Abstract
Background
Primary hyperoxaluria type 2 (PH2) is a rare monogenic disorder characterized by an elevated urinary excretion of oxalate. Increased oxalate excretion in PH2 patients can cause nephrolithiasis and nephrocalcinosis, and can, in some cases, result in renal failure and systemic oxalate deposition. The disease is due to a deficiency of glyoxylate reductase/hydroxypyruvate reductase (GRHPR) activity. A definitive diagnosis of PH2 is currently made by the analysis of GR activity in a liver biopsy. GRHPR is expressed in virtually every tissue in the body, suggesting that utilization of more readily available cells could be used to determine GRHPR deficiency. In this study, we have evaluated the potential of determining GR and D-glycerate dehydrogenase (DGDH) activity in blood mononuclear cells (BMC) as a diagnostic indicator of PH2.
Methods
Blood samples were obtained from 10 male and 10 female normal subjects, median age 31, range 21–63, at the Wake Forest University Medical Center and from primary hyperoxaluria patients at the Mayo Clinic. The BMC were isolated and GR and DGDH activities measured in cell lysates.
Results
An assay of 20 normal individuals indicated that BMC contained a DGDH and GR activity of 0.97±0.20 (range 0.62–1.45), and 10.6±3.3 (range 8.3–16.6) nmol/min/mg protein, respectively. The intra-assay coefficient of variation for DGDH and GR activity was 8.2 and 11.5%, respectively. The BMC lysates from normal adult subjects and patients with PH1 showed similar GR and DGDH activities. This was confirmed by the presence of immunoreactive GRHPR protein by western blot analysis. In contrast, PH2 BMC lysates did not exhibit DGDH or GR activity, and showed no immunoreactive GRHPR by western blot analysis.
Conclusion
These results suggest that the assay of DGDH or GR activity in BMC could be used as a minimally invasive diagnostic test for PH2.
Keywords: blood mononuclear cells, D-glycerate dehydrogenase, glyoxylate reductase, primary hyperoxaluria type 2
Introduction
Primary hyperoxaluria type 2 (PH2) is a rare monogenic disorder characterized by an elevated urinary excretion of oxalate and, in nearly all cases, L-glycerate [1]. Increased oxalate excretion in PH2 patients can cause nephrolithiasis and nephrocalcinosis, and can, in some cases, result in renal failure and systemic oxalate deposition. The disease is due to a deficiency of glyoxylate reductase/hydroxypyruvate reductase (GRHPR) (EC 1.1.1.79) activity, an enzyme that has glyoxylate reductase (GR), hydroxypyruvate reductase (HPR) and D -glycerate dehydrogenase (DGDH) activities [2,3]. Several mutations have been identified in the GRHPR gene in affected individuals, confirming the genetic origin of the disease [4,5].
Methods for establishing the diagnosis of PH2 include urine oxalate and L-glycerate measurements, DNA screening for mutations of the GRHPR gene and liver biopsy for the measurement of enzymatic activity. Since urinary L-glycerate excretion can occasionally be normal in PH2 [6], DNA screening of the most common mutation of the GRHPR gene currently detects only 33% of patients with PH2 [7], and liver enzyme measurement requires invasive testing, additional diagnostic approaches are needed. GRHPR is expressed in virtually every tissue in the body [2], suggesting that utilization of more readily available cells could be used to determine GRHPR deficiency.
The original description of the biochemical basis for the disease by Williams and Smith [3] was based on the DGDH activity in leucocytes. In this study, we have evaluated the potential of measuring GR and DGDH activities in blood mononuclear cells (BMC), as a diagnostic indicator of PH2. We have determined the reproducibility of the assay, the variability of this parameter in isolates obtained on different days, and have established a normal range in adults. We have verified the utility of the assay by showing an absence of activity in patients with PH2, but not in those with PH1.
Materials and methods
Samples were obtained from 10 male and 10 female normal subjects, median age 31, range 21–63, at the Wake Forest University Medical Center and from four patients with PH1 and three patients with PH2 at the Mayo Clinic. The PH1 patients and one PH2 patient were diagnosed by a liver biopsy. One PH2 patient was diagnosed by elevated urinary and L-glycerate excretions, and the third PH2 patient was a sibling of a PH2 patient and had elevated urinary oxalate.
This study was approved by the Institutional Review Boards of each institution and the subjects signed an informed, written consent. The BD vacutainer sodium citrate CPT cell preparation tubes (Becton Dickinson & Company, NJ) were used for separation of mononuclear cells from 8ml whole blood in a single centrifugation step. The BMC recovered were washed three times in phosphate buffered saline (PBS), pH 7.4, to reduce contamination from platelets and remove traces of plasma and sodium citrate. The BMC pellets were stored at −70°C until analysis. Storage of samples for up to 3 months at −70°C did not show any loss of enzyme activity. Samples obtained at the Mayo Clinic were shipped to Wake Forest on dry ice.
The BMC lysates were prepared by incubating pellets with 10 volumes of hypotonic detergent lysis buffer (25 mmol/l HEPES, pH 7.0, 0.1% Triton X-100) for 20 min on ice. Lactate dehydrogenase (LDH) activity was measured spectrophotometrically for 5 min at 37°C by following the formation of NADH at 340nm in an assay mixture containing 100 mmol/l Tris-HCl (pH 9.0), 2 mmol/l NAD+ and 20 mmol/l lithium lactate. The GR activity was measured spectrophotometrically for 5 min at 37°C using assay conditions modified from Giafi and Rumsby [8] (50 mmol/l HEPES, pH 7.1, 0.2 mmol/l NADPH, 100 mmol/l KCl, 6 mmol/l glyoxylate). The DGDH activity was measured by the conversion of 20 mmol/l D-glycerate to hydroxypyruvate in 10 min at 37°C in an incubation mixture containing 20 mmol/l Tris-HCl, pH 8.5, and 1 mmol/l NADP+. The reaction was stopped with ice cold 1M perchloric acid. The assay mixture was incubated in the dark for 15 min with 1 mmol/l phenylhydrazine, and the phenylhydrazones formed were immediately measured in the supernatant by reversedphase HPLC, as previously described [9]. A Coomassie Plus protein assay kit (Pierce, Rockford, IL), with bovine serum albumin as the standard, was used to determine protein concentrations in BMC lysates.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) of BMC lysates was performed with a Bio-Rad Ready Gel System (Bio-Rad Laboratories, Hercules, CA) under reducing conditions using a 6% stacking gel and a 12% resolving gel of acrylamide. After electrophoresis, samples were electroblotted from 12% (w/v) acrylamide gels onto Hybond-C nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Membranes were incubated for 1 h in 5% (w/v) non-fat dried milk dissolved in PBS, pH 7.4, containing 1% (v/v) Tween-20, followed by incubation for 1.5 h at room temperature with affinity-purified rabbit polyclonal antibodies to human recombinant GRHPR [1 μg/ml]. The membrane was then washed and incubated for 1 h at room temperature with peroxidase-conjugated goat anti-rabbit immunoglobulin G (0.2 μg/ml) (Dako Corporation, Carpenteria, CA). The GRHPR was visualized by using enhanced chemiluminescence (Amersham Biosciences).
Results are expressed as mean ± SD.
Results
The mean protein yield of BMC recovered from 8ml whole blood was 152±66 μg, range 67–285 μg. The lactate dehydrogenase (LDH) and DGDH activities were linear between 0.5–5 μg and 1–12 μg protein, respectively. In contrast, the GR assay required a minimum of 20 μg protein to achieve a GR activity three SD greater than the blank rate. The GR activity was linear up to 200 μg protein, which was the highest protein concentration tested. The minimal detectable activity for DGDH and GR was 0.02 and 0.7 nmol/min/mg protein, respectively. The intra-assay coefficients of variation (CV) for the measurement of DGDH and GR activities in BMC were determined by assaying activities in six BMC preparations isolated from six separate CPT tubes of blood obtained from one individual in a single blood draw. The CVs were 8.2 and 11.5%, respectively. The CVs of DGDH and GR activities in BMC isolated from whole blood taken from the same individual on six different days were 19 and 20%, respectively. These higher CVs of DGDH and GR activities may be due to actual differences in BMC composition or other factors.
Table 1 shows the enzyme activities of BMC separated from 8ml whole blood of normal subjects and primary hyperoxaluria patients. The BMC lysates from normal adult subjects and patients with PH1 showed similar GR and DGDH activities. This was confirmed by the presence of immunoreactive GRHPR protein by western blot analysis (Figure 1). In contrast, PH2 BMC samples did not exhibit DGDH or GR activity, and showed no immunoreactive GRHPR by western blot analysis (Figure 1).
Table 1.
Enzyme activities of blood mononuclear cell lysates isolated from 8ml whole blood of normal adult subjects and primary hyperoxaluria (PH) patients.
| n | Enzyme activities (nmol/min/mg protein)
|
|||
|---|---|---|---|---|
| LDH | GR | DGDH | ||
| Normal | 20 | 1025±137 (832–1346) | 10.6±3.3 (8.3–16.6) | 0.97±0.20 (0.62–1.45) |
| PH1 | 4 | 1254±93 (1153–1337) | 12.3±2.9 (9.4–15.3) | 0.66±0.07 (0.48–0.84) |
| PH2 | 3 | 1072±323 (825–1439) | <0.7 | <0.02 |
n, number of unique individuals.
Results are expressed as mean ± SD. The range of enzymatic activity is given in parentheses. LDH, lactate dehydrogenase, GR, glyoxylate reductase, DGDH, D -glycerate dehydrogenase.
Fig. 1.
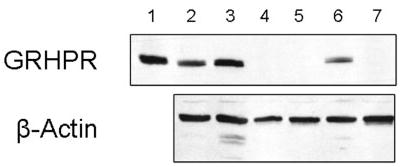
Western blot analysis of blood mononuclear cell lysates (5 μg total protein) using affinity-purified rabbit polyclonal antibodies to GRHPR, shown in top panel. A monoclonal mouse antibody to human α-actin shows protein loading in bottom panel. Lane 1, purified recombinant human GRHPR protein; 2, PH1; 3, PH1; 4, PH2; 5, PH2; 6, BMC from normal adult subject; 7, PH2. All samples were run on the same gel and blotted to the same membrane.
The effect of storage of whole blood for 24 h in heparinized blood tubes on BMC DGDH activity was assessed. Blood was collected from four individuals and stored in an ice box containing ice blocks for 24 h. Care was taken not to have any ice blocks in direct contact with the blood tubes as this leads to haemolysis. There was no loss of DGDH activity after storage of whole blood for 24 h in an ice box, although total BMC protein yield decreased 10–30%. These results suggest that whole blood from patients suspected of having PH2 could be sent within 24 h to facilities that can carry out BMC separation from whole blood. However, effects of shipping are possible and are yet to be determined.
LDH has been reported to have significant GR activity when NADH is used as a cofactor [10–12]. Its contribution under the assay conditions described here, which uses NADPH, the preferred cofactor for GRHPR [11], was assessed using purified beef heart LDH (Sigma-Aldrich, St Louis, MO). The GR activity of purified beef heart LDH was found to be only 0.08% of its total LDH activity. This would indicate that less than 10% of total GR activity in BMC could be attributable to LDH activity (Table 1). This data is in keeping with a recent report by Mdluli and coworkers [11] who showed purified recombinant human LDH not to have significant GR activity when utilizing NADPH as the cofactor. Purified beef heart LDH lacked DGDH activity, when utilizing NADP+ as a cofactor.
Discussion
Our results confirm that BMC contain readily detectable GRHPR activity. We have established a normal range of GR and DGDH activities in BMC, 10.6±3.3 and 0.97±0.20 nmol/min/mg protein, respectively, and verified the utility of the assay by showing an absence of activity in patients with PH2, but not in those with PH1.
Our results are in keeping with the previous findings of Williams and Smith [3] and Chalmers et al. [13], who demonstrated that leucocyte homogenates from four PH2 patients and two PH2 patients, respectively, were deficient in DGDH activity. In their studies, DGDH activity was measured spectrophotofluorometrically and required large amounts of protein (0.5–1 mg). In contrast, the methods described here for measurement of DGDH activity require low microgram amounts of protein, which would equate to significantly less whole blood (only a few millilitres) needed for enzymatic analysis. Furthermore, BMC can now be isolated in a single centrifugal step with the new sodium citrate CPT cell preparation tubes introduced by Becton Dickinson & Company. The leucocyte DGDH activities reported by Williams and Smith [3] (mean activity 18.2, with a range of 12.2–24.3 nmol/mg protein/h, at room temperature) and Chalmers et al. [13] (41±9 nmol/mg protein/h at 37°C) for normal control subjects are in reasonable agreement with the results presented here.
The GR activities reported by Giafi and Rumsby [8] in lymphocytes from normal adults (mean activity 10 nmol/min/mg protein) show good agreement with our data. Furthermore, they showed a lack of GR activity in leucocytes from two PH2 patients, which is in keeping with the results presented here.
A recent publication reported the detection of a leucocyte cDNA from PH2 patients with sequence similarity to wild-type GRHPR, despite only mutant GRHPR in genomic DNA or liver cDNA from the same individuals [14]. Although the existence of this transcript is of interest, it may be premature to preclude BMC GRHPR enzymatic analysis as a diagnostic measure based on the data they presented for the following reasons. Firstly, no data were presented to show that this transcript in leucocytes had enzymatic activity. Secondly, sequence data was presented only for regions of exons 6 and 7. The sequence information of the rest of the cDNA has not been reported. Thirdly, other studies have shown that leucocytes from PH2 patients lack GRHPR activity [3,8,13]. Fourthly, their data suggests that the transcript may be a transcribed pseudogene, which, by definition, is non-functional.
The functional significance of GR and HPR activities in BMC is not clear. A possible hypothesis is that these cells produce glyoxylate in the course of their normal metabolism and that GRHPR activity acts to convert this glyoxylate to glycolate and limit oxalate production. However, to date, neither dysfunctional metabolism nor oxalate accrual has been reported in BMC of individuals with PH2.
Currently, a definitive diagnosis of PH2 is made by measurement of GR activity in a liver biopsy [8]. Although liver biopsy is generally regarded as a safe procedure, complications including mortality have been reported. The rate of complications requiring hospitalization is around 4%, and the rate of mortality is between 0.0088 and 0.3% [15]. The measurement of either GR or DGDH activity in BMC could potentially be used as a minimally invasive diagnostic test for PH2, although measuring DGDH activity may be preferable because of the lower requirement for BMC protein.
Acknowledgments
The authors gratefully acknowledge the invaluable contribution of Ying Yang for assisting with these experiments. This research was supported by NIH grants DK54468 and DK73354, and the authors thank Oxalosis and Hyperoxaluria Foundation, which supported the Mayo Clinic Hyperoxaluria Center.
Footnotes
Conflict of interest statement. None declared.
(See related article by Rumsby. Nephrol Dial Transplant 2006; 21: 2063–2064.)
References
- 1.Danpure CJ. Primary hyperoxaluria. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3323–3367. [Google Scholar]
- 2.Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with Primary Hyperoxaluria Type II. Hum Mol Genet. 1999;11:2063–2069. doi: 10.1093/hmg/8.11.2063. [DOI] [PubMed] [Google Scholar]
- 3.Williams HE, Smith LHJ. L-glyceric aciduria. A new genetic variant of primary hyperoxaluria. New Engl J Med. 1968;278:233–239. doi: 10.1056/NEJM196802012780502. [DOI] [PubMed] [Google Scholar]
- 4.Cregeen DP, Williams EL, Hulton S, Rumsby G. Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat. 2003;22:497–505. doi: 10.1002/humu.9200. [DOI] [PubMed] [Google Scholar]
- 5.Webster KE, Ferree PM, Holmes RP, Cramer SD. Identification of missense, nonsense, and deletion mutations in the GRHPR gene in patients with primary hyperoxaluria type II (PH2) Human Gen. 2000;107:176–185. doi: 10.1007/s004390000351. [DOI] [PubMed] [Google Scholar]
- 6.Rumsby G, Sharma A, Cregeen DP, Solomon LR. Primary hyperoxaluria type 2 without L-glycericaciduria: is the disease under-diagnosed? Nephrol Dial Transplant. 2001;16:1697–1699. doi: 10.1093/ndt/16.8.1697. [DOI] [PubMed] [Google Scholar]
- 7.Rumsby G, Williams E, Coulter-Mackie M. Evaluation of mutation screening as a first line test for the diagnosis of the primary hyperoxalurias. Kidney Int. 2004;66:959–963. doi: 10.1111/j.1523-1755.2004.00842.x. [DOI] [PubMed] [Google Scholar]
- 8.Gia CF, Rumsby G. Kinetic analysis and tissue distribution of human D-glycerate dehydrogenase/glyoxylate reductase and its relevance to the diagnosis of primary hyperoxaluria type 2. Ann Clin Biochem. 1998;35:104–109. doi: 10.1177/000456329803500114. [DOI] [PubMed] [Google Scholar]
- 9.Holmes RP, Hurst CH, Assimos DG, Goodman HO. Glucagon increases urinary oxalate excretion in the guinea pig. Amer J Physiol. 1995;269:E568–E574. doi: 10.1152/ajpendo.1995.269.3.E568. [DOI] [PubMed] [Google Scholar]
- 10.Duncan RJS, Tipton KF. The oxidation and reduction of glyoxylate by lactic dehydrogenase. Eur J Biochem. 1969;11:58–61. doi: 10.1111/j.1432-1033.1969.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 11.Mdluli K, Booth MP, Brady RL, Rumsby G. A preliminary account of the properties of recombinant human Glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochim Biophys Acta. 2005;1753:209–216. doi: 10.1016/j.bbapap.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Warren WA. Catalysis of both oxidation and reduction of glyoxylate by pig heart lactate dehydrogenase isozyme 1. J Biol Chem. 1970;245:1675–1681. [PubMed] [Google Scholar]
- 13.Chalmers RA, Tracey BM, Mistry J, Griffiths KD, Green A, Winterborn MH. L-glyceric aciduria (primary hyperoxaluria type 2) in siblings in two unrelated families. J Inher Metab Dis. 1984;7(Suppl 2):133–134. doi: 10.1007/978-94-009-5612-4_41. [DOI] [PubMed] [Google Scholar]
- 14.Bhat S, Williams EL, Rumsby G. Tissue differences in the expression of mutations and polymorphisms in the GRHPR gene and implications for diagnosis of primary hyperoxaluria type 2. Clin Chem. 2005;51:2423–2425. doi: 10.1373/clinchem.2005.058305. [DOI] [PubMed] [Google Scholar]
- 15.Malnick S, Melzer E. Routine ultrasound-guided liver biopsy: a time whose idea has come? J Clin Gastroenterol. 2005;39:900–903. doi: 10.1097/01.mcg.0000180803.49328.a7. [DOI] [PubMed] [Google Scholar]


