Abstract
Purpose
Ex vivo expansion of limbal epithelial progenitor cells on amniotic membrane (AM) without 3T3 fibroblasts is a new surgical approach to treat limbal stem cell deficiency. Such expansion requires NGF-TrkA-mediated signaling, and this study was conducted to delineate the downstream signaling pathways.
Methods
The human corneolimbal ring was cut into explants and cultured on intact human AM. At day 0 or 10, low-molecular-weight inhibitors were added, whereas the control group received dimethyl sulfoxide (DMSO). The epithelial outgrowth rate was monitored for 17 days, and the epithelial cells were collected for Western blot analysis.
Results
In the control, most expansion of human limbal epithelial cells started from the limbus from days 5 to 7 and reached ~80% confluence at day 17. Compared with the control, the outgrowth was completely inhibited by 50 μM LY294002 or 50 μM SR13668 and was significantly suppressed by 10 μM U0126, but was not affected by 10 μM of either SB203580 or JNK inhibitor 1. The inhibition of outgrowth by LY294002, SR13668, and U0126 was reversible. Western blot analysis showed that phosphorylation of Akt and FKHRL1was abolished by LY294002 and SR13668, but downregulated by U0126, which also abolished phosphorylation of p44/42 mitogen-activated protein kinase (MAPK). The phosphorylation of p38 and JNK MAPK were downregulated or abolished during ex vivo expansion.
Conclusions
Ex vivo expansion of human limbal epithelial progenitor cells on intact AM is mediated by the survival signaling pathway mediated by PI3K-Akt-FKHRL1 and by the mitogenic MAPK pathway mediated by p44/42 at the expense of p38 and JNK MAPK.
One major advance made in treating patients with total limbal stem cell deficiency is to transplant epithelial cells that have been expanded from a small limbal biopsy specimen by culturing on amniotic membrane (AM) with or without the use of 3T3 fibroblast feeder layers.1–4 In a rabbit model of unilateral total limbal stem cell deficiency, we have recently demonstrated the long-term efficacy of this new surgical procedure5 and that clinical success correlates with the recovery of a normal corneal epithelial phenotype, and clinical failure correlates with the maintenance of a conjunctival epithelial phenotype.6 Furthermore, restoration of a clear and transparent cornea is associated with a normal corneal epithelium and complete wound remodeling.7
The success of this new surgical procedure can further be attributed to the fact that ex vivo expanded limbal epithelial cells on intact amniotic membrane (without 3T3 fibroblast feeder layers or air lifting) preserve characteristics of limbal epithelial stem cells such as negative expression of K3 keratin and connexin 43 (Cx 43), label-retaining properties, and p63 expression.4,8–10 As a first step to exploring the molecular mechanism governed by AM, we have noted that nerve growth factor (NGF) is abundantly present in AM stromal matrix and that TrkA (the NGF high-affinity receptor) but not p75NTR (the NGF low-affinity receptor), is expressed at the limbal basal epithelial cell layer, suggesting that the limbal epithelial stem cells may preferentially use TrkA but not p75NTR to sustain its unique antiapoptotic survival. Moreover, addition of K252a, which inhibits TrkA-mediated phosphorylation,11 suppresses epithelial outgrowth expanded on AM.12
It has been reported that NGF/TrkA signaling leads to the PI3K-Akt-FKHRL1 pathway,13 which is known to govern cell survival (for review, see Ref. 14), and to the mitogen-activated protein kinase (MAPK) pathway,15,16 which is known to control cell mitosis (for review, see Ref. 17). In the MAPK pathway, extracellular signals such as growth factors, mitogens, cytokines, and various forms of environmental stresses activate phosphorylation of one or several of the three members: extracellular signal-regulated kinases (ERKs), also referred to as p44/42 MAP kinase; p38 MAP kinases; and c-Jun N-terminal kinases (JNKs, also termed stress-activated protein kinases).18,19 In this study, we wanted to determine whether downstream signaling pathways mediated by PI3K-Akt-FKHRL1 and MAPKs are involved in ex vivo expansion of limbal epithelial progenitor cells on AM.
MATERIALS AND METHODS
Dulbecco’s modified Eagle’s medium (DMEM), F-12 nutrient mixture, fetal bovine serum (FBS), HEPES buffer, phosphate-buffered saline (PBS), amphotericin B, gentamicin, and dispase II were purchased from Invitrogen (Carlsbad, CA); mouse-derived epidermal growth factor, cholera-toxin (subunit A), dimethylsulfoxide (DMSO), hydrocortisone, and insulin-transferrin-sodium selenite medium supplement from Sigma-Aldrich (St. Louis, MO); plastic cell culture dishes from BD Biosciences (Lincoln Park, NJ); culture plate inserts (30 mm diameter, 0.4-μm pore size; Millicel-CM) and polycarbonate filters from Millipore (Bedford, MA); LY294002 and antibodies to Akt, FKHRL1, and MAPKs of p44/42, p38, and JNK from Cell Signaling Technology (Beverly, MA); SR13668 (a phosphor-Akt inhibitor) from SRI International (Menlo Park, CA); U0126 from Promega (Madison, WI); SB203580 from Upstate (Charlottesville, VA); and JNK inhibitor 1 from Calbiochem (San Diego, CA).
Preparation of Human Amniotic Membrane Insert
Human AM was kindly provided by Biotissue (Miami, FL) and stored at −80°C before use. AM was devitalized by freezing and thawing and washed three times with Hanks’ balanced saline solution (HBSS) before being fastened onto a 30-mm culture insert (Millicel-CM; Millipore), which generated an insert with 23-mm diameter covered by AM, and placed in a six-well plate, as previously described.20
Preparation of Human Corneolimbal Tissue
Human tissue was handled according to the tenets of the Declaration of Helsinki. After removal of excessive sclera, iris, corneal endothelium, conjunctiva, and Tenon’s capsule, the limbal ring was separated by a 7.5-mm trephine from donor corneas that were obtained from the Medical Eye Bank of Florida (Orlando, FL) and were of transplant quality but had been excluded from clinical use for nonocular reasons. The limbal ring was also obtained after transplantation of donor corneas obtained from Florida Lions Eye Bank (Miami, FL).
Human Limbal Explant Culture on Amniotic Membrane
Each limbal ring was rinsed three times with supplemented hormonal epithelial medium (SHEM) made of an equal volume of HEPES-buffered DMEM containing bicarbonate and Ham’s F12 and supplemented with 5% FBS, 0.5% DMSO, 50 μg/mL gentamicin, 1.25 μg/mL amphotericin B, 2 ng/mL mouse EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 0.5 μg/mL hydrocortisone, and 30 ng/mL cholera toxin subunit A. The limbal ring was then exposed for 10 minutes to 1.2 U/mL Dispase II in Mg2+- and Ca2+-free HBSS at 37°C under 95% humidity and 5% CO2. After three rinses with SHEM, each limbal ring was subdivided into two halves and each half further subdivided into six pieces of 1 × 1.5 × 2.5-mm explants. To eliminate variations of age, sex, and race, explants from the corresponding position of the same donor cornea were selected for the control and the experimental group, respectively. An explant was placed on the center of intact AM or plastic with the epithelial side facing up and cultured in SHEM. The experimental group was added with the inhibitor of desired concentration, whereas the control group was added with the same concentration of DMSO as the vehicle that was used to dissolve each inhibitor. The culture was maintained at 37°C under 95% humidity and 5% CO2, the medium was changed every other day, and the outgrowth was monitored daily for 17 days by inverted phase microscope (Nikon, Tokyo, Japan). The outgrowth area was digitized every other day (Photoshop 5.5; Adobe Systems, Mountain View, CA) and analyzed by NIH ImageJ 1.30v (available by ftp at zippy.nimh.nih.gov/ or at http://rsb.info.nih.gov/nih-image; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD).
Western Blot Analysis
Expanded epithelial sheets from three explants cultured on plastic with DMSO treatment or on AM after treatment with DMSO, SB203580, or JNK inhibitor 1 for 17 days or treatment with LY294002, SR13668 or U0126 for 24 hours at day 10 (at this time point, cell outgrowth was rapid and there were enough cells to be collected for Western blot) were scraped off with a spatula. Cells were washed once with PBS, resuspended in 300 μL RIPA buffer (50 mM Tris Cl [pH 7.5]; 150 mM NaCl; 1% Nonidet P-40; 0.5% sodium deoxycholate; 0.1% SDS with 1 μg/mL of aprotinin, leupeptin, and pepstatin A; 1 mM phenylmethyl-sulfonyl fluoride [PMSF]; 50 mM sodium fluoride; and 0.2 mM sodium vanadate), and mixed at 4°C for 30 minutes. Cell lysates were cleared by centrifuging at 15,000g for 10 minutes at 4°C. The protein concentration in the supernatant was quantitated by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). Ten to 20 μg total protein from each sample was mixed with an equal volume of 2× SDS sample buffer, boiled for 5 minutes, electrophoresed on a 4% to 15% gradient SDS-PAGE gel and transferred to a nitrocellulose membrane. These membranes were preincubated with blocking buffer (5% BSA or nonfat milk in TBS/0.1% Tween-20), probed with an affinity-purified rabbit polyclonal (anti-Akt-pSer473, anti-FKHRL1-pThr32, anti-JNK MAPK, and anti-p44/42 MAPK pThr202/pTyr204), or mouse monoclonal antibody (anti-Akt-pThr308, anti-JNK-pThr183/Tyr185, anti-p38 MAPK, anti-p38 MAPK-pThr180/Tyr182, and anti-β-actin; all at 1:1000 dilution) overnight at 4°C. The membranes were then washed three times with TBS/0.1% Tween-20, incubated with goat anti-rabbit or rabbit anti-mouse IgG-peroxidase–conjugated antibody (Bio-Rad, Hercules, CA) for 1 hour at room temperature, and developed with enhanced chemiluminescence reagent (PerkinElmer Life Sciences, Boston, MA).
Statistical Analysis
All experiments were performed at least in triplicate. Summary data were reported as the mean ± SD and were compiled and analyzed on computer (Microsoft Excel; Microsoft, Redmond, WA). The mean and SD were calculated for each group by using the appropriate version of Student’s unpaired t-test. Test results were reported as two-tailed probabilities, where P < 0.05 was considered statistically significant.
RESULTS
Epithelial Outgrowth Rate
The epithelial morphology of the human limbal outgrowth has been reported.4,8,21 To make sure that the control without treatment had a consistent growth rate and pattern, we examined 33 limbal explants from 11 donors ranging from 37 to 61 years of age. Under microscopic observation, we noted epithelial cells started to migrate from the limbal edge to AM in 28 (85%) of the 33 explants, at days 3 to 4, and from the corneal or scleral edge in the rest. At day 5, cell outgrowth could be discerned by the naked eye. The surface area was scanned and digitized every other day until day 17 when the outgrowth reached ~80% confluence (i.e., ~ 340 mm2 of 415 mm2 of the AM insert). We terminated the culture before reaching confluence to avoid possible underestimation caused by cell contact inhibition. As shown in Figure 1, the outgrowth rate of the control showed a consistent pattern as a group. The outgrowth rate was gradually increased from days 5 to 9, but rapidly increased from days 9 to 13 and gradually slowed down from days 13 to 17.
Figure 1.
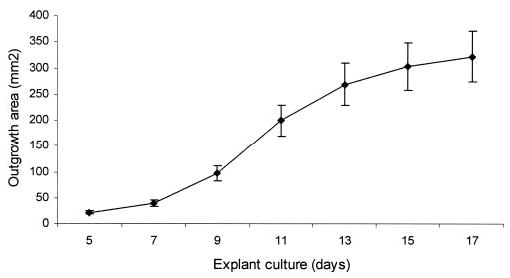
The outgrowth rate of human limbal explants on intact AM. Human limbal explants were cultured in SHEM and monitored for 17 days. A consistent pattern of epithelial outgrowth from a total of 33 explants was noted. The surface area gradually increased from days 5 to 9, rapidly expanded from days 9 to 13, and then gradually decreased from days 13 to 17, when it reached ~ 80% confluence of the AM insert.
Inhibition of the PI3K-Akt Pathway
The PI3K-Akt pathway controls cell survival, and inhibition of this pathway frequently leads to apoptosis (for review, see Refs. 14,22). LY294002 is a specific inhibitor of PI3K, and one of the downstream targets of PI3K is phosphorylation and activation of Akt kinase. The epithelial outgrowth was not significantly inhibited by 5 and 10 μM of LY294002 (P = 0.85 and 0.09, respectively), but was significantly or completely inhibited by 20 and 50 μM of LY294002 (P = 0.0008 and 0.0007, respectively; Figs. 2A, 2B). Addition of 10 μM SR13668, a potent phosphor-Akt inhibitor, resulted in 50% reduction of the outgrowth rate from days 5 to 11 and a 60% reduction from then on (Fig. 2C). Addition of 50 μM SR13668 completely inhibited epithelial outgrowth (Fig. 2D). These results indicate that inhibition of either PI3K or Akt could completely abolish epithelial outgrowth from a limbal explant cultured on AM.
Figure 2.
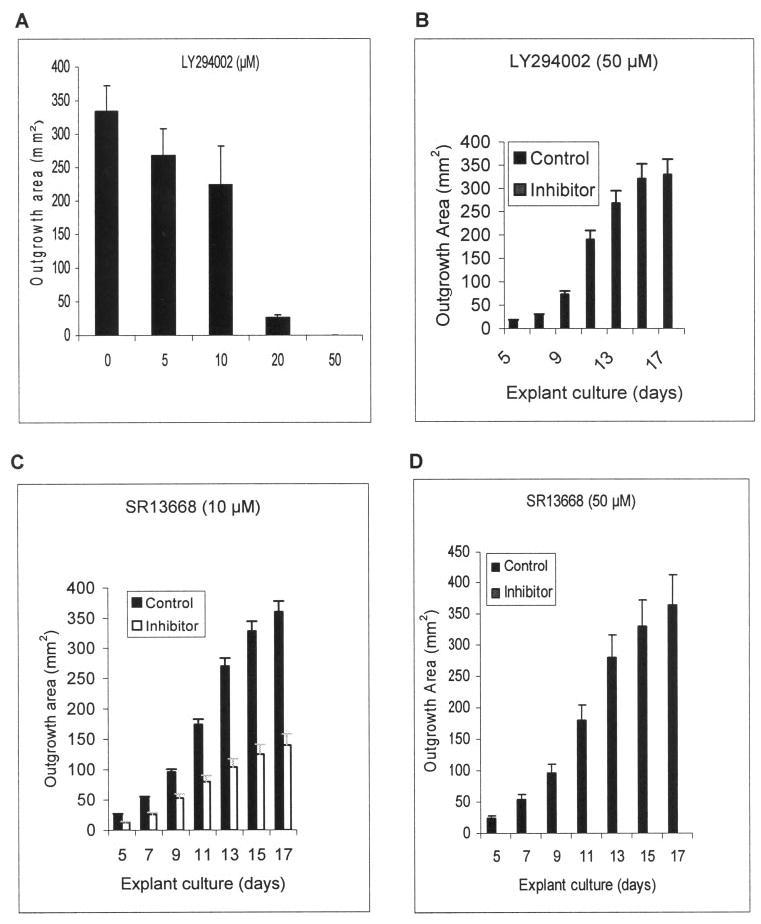
Inhibition of epithelial outgrowth by LY294002 and SR13668. Epithelial outgrowth was dose-dependently inhibited by LY294002, an inhibitor of PI3K, from 5 to 50 μM (A). Compared with the control, complete inhibition of epithelial outgrowth was noted at 20 (not shown) and 50 μM of LY294002 (B). The addition of 10 μM SR13668, a phosphor-Akt inhibitor, partially suppressed the outgrowth (C), but 50 μM of SR13668 completely abolished the outgrowth (D).
Inhibition of the MAPK Pathways
The MAPK pathway controls cell proliferation, migration, and differentiation (for review, see Ref. 23). U0126 is a specific inhibitor of the MAPK kinase MEK1/2. At 10 μM, it completely inhibits p44/42 MAPK phosphorylation in many cells.24 When 10 μM of U0126 was added, epithelial outgrowth was noted at day 8, which was significantly more delayed than the control. From day 13 on, the outgrowth from the U0126-treated explants was almost halted. At day 17, the average outgrowth area of the control and U0126-treated group was 334 ± 34.3 and 17.0 ± 3.0 mm2, respectively (Fig. 3A; P = 0.0077). SB203580 and JNK inhibitor 1 are specific inhibitors of MAPK p38 kinase and JNK kinase, respectively. Addition of 10 μM of either SB203580 or JNK inhibitor 1 did not change the outgrowth that started at day 5 and reached similar rates when compared with the control (Figs. 3B, 3C). There appeared to be some promotion of epithelial outgrowth in SB203580-treated explants, but the difference did not reach statistical significance (P = 0.89). Collectively, these results indicate that inhibition of p44/42 MAPK, but not p38 kinases or JNK, of the MAPK family also completely abolishes epithelial outgrowth from a limbal explant cultured on AM.
Figure 3.
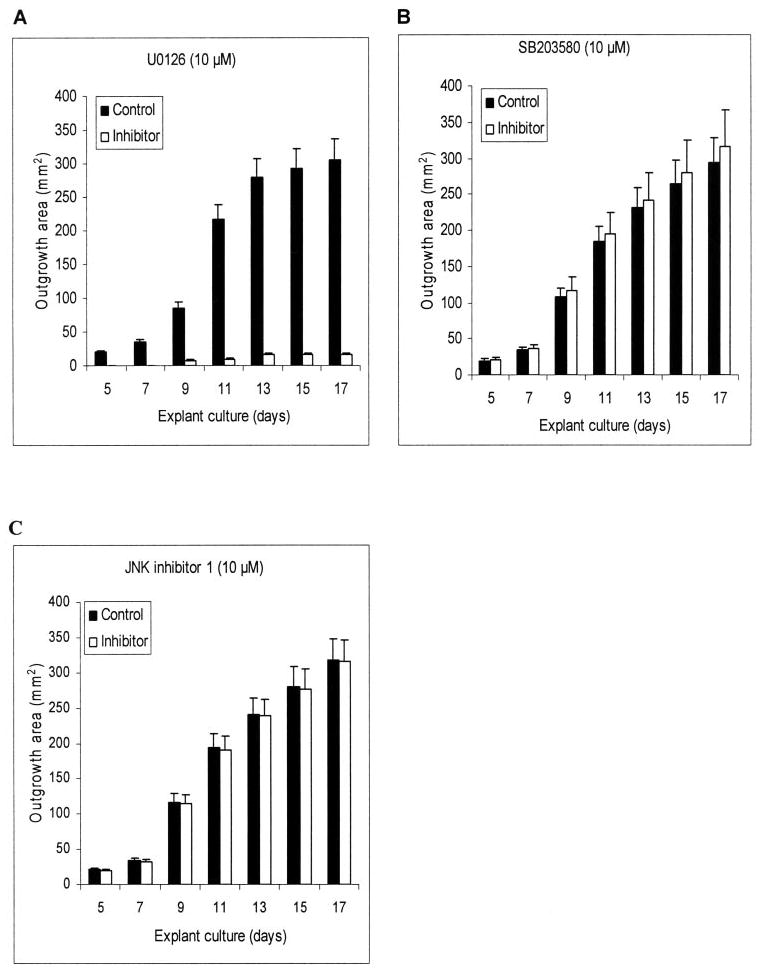
Inhibition of epithelial outgrowth by U0126, U203580, and JNK Inhibitor 1. Compared with the control, addition of 10 μM U0126, a MEK1/2 inhibitor, almost completely inhibited the outgrowth (A). In contrast, no inhibition was noted with 10 μM U203580, a p38 MAPK inhibitor (B), or 10 μM JNK inhibitor 1 (C).
Reversibility of Inhibition by LY294002, SR13668, or U0126
Because addition of LY294002, SR13668, or U0126 led to complete or significant inhibition of ex vivo expansion of limbal epithelial cells, we thus removed these inhibitors after the explants was treated with 50 μM LY294002, 50 μM SR13668, or 10 μM U0126 for 17 days, respectively. We noted that the inhibition of epithelial outgrowth was reversible, because the outgrowth reinitiated in 2 days after the culture medium containing the inhibitor was switched to the fresh medium. However, the outgrowth was resumed at a much slower rate and took a significantly longer time (25–30 days to reach ~80% confluence). The reversible outgrowth from 10 μM U0126 treatment was faster than those treated with 50 μM LY294002 or 50 μM SR13668 (25 days vs. 30 days to reach ~80% confluence; Table 1). These results indicate that such inhibition is reversible and that the progenitor cells in the limbal explant remain viable and can resume proliferation and migration, even after being treated by these inhibitors for 17 days.
Table 1.
Outgrowth Area before and after Removal of an Inhibitor
| LY294002 (50 μM) | SR13668 (50 μM) | U0126 (10 μM) | |
|---|---|---|---|
| Outgrowth area (mm2) with inhibitor present (at day 17) | 0 | 0 | 16 ± 1.8 |
| Outgrowth area (mm2) after inhibitor removal (at day 25 or 30) | 330 ± 23.9 (day 30) | 336 ± 16.1 (day 30) | 344 ± 34.4 (day 25) |
Human limbal explants on AM inserts were cultured for 17 days in the present of 50 μM LY294002, 50 μM SR13668, or 10 μM U0126. At day 17, explants treated with LY294002 or SR13668 did not show any outgrowth, whereas those treated with U0126 outgrew very little. From this day on, the SHEM medium with the inhibitor was replaced with the fresh medium without the inhibitor. As a result, all explants resumed outgrowth 2 days later, but the outgrowth rate was slower than the control (see Figure 1). Explants previously treated with LY294002 or SR13668 reached 80% confluence by day 30 after the inhibitor was removed, whereas explants previously treated with U0126 reached the similar confluence by day 25.
Inhibition of Phosphorylation of Akt, FKHRL1, p44/42 MAPK, p38 MAPK, and JNK MAPK
Western blot analysis was performed to verify that the respective phosphorylation of these kinases was indeed inhibited after the treatment of the aforementioned inhibitors (Fig. 4A). The results showed that addition of 50 μM of LY294002 or SR13668 abolished phosphorylation of Akt at Thr308 and Ser473. SR13668 (50 μM) also abolished, whereas 50 μM LY294002 decreased, Thr32 phosphorylation of FKHRL1, a downstream target of Akt (for review, see Refs. 25–27). U0126 (10 μM) eliminated phosphorylation of Akt at Thr308 and decreased phosphorylation of Akt at Ser473 and FKHRL1 at Thr32. Only 10 μM U0126 abolished phosphorylation of p44/42 MAPK at both Thr202 and Tyr204, whereas 50 μM LY294002 and 50 μM SR13668 did not change p44/42 MAPK phosphorylation. SB203580 and JNK inhibitor 1 (10 μM) increased the phosphorylation of p44/42 MAPK. Of interest, phosphorylation at Thr180/Tyr182 of p38 MAPK was expressed by cells expanded on plastic, but markedly downregulated in those expanded on intact AM, and was abolished with the addition of 10 μM SB203580 (Fig. 4B). Likewise phosphorylation of Thr183/Tyr185 of JNK MAPK was expressed by cells expanded on plastic, but was abolished in those expanded on intact AM, with or without addition of JNK inhibitor 1 (Fig. 4C).
Figure 4.
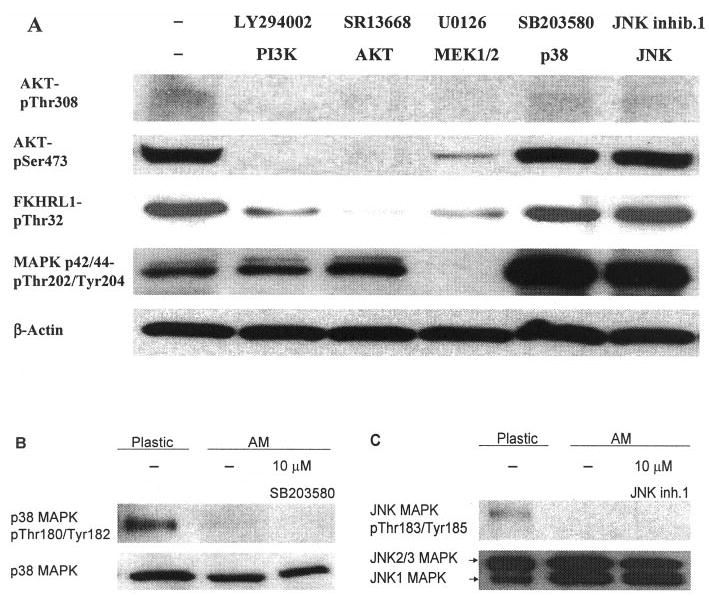
Western blot analysis. (A) LY294002, SR13668, and U0126 abolished phosphorylation of Akt at Thr308. LY294002 and SR14668 completely, but U0126 partially, abolished phosphorylation of Akt at Ser473. SR13668 completely but LY29004 and U0126 partially eliminated FKHRL1 phosphorylation at Thr32. Among all inhibitors, only U0126 abolished phosphorylation of p44/42 MAPK at both Thr202 and Tyr204.(B) Phosphorylation at Thr180/Tyr182 of p38 MAPK was expressed by cells expanded on plastic, but was markedly downregulated in those expanded on intact AM and was abolished with addition of 10 μM SB203580. (C) Phosphorylation of Thr183/Tyr185 of JNK MAPK was expressed by cells expanded on plastic, but was abolished in those expanded on intact AM, with or without addition of JNK inhibitor 1.
These data collectively further supported that selective activation of Akt and/or p44/42 MAPK without concomitant activation of p38 and JNK MAPKs is uniquely involved in ex vivo expansion of human limbal epithelial progenitor cells on intact AM without 3T3 fibroblast feeder layers.
DISCUSSION
We and others have demonstrated that human limbal epithelial progenitor cells can be expanded and maintained on intact, but not denuded, AM or plastic in the absence of 3T3 fibroblast feeder layers.4,8–10 We have further reported that the signaling mediated by NGF is involved in such ex vivo expansion.12 Herein, using specific inhibitors of PI3K, Akt, and the MAPKs (p44/42, p38, and JNK), we showed that both the PI3K-Akt-FKHRL1 and the p44/p42 MAPK pathways provide the downstream signaling.
The PI3K-Akt-FKHRL1 signaling pathway plays a central role in promoting the survival of a wide range of cell types by upregulating expression of antiapoptotic genes or by downregulating expression of apoptotic genes (for review, see Refs. 14,22). PI3K activation is triggered by the binding of such survival factors as insulin-like growth factor (IGF)-1 and neurotrophins (e.g., NGF) to their cell surface receptors.28 Activated PI3K in turn leads to the activation of a serine/threonine kinase, termed Akt or PKB, by phosphorylation at Thr308 and Ser473 (for review, see Ref. 14), which then phosphorylates its downstream target FKHRL1 to be translocated into the nucleus to transactivate proapoptotic genes such as tumor necrosis factor-α, TGF-β1, and Bad (for review, see Ref. 22). In this study, we noted that 50 μM of both LY294002 (an inhibitor of PI3K) and SR13668 (an inhibitor of phosphor-Akt) completely inhibited limbal epithelial outgrowth (Fig. 2), and such inhibition correlated with the complete inhibition of phosphorylation of Akt at Thr308 and Ser473 by both LY294002 and SR13668 (Fig. 4A). As a result, FKHRL1 was partially or completely dephosphorylated at Ser32 by LY294002 or SR13668, respectively. The finding that U0126, a MEK1/2 inhibitor, also inhibited the AKT-pThr308, AKT-pSer473, and FKHRL1-pThr32 bands (Fig. 4A) may be caused by crosstalk between these two pathways, as reported in other cell systems.29–33 Future studies are needed to delineate this hypothesis and identify the exact mechanism.
The findings in the present study lead us to speculate that the antiapoptotic survival signaling mediated by the PI3K-Akt-FKHRL1 pathway is necessary to sustain ex vivo expansion of limbal epithelial progenitor cells including stem cells. Because self-renewal of murine embryonic stem cells maintained by leukemia inhibitory factor is also reduced by LY294002,34 future studies are needed to determine whether PI3K-Akt-FKHRL1 is involved in the self-renewal of limbal epithelial stem cells, and whether antiapoptosis is the key mechanism governing stem cell renewal.
Our data also demonstrated that the limbal epithelial outgrowth was inhibited significantly by U0126, a specific inhibitor to MEK1/2, but not by SB203580 and JNK inhibitor 1, inhibitors of p38 and JNK MAPK, respectively (Figs. 3A, 3B, 3C). This finding was further supported by the Western blot analysis data showing that 10 μM U0126 completely eliminated phosphorylation of p44/42 MAPK at Thr202/Tyr204 (Fig. 4A). Ten micromolar of either SB203580 or JNK inhibitor 1 actually upregulated the phosphorylation of p44/42 MAPK (Fig. 4A), a finding that can be explained by crosstalk among the three MAPK subfamilies of p44/42, p38, and JNK, as reported in other cell systems.35–39
Although JNK inhibitor 1 did not affect limbal epithelial outgrowth, p38 MAPK inhibitor SB203580 did promote the outgrowth, although not significantly (Fig. 3B; P = 0.89). It has been reported that p38 MAPK signaling promoted cell death in CD4+ T cell.40,41 Saika et al.35 and Sharma et al.36 noted that migration of human, rabbit, and mouse corneal epithelial wound healing depends on p38 MAPK. In the current study, we noted that phosphorylation of p38 MAPK and JNK MAPK was expressed by cells expanded on plastic, but was markedly downregulated in those expanded on intact AM (Figs. 4B, 4C, respectively). A similar phenomenon was observed in mouse corneal epithelial wound healing, in which SB203580 induced p44/42 MAPK activity while downregulating p38 MAPK phosphorylation.35 Therefore, it is tempting to speculate that ex vivo expansion of limbal epithelial progenitor cells by intact AM correlates with cellular proliferation without apoptosis and migration mediated by p38 and JNK MAPKs. Further studies are needed to look into the mechanisms by which AM exerts such a selective upregulation of the p44/42 MAPK pathway without concomitant upregulation of the p38 and JNK MAPK pathways.
Notwithstanding, the aforementioned inhibition of limbal epithelial outgrowth was fully reversible on withdrawal of LY294002, SR13668, and U0126 (Table 1). This finding suggests that some epithelial progenitor cells (perhaps stem cells) in the limbal explant could resist the inhibitory actions by these inhibitors regarding regulation of cell survival and mitosis. Previously, it has been shown that the side population (SP) cells of mouse bone marrow, which are enriched in hematopoietic stem cells, could be reduced by treatment with LY294002 or Akt1 knockout. But such SP cells could be reversibly back to the normal level by removing LY294002 or over-expressing Akt1.42 Future studies are needed to determine whether the p44/42 MAPK signaling cascade is involved in cell differentiation, whereas the PI3K-Akt signaling pathway is more important for progenitor cell survival and renewal of limbal epithelial stem cells.
Footnotes
Disclosure: H. He, TissueTech, Inc. (F, E); H.-T. Cho, None; W. Li, TissueTech, Inc. (F, E); T. Kawakita, TissueTech, Inc. (F, E); L. Jong, SRI (E); S.C.G. Tseng, TissueTech, Inc. (E, F, I, P), Biotissue (I)
Supported by Grants R01 EY06819 and R01 EY015735 from National Institutes of Health, National Eye Institute, Bethesda, MD; a research grant from TissueTech, Inc., and an unrestricted grant from Ocular Surface Research & Education Foundation, Miami, FL (all SCGT).
References
- 1.Tsai RJF, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction in the acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119:298–300. [PubMed] [Google Scholar]
- 3.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 4.Grueterich M, Tseng SCG. Human limbal progenitor cells expanded on intact amniotic membrane. Arch Ophthalmol. 2002;120:783–790. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 5.Ti S-E, Anderson DF, Touhami A, et al. Factors affecting outcome following transplantation of ex vivo expanded limbal epithelium on amniotic membrane for total limbal deficiency in rabbits. Invest Ophthalmol Vis Sci. 2002;43:2584–2592. [PubMed] [Google Scholar]
- 6.Ti S-E, Grueterich M, Espana EM, et al. Correlation of long-term phenotypic and clinical outcomes following limbal epithelial transplantation cultivated on amniotic membrane in rabbits. Br J Ophthalmol. 2003;88:422–427. doi: 10.1136/bjo.2003.026054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espana EM, Grueterich M, Touhami A, Tseng SCG. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br J Ophthalmol. 2003;87:1509–1514. doi: 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res. 2003;76:303–314. doi: 10.1016/s0014-4835(02)00314-7. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Expression of Delta Np63 in response to phorbol ester in human limbal epithelial cells expanded on intact human amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:2959–2965. doi: 10.1167/iovs.02-0776. [DOI] [PubMed] [Google Scholar]
- 11.Koizumi S, Contreras ML, Matsuda Y, et al. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J Neurosci. 1988;8:715–721. doi: 10.1523/JNEUROSCI.08-02-00715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–994. [PubMed] [Google Scholar]
- 13.Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002;80:1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- 14.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 15.Amino S, Itakura M, Ohnishi H, et al. Nerve growth factor enhances neurotransmitter release from PC12 cells by increasing Ca(2+)-responsible secretory vesicles through the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. J Biochem (Tokyo) 2002;131:887–894. doi: 10.1093/oxfordjournals.jbchem.a003179. [DOI] [PubMed] [Google Scholar]
- 16.Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MD, Okedli E, Woodard A, et al. Evidence for phosphatidylinositol 3-kinase-Akt-p7S6K pathway activation and transduction of mitogenic signals by platelet-derived growth factor in meningioma cells. J Neurosurg. 2002;97:668–675. doi: 10.3171/jns.2002.97.3.0668. [DOI] [PubMed] [Google Scholar]
- 18.Graves JD, Draves KE, Craxton A, et al. Involvement of stress-activated protein kinase and p38 mitogen-activated protein kinase in mIgM-induced apoptosis of human B lymphocytes. Proc Natl Acad Sci USA. 1996;93:13814–13818. doi: 10.1073/pnas.93.24.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 20.Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- 21.Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- 22.Franke TF, Hornik CP, Segev L, et al. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 24.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18532. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 25.Kops GJ, Burgering BM. Forkhead transcription factors are targets of signalling by the proto-oncogene PKB (C-AKT) J Anat. 2000;197:571–574. doi: 10.1046/j.1469-7580.2000.19740571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 28.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 29.Moelling K, Schad K, Bosse M, et al. Regulation of Raf-Akt Crosstalk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 30.Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Fujita N, Tsuruo T. Involvement of 3-phosphoinositide-dependent protein kinase-1 in the MEK/MAPK signal transduction pathway. J Biol Chem. 2004;279:33759–33767. doi: 10.1074/jbc.M402055200. [DOI] [PubMed] [Google Scholar]
- 32.Campbell M, Allen WE, Sawyer C, et al. Glucose-potentiated chemotaxis in human vascular smooth muscle is dependent on crosstalk between the PI3K and MAPK signaling pathways. Circ Res. 2004;95:380–388. doi: 10.1161/01.RES.0000138019.82184.5d. [DOI] [PubMed] [Google Scholar]
- 33.Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology. 2004;145:4522–4531. doi: 10.1210/en.2004-0488. [DOI] [PubMed] [Google Scholar]
- 34.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 35.Saika S, Okada Y, Miyamoto T, et al. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- 36.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 37.Xiao YQ, Malcolm K, Worthen GS, et al. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem. 2002;277:14884–14893. doi: 10.1074/jbc.M111718200. [DOI] [PubMed] [Google Scholar]
- 38.Shen YH, Godlewski J, Zhu J, et al. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J Biol Chem. 2003;278:26715–26721. doi: 10.1074/jbc.M303264200. [DOI] [PubMed] [Google Scholar]
- 39.McMullen ME, Bryant PW, Glembotski CC, et al. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J Biol Chem. 2005;280:20995–21003. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar D, Su ZZ, Lebedeva IV, et al. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porras A, Zuluaga S, Black E, et al. P38 alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol Biol Cell. 2004;15:922–933. doi: 10.1091/mbc.E03-08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogi M, Yang J, Lambert JF, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]


