton D F. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mello C V, Clayton D F. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin H, Clayton D F. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 17.Mello C V, Ribeiro S. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Clayton D F. J Neurobiol. 1997;33:549–571. [PubMed] [Google Scholar]
- 19.Ribeiro S, Cecchi G A, Magnasco M O, Mello C V. Neuron. 1998;21:359–371. doi: 10.1016/s0896-6273(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis E D, Nottebohm F. Proc Natl Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimpo R R, Doupe A J. Neuron. 1997;18:315–325. doi: 10.1016/s0896-6273(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 22.Bolhuis J J, Van Mil D P, Houx B B. Anim Behav. 2000;58:1285–1292. doi: 10.1006/anbe.1999.1266. [DOI] [PubMed] [Google Scholar]
- 23.McCabe B J, Horn G. Proc Natl Acad Sci USA. 1994;91:11417–11421. doi: 10.1073/pnas.91.24.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vates G E, Broome B M, Mello C V, Nottebohm F. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Chew S J, Vicario D S, Nottebohm F. Proc Natl Acad Sci USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janata P, Margoliash D. J Neurosci. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mello C V, Nottebohm F, Clayton D F. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown M W. Semin Neurosci. 1996;8:23–32. [Google Scholar]
- 29.Horn G. Memory, Imprinting, and the Brain. Oxford: Clarendon; 1985. [Google Scholar]
- 30.Horn G. Trends Neurosci. 1998;21:300–305. doi: 10.1016/s0166-2236(97)01219-8. [DOI] [PubMed] [Google Scholar]
- 31.Bolhuis J J, Honey R C. Trends Neurosci. 1998;21:306–311. doi: 10.1016/s0166-2236(98)01258-2. [DOI] [PubMed] [Google Scholar]
- 32.Horn G, Bradley P, McCabe B J. J Neurosci. 1985;5:3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheu F-S, McCabe B J, Horn G, Routtenberg A. Proc Natl Acad Sci USA. 1993;90:2705–2709. doi: 10.1073/pnas.90.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebb D O. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]