Abstract
Nav1.5 or SCN5A is a member of the voltage-dependent family of sodium channels. The distribution of Nav1.5 protein was investigated in the mouse brain using immunohistochemistry. Immunostaining with a Nav1.5-specific antibodyrevealed that Nav1.5 protein was localizedin certain distinctregions of brain including the cerebral cortex, thalamus, hypothalamus, basalganglia, cerebellum and brain stem. Notably, we found that Nav1.5 protein co-localized with neurofilaments and clustered at a high density in the neuronal processes, mainly axons.These results suggest that Nav1.5 protein may play a role in the physiology of the central nervous system (generation and propagation of electrical signals by axons).
Keywords: Axon, Brain, Cardiac sodium channel, Nav1.5/SCN5A, Neuronal process, Seizure, Sudden death
INTRODUCTION
Voltage-dependent sodium channels are transmembrane proteins that are responsible for generating action potentials and for rapid conduction of electrical signals in excitable cells [1]. Sodium channels are classified into three types based on their sensitivity to blockade by tetrodotoxin (TTX): TTX-sensitive Na channels (TTXs), TTX-resistant Na channels (TTXr), and TTX-insensitive Na channels (TTXi) [2,3]. In the brain, six TTXs Na channels have been identified, including Nav1.1, Nav1.2, Nav1.3, Nav1.6, Nav1.7. Only one TTXr channel, Nav1.9, has been identified in the brain [4]. TTXr Na channels generate currents that are slower, but recover from inactivation much faster than TTXs channels [5], suggesting a possible role of TTXr Na channels in sustained firing of neurons or as pace-makers. In this study, we investigated the localization pattern of another TTXr Na channel Nav1.5 in the mouse brain using immunohistochemistry.
Nav1.5 or SCN5A encodes the cardiac sodium channel with 2016 amino acids and a calculated mol. wt of 227 kDa [6]. The putative structure of Nav1.5 consists of four homologous domains (I-IV), each containing six transmembrane segments (S1-S6). Nav1.5 mutations cause syncope, seizures, and sudden death triggered by lethal cardiac arrhythmias associated with long QT syndrome (LQTS), idiopathic ventricular fibrillation including Brugada syndrome and cardiac conduction disease [7–11]. Nav1.5 was originally identified as a cardiac sodium channel. Subsequently, it was shown to be expressed in the brain at the mRNA level [2,12], and these findings may explain whyNav1.5 mutations are associated with seizures. However, the distribution of Nav1.5 in the brain at the protein level has not been investigated previously. Here, we demonstrate selective expression of Nav1.5 at the protein level in regions of the mouse central nervous system.
MATERIALS AND METHODS
A Nav1.5-specific polyclonal antibody was developed against a synthetic polypeptide (AC-RPQLDLQASKKLP-DLYC-Amide) corresponding to a unique portion of the less conserved N-terminus of Nav1.5 as described [13]. The mouse anti-neurofilament 200 antibody (clone NE14) was from Sigma (St. Louis, MO), and fluorescein-conjugated secondary antibodies were from Jackson Immuno Research Laboratories (West Grove, PA). The tissues for Western blotting and immunohistochemical analyses were from the mouse strain C57BL.
For Western blot analysis, lysates from cells transfected with a Nav1.5 expression construct (SCN5A in pcDNA3) or the protein extracts from heart, brain, kidney, liver, and skeletal muscle were separated by SDS-PAGE and probed with the anti-Nav1.5 antibody. The signal was visualized with ECL Western blotting detection reagents (Amersham Pharmacia Biotech, Inc., Piscataway, NJ).
Immunohistochemistry was carried out as described previously [14]. Double immunofluorescence staining with the anti-Nav1.5 antibody and the anti-neurofilament antibody was carried out as described [15]. The immunostained slides were mounted with anti-fading media, and visualized with a Zeiss Axiophot photomicroscope or a confocal laser-scanning microscope (SP2; Leica, Heidelberg, Germany).
RESULTS
We have developed an antibody to the Nav1.5 channel. Several lines of evidence strongly indicates that this antibody is specific to Nav1.5. First, the anti-Nav1.5 antibody was generated using a peptide immunogen located at the less-conserved N-terminus of Nav1.5 protein and database searches indicate that it is unique to Nav1.5. Second, the specificity of the anti-Nav1.5 antibody was confirmed by Western blot analysis with protein extracts from mouse heart, kidney, liver, brain, skeletal muscle, and HEK293 cells expressing human Nav1.5. The antibody detected a band of the expected size of 227 kDa [16] strongly in the heart, weakly in the brain, but not in other tissues (Fig. 1a). These data are consistent with the results from Northern blot analysis [6]. The weak Western blotting signal from the brain suggests that our anti-Nav1.5 antibody does not cross-react with major brain sodium channel proteins, indicating the specificity of our antibody. The antibody also yielded the expected 227 kDa band in the lane loaded with protein extract from the transfected cells over-expressing Nav1.5 (Fig. 1b). No equivalent band was detected in the control non-transfected cells (Fig. 1b). Thirdly, as expected for a channel protein, immunofluorescence staining with HEK293 and P19CL6 cells with Nav1.5 expression detected SCN5A-imunoreactivity only on the cell surface (Fig. 1c). Collectively, these data indicate that our antibody is specific to Nav1.5.
Fig. 1.
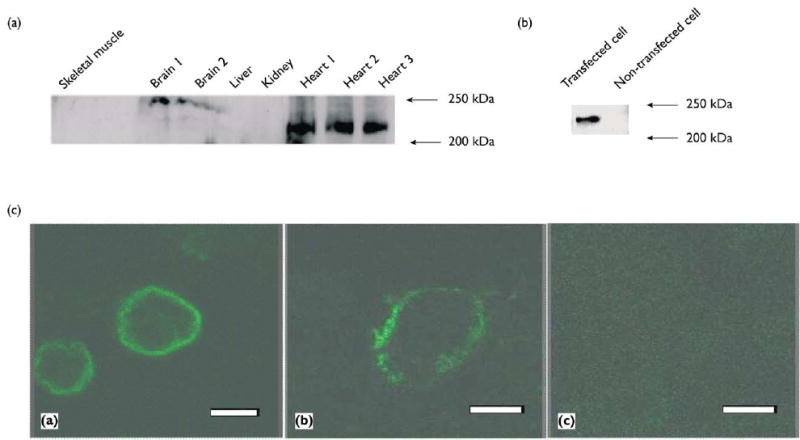
Characterization of the anti-Nav1.5 antibody. (a) Western blot analysis of mouse membrane proteins from the heart, kidney, liver, brain, and skeletal muscle tissues with the anti-Nav1.5 antibody.The positions of mol w markers are indicated. (b) Western blot analysis with protein extracts from HEK293 cells expressing Nav1.5 (transfected cell), and control cells that were not transfected with the Nav1.5 expression construct (non-transfected cell).(c) Cellular localization of wild type human SCN5A protein on plasma membrane (green signal). HEK293 cells (left) and P19CL6 cells (middle) transfected with the human SCN5A expression construct were immunostained with the anti-SCN5A antibody. No detectable immuno£uorescence staining was observed with the anti-SCN5A antibody pre-absorbed with the peptide antigen (right). Bar = 5 μm.
Immunohistochemistry using this anti-Nav1.5 antibody was performed to determine the distribution of Nav1.5 in the normal mouse brain. A negative control experiment was carried out on the adjacent brain sections with the anti-Nav1.5 antibody pre-absorbed with the antigen peptide. Elimination of Nav1.5-immunoreactivity in the negative control experiments (compare Fig. 2a and 2b) indicated that positive staining signal was specific to Nav1.5.
Fig. 2.
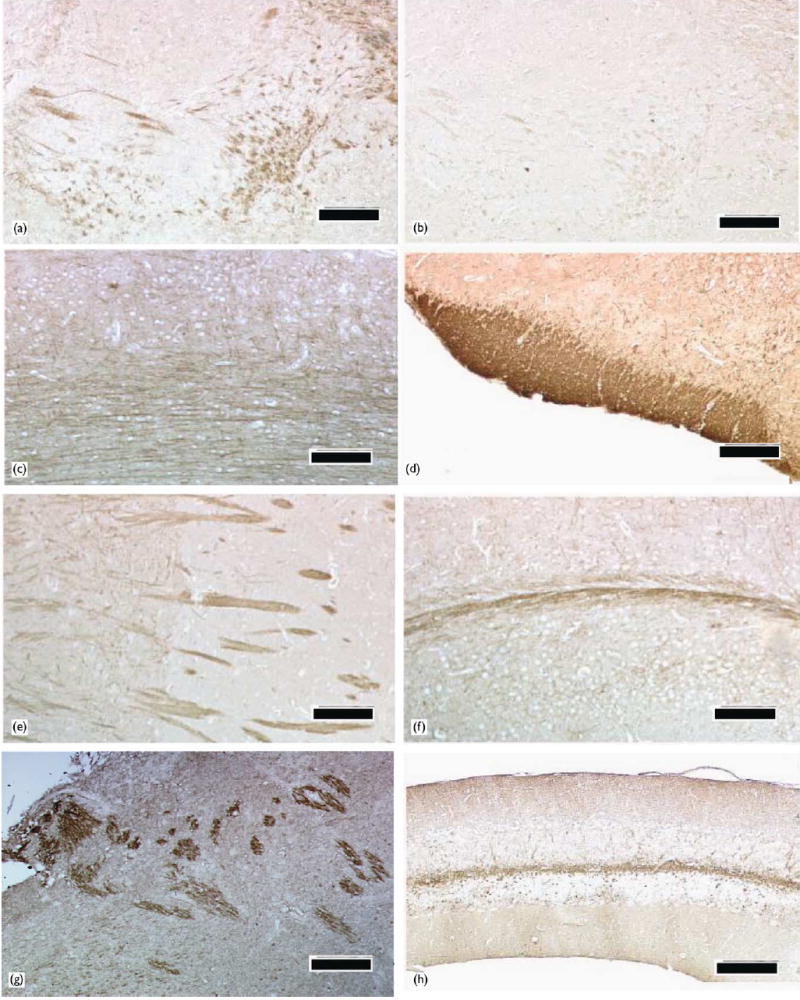
Regional distribution of Nav1.5 protein in the mouse brain. (a) Some nerve fibers in the limbic system express Nav1.5 protein, whereas neuronal and glial cell bodies were not stained by the anti-Nav1.5 antibody. (b) An adjacent section to the section used in (a) was stained with the anti-Nav1.5 antibody pre-absorbed with the immunogen peptide. (c) In the cerebral cortex, the neuronal processes, mainly axons, were positive for Nav1.5 protein, but little or no signal was observed in the cell bodies. (d) The olfactory tract had strong Nav1.5 signal. (e) In the striatum, Nav1.5 protein was mainly localized to the nerve fibers (the pencil fibers). (f) The cerebral peduncle was stained by the anti- Nav1.5 antibody. (g) In the brain stem, some nerve fibers in the lateral medulla were moderately positive for Nav1.5. (h) In the cerebellum, the white matter consisting of nerve fibers was strongly stained for Nav1.5, but there was no signal in the granular layer and the Purkinje cell layer. Bars = 100 μm.
In the majority of cerebral cortex regions (frontal cortex, parietal cortex, temporal cortex and occipital cortex), a weak, but significant, Nav1.5 immunoreactivity was observed (Fig. 2c). Within these regions, the neuronal processes originating from cerebral cortical neurons were the structures positively stained for Nav1.5, whereas little or no immunoreactivity was detected in the neuronal cell bodies or any glial components (Fig. 2c). In the forebrain, we also observed strong Nav1.5 immunoreactivity in the olfactory tract (Fig. 2d), and weak staining in the olfactory tubercule (data not shown). Meanwhile, in the limbic system where Nav1.5 mRNA was reported to be strongly expressed [2,12], moderate Nav1.5 immunoreactivity was detected in the nerve fibers, but no or little Nav1.5 immunoreactivity was detected in the cell bodies in any of the limbic nuclei (Fig. 2a). In the striatum, the anti-Nav1.5 antibody detected strong staining in the nerve bundles, also known as the pencil fibers, whereas the cell bodies in the striatal nuclei showed little or no signal (Fig. 2e; Fig. 3a–c). Interestingly, Nav1.5 immunoreactivity in the pencil fibers was significantly stronger within the caudate putamen (Fig. 2e, right half) than within the globus pallidus (Fig. 2e, left half). In the thalamus and hypothalamus, nerve fibers expressed Nav1.5, while a low level of Nav1.5 immunoreactivity was localized to the hypothalamic and thalamic nuclei (data not shown).
Fig. 3.
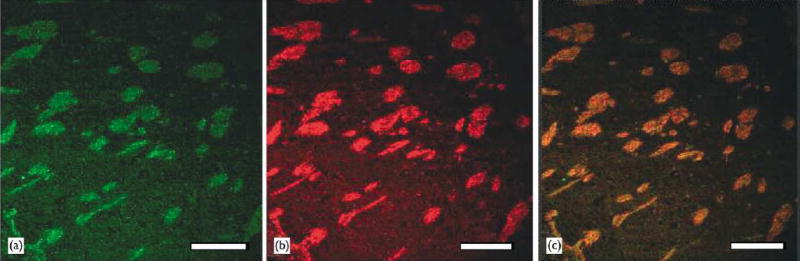
Co-localization of Nav1.5 and neurofilaments.Confocal microscopic images of caudate putamen immunostained with both the anti-Nav1.5 antibody and the monoclonal anti-neurofilament 200 antibody. (a) immuno£uorescent image for Nav1.5 protein, green; (b) immuno£uorescent image for neurofilaments, red; (c) overlay of image A and image B. Bar = 200 μ m.
In the diencephalon of the adult mouse brain, Nav1.5 was also localized to the nerve fiber tracts. The cerebral peduncle was intensively stained for Nav1.5 (Fig. 2f).
In the brain stem, intense Nav1.5 immunoreacitivy was localized to some spotty regions in the lateral medulla (Fig. 2g). These signals seemed to correspond to discrete nerve tracts in the medulla. Based on a report showing that Nav1.5 mRNA expression is located in the lateral paragigantocellular nuclei (LPGi) [2], the nerve tracts with Nav1.5 immunoreactivity shown in this study may be assumed to be originated from LPGi.
In the cerebellum, no signal was detected in the granular layer and the Purkinje cell layer. However, weak Nav1.5 immunoreactivity on some fibrous structures was observed in the cerebellar molecular layer, and strong Nav1.5 immunoreactivity was detected in the white matter of the cerebellum (Fig. 2h).
It is important to note that not all the nerve tracts or nerve bundles through the mouse brain were positively stained by the anti-Nav1.5 antibody, for example, a huge nerve tract connecting both hemispheres in the corpus callosum was not positively stained (data not shown). These data suggest that the distribution of Nav1.5 protein is restricted to some specific nerve tracts like those in the limbic system, olfactory system, and striatum.
To characterize the localization of Nav1.5 at the cellular level, we performed double immunostaining using the rabbit anti-Nav1.5 antibody and the anti-neurofilament antibody. Figure 3 shows confocal microscopic images of the caudate putamen of the basal ganglion. Co-localization of Nav1.5 and neurofilaments (the major intermediate filament proteins in axons) indicates that Nav1.5 is mainly localized in axons (Fig. 3a–c). Furthermore, because neuro-filaments are synthesized in cell bodies and then transported into axons [17], co-localization of Nav1.5 and neurofilaments may suggest that Nav1.5 is synthesized in cell bodies and then transported into axons.
DISCUSSION
Increasing evidence suggests that the distinctive roles played by different brain Na channels are often dictated by their unique localization in excitable tissues. We observed the striking and distinct localization of the Nav1.5 Na channels in axons of certain distinct regions of the brain. Axons are specialized for conducting electrical signals. These results are consistent with the functional role of sodium channels, i.e. generation and propagation of the action potentials in the neurons and for conducting electrical signals throughout the nervous system. Together with Nav1.9, Nav1.5 channels may generate the TTX-resistant sodium currents in the brain, which may play a role in sustained firing of neurons or as pace-makers.
The distribution of the cardiac sodium channel Nav1.5 in the brain has not been previously characterized at the protein level. Our study represents the first detailed characterization of Nav1.5 protein distribution in the mouse brain. Consistent with the distribution of Nav1.5 transcripts in rat and human brain [2,12], the mouse Nav1.5 protein was present in the limbic system, olfactory system, thalamus, and hypothalamus. However, our study revealed localization of Nav1.5 protein in several brain regions that were not described or analyzed in previous studies. These regions include the cerebral cortex, striatum, and cerebellum. In the limbic system, Hartmann et al. [12] and Donahue et al. [2]showed strong expression of Nav1.5 mRNA in the piriform cortex, septal nuclei, the diagonal band of Broca, amygdala, and habenular nuclei. We found weak or moderate presence of Nav1.5 protein in the nerve fibrous structures in these regions. A possible explanation for the difference may be that Nav1.5 mRNA is localized to the neuronal cell bodies, whereas the Nav1.5 protein molecules are synthesized in these locations, and are transported to and accumulate in the nerve fibers. An alternative explanation is that Nav1.5 mRNA in some regions may not be fully translated, as reported for the GABA A receptor mRNA and the NMDA R1 mRNA [2]. Our results imply that it is important to investigate the distribution of ion channels in the brain at the protein level.
Mutations in Nav1.5 cause cardiac arrhythmias and sudden cardiac death [7–11]. It has been recognized that the nervous system plays a role in the genesis of cardiac arrhythmias. Although it is speculative and remains to be investigated, it is possible that mutant Nav1.5 proteins expressed in certain brain regions of patients with cardiac arrhythmias may play a role in generating the trigger to provoke arrhythmias in these patients. Of particular interest is the expression and localization of Nav1.5 in the brainstem, which is known to be involved in regulating cardiac activities through autonomic motor neurons.
CONCLUSION
This study represents the first detailed characterization of Nav1.5 protein distribution in the mouse brain. Nav1.5 protein is widely distributed throughout the central nervous system, including the cerebral cortex, thalamus, hypothalamus, striatum, cerebellum and brainstem. At the cellular level, Nav1.5 protein is mainly localized to the axons. Our results are consistent with the hypothesis that Nav1.5 protein may play an important role in regulating neuronal excitability.
Acknowledgments
We thank Larry Oliver for brain tissue processing, Xiao-Li Tian for help, and Mary E.Rayborn, Bruce D.Trapp, and Hitoshi Komuro for comments and discussions.This work was supported in part by the American Heart Association Ohio-A/liate grant-in-aid (Q.W.), and a grant from NIH (R0I HL66251 (Q.W.).
References
- 1.Catterall WA. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 2.Donahue LM, Coates PW, Lee VH, et al. Brain Res. 2000;887:335–343. doi: 10.1016/s0006-8993(00)03033-x. [DOI] [PubMed] [Google Scholar]
- 3.Fozzard HA, Hanck DA. Physiol Rev. 1996;76:887–926. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- 4.Jeong SY, Goto J, Hashida H, et al. Biochem Biophys Res Commun. 2000;267:262–270. doi: 10.1006/bbrc.1999.1916. [DOI] [PubMed] [Google Scholar]
- 5.Elliott AA, Elliottv JR. J Physiol. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellens ME, Jr, George AL, Chen LQ, et al. Proc Natl Acad Sci USA. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Pyeritz RE, Seidman CE, et al. Genetic studies of myocardial and vascular disease Topol EJ.Textbook of Cardiovascular Medicine Philadelphia: Lippincott Williams and Wilkins; 2001. 19671990 [Google Scholar]
- 8.Wang Q, Shen J, Li Z, et al. Hum Mol Genet. 1995;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Shen J, Splawski I, et al. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Kirsch GE, Zhang GE, et al. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 11.Tan HL, Bink-Boelkens MTE, Bezzina CRP, et al. Nature. 2001;409:1043–1047. doi: 10.1038/35059090. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann HA, Colom LV, Sutherland ML, et al. Nature Neurosci. 1999;2:593–595. doi: 10.1038/10147. [DOI] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1988. p. 53. [Google Scholar]
- 14.Nishiyama K, Murayama S, Kwak S, et al. Ann Neurol. 1997;41:551–556. doi: 10.1002/ana.410410420. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama K, Trapp BD, Ikezu T, et al. J Neurosci. 1999;19:6538–6548. doi: 10.1523/JNEUROSCI.19-15-06538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Li Z, Shen J, et al. Genomics. 1996;34:9–16. doi: 10.1006/geno.1996.0236. [DOI] [PubMed] [Google Scholar]
- 17.Miller CCJ, Ackerley S, Brownlees J, et al. Cell Mol Life Sci. 2002;59:323–330. doi: 10.1007/s00018-002-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


