Abstract
Experimental evidence demonstrates that cerebellar networks are involved in spatial learning, controlling the acquisition of exploration strategies without blocking motor execution of the task. Action learning by observation has been considered somehow related to motor physiology, because it provides a way of learning performances that is almost as effective as the actual execution of actions. Neuroimaging studies demonstrate that observation of movements performed by others, imagination of actions, and actual execution of motor performances share common neural substrates and that the cerebellum is among these shared areas. The present paper analyzes the effects of observation in learning a spatial task, focusing on the cerebellar role in learning a spatial ability through observation. We allowed normal rats to observe 200 Morris water maze trials performed by companion rats. After this observation training, “observer” rats underwent a hemicerebellectomy and then were tested in the Morris water maze. In spite of the cerebellar lesion, they displayed no spatial defects, exhibiting exploration abilities comparable to controls. When the cerebellar lesion preceded observation training, a complete lack of spatial observational learning was observed. Thus, as demonstrated already for the acquisition of spatial procedures through actual execution, cerebellar circuits appear to play a key role in the acquisition of spatial procedures also through observation. In conclusion, the present results provide strong support for a common neural basis in the observation of actions that are to be reproduced as well as in the actual production of the same actions.
Recent findings demonstrated that the cerebellum must be included in the neural substrates that process spatial information (1–4). Whereas neocortical and hippocampal regions are involved in memory for spatial and object information and in locating objects in a given environment (5–8), the cerebellum appears to be involved in the navigational system with overt procedural features that control the way to reach an object, explore a new environment, and acquire spatial knowledge in relation to personal orientation. Thus, the cerebellar function is specifically linked to “how to find an object” rather than “where the object is” (9). This evidence mainly results from experimental data reporting that in the Morris water maze (MWM), hemicerebellectomized (HCbed) rats are impaired in developing efficient exploration strategies, but not in locating platform position (3). When the task is proposed for the first time postoperatively, HCbed animals display only peripheral circling around the pool, a rather ineffective way of acquiring spatial information. Conversely, when spatial procedures are acquired by executing the MWM task preoperatively, the cerebellar lesion does not alter spatial task execution.
Thus, these findings demonstrate that cerebellar circuitry is involved only (or primarily) in the acquisition of procedural components of spatial tasks and that a cerebellar lesion blocks the learning of spatial task requirements without blocking motor execution of the task (3).
There is a great deal of psychological evidence that the actual execution of a task is not the only way of acquiring an ability (10, 11). In fact, even observation of actions provides an effective way of learning new skills. This is a very important evolutionary mechanism in the social life. Just because perception of actions provides cues for interpreting the intention of the subject under observation, humans, as well as animals, have the ability of imitating a large range of actions (12). As part of the broader phenomenon related to recognizing, intending, and preparing a movement, observational learning of actions can be considered somehow related to motor physiology. However, its mechanisms are rather controversial and little is known about the neural structures involved. By taking advantage of the specific role of the cerebellum in procedural acquisition (3, 13), we developed a paradigm of observational learning apt for rats to analyze the effects of observation in learning a spatial task and cerebellar involvement in it.
Materials and Methods
Subjects.
Useful data were collected from 39 adult male Wistar rats (250–300 g) that were housed two animals to a cage with free access to food and water throughout the experiment and standardized dark/light schedule (10 h dark/14 h light).
Surgery and Motor Assessment.
The rats were anaesthetized with sodium pentobarbital (Nembutal, 40 mg/kg, i.p.). A craniotomy was performed over the right hemicerebellum. The dura was excised and the right cerebellar hemisphere and hemivermis were ablated by suction; care was taken not to lesion extracerebellar structures. The cavity was filled with sterile gel foam, and the wound edges were sutured. After recovery from anesthesia, the animals were housed two per cage. Testing was performed 2 weeks after the hemicerebellectomy (HCb), when no changes in cerebellar symptomatology were observed. The following aspects were taken into account: head and body tilts, positions of either hindlimb in relation to trunk, presence of ataxia, tremor, rearing behavior, falls to lesion side, wide-based locomotion, collapsing on the belly, pivoting, vestibular drop reactions, and abilities to traverse a narrow path and to be suspended on a wire. Details and time course of these cerebellar symptoms in rats were described elsewhere (3, 14, 15). It is noteworthy that these previous reports already demonstrated that HCbed animals are very competent swimmers and that motor and postural disturbances after a cerebellar lesion do not significantly correlate with spatial abilities in the water maze task.
MWM Testing.
The rats were placed in a circular plastic pool (diameter, 120 cm) with white inside walls, located in a normally equipped laboratory room, uniformly lighted by four neon lamps (40 W each) suspended from the ceiling (3 m). No care was taken to enhance (or, vice versa, to impoverish) extra-maze cues, which were held in constant spatial relations throughout the experiments. The pool was filled with water (24°C), which was 50 cm deep and made opaque by the addition of 2 liters of milk. A white, steel escape platform (10 cm in diameter) was placed in the middle of one cardinal quadrant (NW, NE, SW, SE) 30 cm from the side walls; it was either submerged 2 cm below or elevated 2 cm above the water level. Each rat was released gently into the water always from the same cardinal wall point (S) facing the center of the pool. The animal was allowed to swim around to find the platform. Blocks of four trials were presented to each rat, two blocks of trials per day. On reaching the platform, each rat was allowed to remain on it for 30 sec before being placed again in the water for the next trial. If a rat failed to locate the platform within 120 sec, it was guided there by the experimenter and allowed to stay there for 30 sec. In the first four sessions (trials 1–16) the platform was hidden in the NW pool quadrant (Place I), in the successive two sessions (trials 17–24) the platform was kept visible in the NE quadrant (Cue phase), and in the final four sessions (trials 25–40) the platform was hidden in the NE quadrant (Place II). The paths taken by the animals in the pool were monitored by a video camera mounted on the ceiling. The resulting video signal was relayed to a monitor, allowing both on- and off-line analyses, and to an image analyzer (Ethovision; Noldus Information Technology, Wageningen, The Netherlands). The x and y coordinates of the rat's position were sampled and stored on disk. The software can provide measures of latency, path length, swimming speed, and so on. Parameters analyzed were successful findings, finding latencies, and swimming trajectories. Exploration behavior was scored on a 1–10 rating scale described in detail elsewhere (16), with a score of 1 attributed to best performance (direct finding of the platform) and a score of 10 attributed to worst performance (unsuccessful peripheral circling).
Observational Training.
The animals were housed separately in small cages suspended over the water maze tank. The cage floor was a metallic grid through which they repeatedly observed companion rats performing the MWM test, consisting of 40 trials distributed over 10 sessions of 4 trials each. This trial sequence was observed 5 times, so that each suspended animal observed 200 trials.
Experimental Protocols.
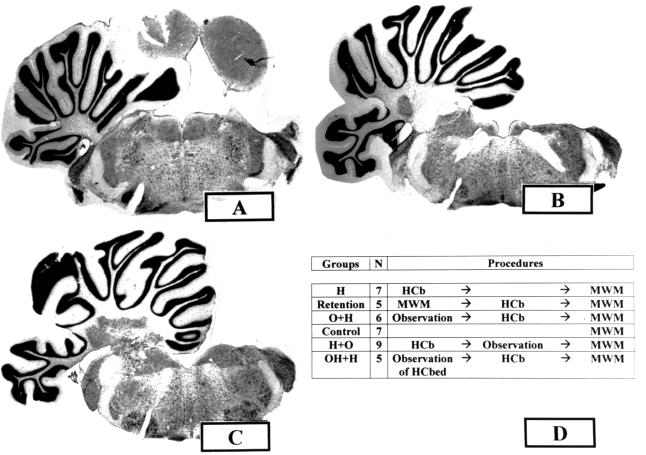
The animals were divided into six experimental groups (Fig. 1): (i) normal rats tested in the MWM used as controls (n = 7) (group name = Control group); (ii) HCbed animals tested in the MWM after cerebellar lesion (n = 7) (group name = H group); (iii) HCbed rats tested in the MWM before and after cerebellar lesion (n = 5) (group name = Retention group); (iv) HCbed rats that had observed control rats performing MWM paradigm before cerebellar lesion and that were tested postoperatively in the MWM (n = 6) (group name = O+H group); (v) HCbed rats that had observed control rats performing MWM paradigm after cerebellar lesion and that were then tested in the MWM (n = 9) (group name = H+O group); and (vi) HCbed rats that had observed HCbed animals performing MWM paradigm before cerebellar lesion and that were tested postoperatively in the MWM (n = 5) (group name = OH+H group).
Figure 1.
Coronal sections [bregma: −9.5 (A), −10.3 (B), and −11 (C)] through the cerebellum and brain stem of a HCbed rat. Note the total absence of the right hemicerebellum and the sparing of any extracerebellar structure. A similar lesion of cerebellar regions, as well as sparing of surrounding areas, was observed in all HCbed animals. (D) Experimental groups, number of subjects (N), and testing procedures.
Histological Controls.
After completion of behavioral testing, the animals were deeply anaesthetized with Nembutal and perfused with saline followed by 10% buffered formalin. The extent of the cerebellar lesion was determined from Nissl-stained, 40-μm frozen sections. Animals were included in the present study if they had received a complete right HCb with a total ablation of deep nuclei (Fig. 1). In all cases reported here, the left side of the cerebellum and all extracerebellar structures were completely spared, except for the dorsal cap of the right Deiters' nucleus, which, in some cases, was slightly affected. The variability in the extent of the floccular and vermal lesions was considered noninfluencing, because in all cases these structures were functionally disconnected because of the ablation of the cerebellar peduncles and deep nuclei of the right side.
Statistical Analysis.
Metric unit results of animals belonging to the different experimental groups first were tested for homoscedasticity of variance and then compared by using one-way or two-way “p × q” ANOVAs with repeated measures, eventually followed by multiple comparisons using Tukey's tests.
Results
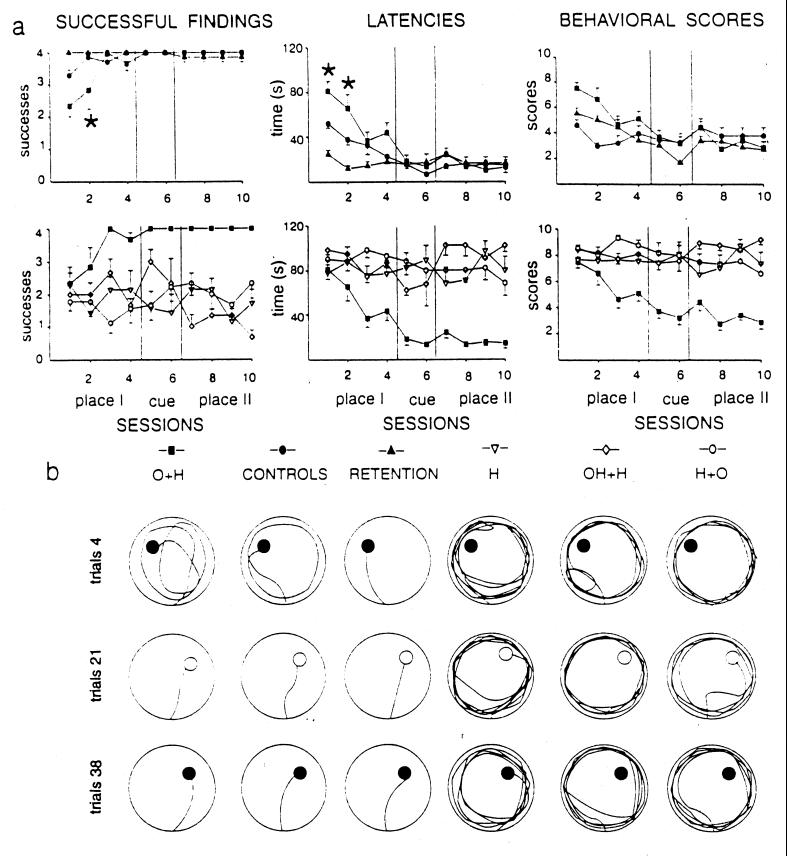
The findings obtained in the present paper indicate that the treatments the animals underwent (presence or absence of observational training, presence or absence of cerebellar lesion, presence or absence of preoperative training) clearly influenced their performances in the MWM. When the time course of the spatial performances of all experimental groups and performance levels reached in Place II sessions were analyzed, two different learning profiles clearly emerged. The first one, displayed by the O+H, Control, and Retention groups, was characterized by high levels of learning, whereas the second one, displayed by the H, OH+H, and H+O groups, was characterized by flattened learning curves (Fig. 2). Two-way ANOVAs (group × session) on successful findings, latencies, and scores revealed highly significant group and session effects. Also, group × session interactions were significant (Table 1).
Figure 2.
(a) Mean (±SE) successful findings, latencies (s), and behavioral scores of exploration strategies of the six experimental groups in the MWM paradigm. On the basis of the performance levels reached in Place II sessions, two different learning profiles were evidenced. The first one [O+H, Control, and Retention groups (Upper)] was characterized by high levels of learning (*, P < 0.05, Tukey's test: O+H vs. Control group), whereas the second one [H, OH+H, and H+O groups (Lower)] was characterized by flattened learning curves (statistical comparisons are reported in Table 1). (b) Individual swimming trajectories of specimens of the six experimental groups during representative trials. O+H and Control rats exhibited searching behaviors extending around the pool in the early trials and direct platform findings in the successive phases. Retention rats exhibited direct trajectories to the platform from the very first trials. Conversely, H, OH+H, and H+O animals displayed peripheral circling in all task phases, with infrequent successful findings.
Table 1.
Statistical comparisons of MWM spatial performances of the experimental groups
| Group effect
|
Session effect
|
Interaction
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Freedom degrees | F value | P | Freedom degrees | F value | P | Freedom degrees | F value | P | |
| 6 × 10 ANOVAs [group (O+H, Controls, Retention, H, OH+H, H+O) × session] | |||||||||
| Findings | 5,36 | 36.91 | 0.0000 | 9,324 | 1.42 | n.s. | 45,324 | 2.33 | 0.0000 |
| Latencies | 5,36 | 45.16 | 0.0000 | 9,324 | 6.96 | 0.0000 | 45,324 | 2.86 | 0.0000 |
| Scores | 5,36 | 41.36 | 0.0000 | 9,324 | 6.99 | 0.0000 | 45,324 | 3.00 | 0.0000 |
| 3 × 10 ANOVAs [group (O+H, Controls, Retention) × session] | |||||||||
| Findings | 2,17 | 7.87 | 0.0038 | 9,153 | 9.86 | 0.0000 | 18,153 | 5.47 | 0.0000 |
| Latencies | 2,17 | 7.64 | 0.0043 | 9,153 | 22.25 | 0.0000 | 18,153 | 6.42 | 0.0000 |
| Scores | 2,17 | 2.31 | n.s. | 9,153 | 12.27 | 0.0000 | 18,153 | 3.54 | 0.0000 |
| 3 × 10 ANOVAs [group (H, OH+H, H+O) × session] | |||||||||
| Findings | 2,19 | 0.01 | n.s. | 9,171 | 0.90 | n.s. | 18,171 | 2.25 | 0.0038 |
| Latencies | 2,19 | 0.31 | n.s. | 9,171 | 0.70 | n.s. | 18,171 | 1.95 | 0.0145 |
| Scores | 2,19 | 0.71 | n.s. | 9,171 | 0.56 | n.s. | 18,171 | 1.96 | 0.0139 |
| 2 × 10 ANOVAs [group (O+H, H) × session] | |||||||||
| Findings | 1,11 | 32.52 | 0.0001 | 9,99 | 1.66 | n.s. | 9,99 | 2.44 | 0.0149 |
| Latencies | 1,11 | 31.65 | 0.0002 | 9,99 | 4.30 | 0.0001 | 9,99 | 4.74 | 0.0000 |
| Scores | 1,11 | 28.43 | 0.0002 | 9,99 | 4.49 | 0.0001 | 9,99 | 4.59 | 0.0000 |
n.s., not significant.
In detail, animals belonging to the H group were seriously defective in solving the MWM. They failed to actively search for the platform whether or not the platform was visible, displaying only peripheral circling in all testing phases (Fig. 2) without overt learning of task requirements. Even in the very final trials they persisted in their ineffective exploration strategy, consisting of repeated circling at the periphery of the pool, as described previously (3).
When rats performed the MWM before HCb and then were tested after recovery from cerebellar lesion (Retention group), no searching deficit was present. These animals successfully reached the platform with very effective exploration patterns and low finding latencies (Fig. 2), maintaining preoperatively acquired performance levels (3).
Animals belonging to the O+H group underwent a HCb after observation of 200 MWM trials performed by intact animals. They then were tested in the MWM according to the same paradigm they had observed. Their performances in the MWM significantly differed from those displayed by HCbed rats without observation training, that is, the H group (Table 1). Even in the very first trials, they did not show any compulsive peripheral circling (Fig. 2). Instead, they immediately detached from pool walls and foraged around the pool to search (and find) the escape platform. No animal displayed either direct trajectories or a searching bias toward the platform position observed in the Cue and Place II phases. As testing proceeded, they very quickly restricted their searching to the correct quadrant and finally exhibited direct finding (Fig. 2). These results clearly indicate the marked ameliorative effect of observation training. The performances of the O+H group significantly differed from successful finding and latency data displayed by the Control and Retention groups (Table 1), because of the different behavior displayed by the O+H group in the first two sessions, as indicated by posthoc comparisons (Fig. 2). From the third session on, O+H animals' performances were indistinguishable from those of the Retention and Control groups, indicating comparable learning. These results demonstrate that both performing (Retention group) and observing (O+H group) a spatial task have “training” value.
Because our previous findings demonstrated the prevalent role of cerebellar circuits in acquisition rather than in storage or recall of procedural exploration strategies (3, 13, 16), we specifically tested acquisition by observation in an experimental group in which the cerebellar lesion preceded observation training (H+O group). When tested in the MWM, these rats completely lacked the learning effects of observation (Fig. 2). They persisted in displaying the peripheral circling without any efficient exploration strategy, so that they reached the platform only in 50% of the trials, even in the last sessions.
To analyze the role played by observation content in affecting spatial learning, that is, the role of the observed exploration behavior, a different experimental approach was used. In this setting, training paradigm and environment remained unchanged, whereas the exploration behavior the animals observed was different. A group of naive animals (OH+H group) observed HCbed animals performing the MWM task in their very defective way, that is, exhibiting only compulsive peripheral circling. After this peculiar observation training, these animals were then HCbed. When tested in the MWM, they displayed the typical impairment of the H group, consisting of peripheral circling, no effective exploration strategy, and a very low number of successful findings (Fig. 2).
Discussion
The present results indicate that rats are able to learn a complex spatial skill by observing companion rats doing it and that a lesion of the cerebellar circuits prevents this acquisition. Just as performing the MWM before the cerebellar lesion has a dramatic ameliorative effect on the spatial performances of the HCbed animals, observing the performance before the cerebellar lesion also allows overcoming the spatial deficits that follow the HCb. In fact, the animals of the O+H group, which had observed companion rats correctly performing the spatial task before the cerebellar lesion, exhibited spatial performances almost equal to those displayed by control animals and by HCbed animals, which had experienced the MWM task before the lesion. The slight difference observed in the first two sessions in the learning curve of the O+H group in comparison with the Control and Retention groups' performances can be ascribed to additive effects of the cerebellar lesion and lack of direct experience of the task. Physical forces (inertia, friction, etc.) are not predictable by observation, and, thus, the subject must adjust its motor production on the basis of proprioceptive feedback available only during the actual swimming experience (17). Complete learning of a motor task then can be gained only through fine-tuning of those aspects available during actual execution. In fact, only in the actual process of self-movement it is possible to integrate the sensory information generated by the vestibular system, the muscle and joint receptors, and the efferent copies of the commands that generate movements, that is, the path integration system, as it has been defined recently (18–20). Just as in learning through execution (H group), in observational learning no improvement was observed in the MWM task when the cerebellar lesion preceded the learning training (H+O group). The close similarity of effects provoked by a cerebellar lesion on learning a spatial task through observation or actual execution demonstrates that both kinds of learning, at least partially, share common neural substrates and that the cerebellum is among these shared structures (21–23).
This notion is supported by neuroimaging and experimental studies. PET findings indicate that observation of movements performed by others, motor imagery, and actual execution of a motor performance share common neural circuits (21, 24–26), suggesting that mental practice and motor learning during observation of movements involve rehearsal of neural pathways related to cognitive stages of motor control (27, 28). Among the cortical and subcortical structures activated in motor learning, the cerebellum appears to be prominent and its activation is closely linked to the first phases of learning (29). After practice, cerebellar activity declines to very low levels; the cerebellum apparently becomes disengaged from the processing loop (30, 31). The present data demonstrate the importance of cerebellar structures in observational learning and indicate that precisely the activity of cerebellar areas during observation allows learning of spatial performances. In fact, the complete absence of spatial learning in the H+O group in which the experimental manipulations were exactly the same as those of the O+H group, but inverted in sequence, strongly supports the idea of the necessity for intact cerebellar networks for observational learning. Also in this form of learning, as proposed for actual motor performance (27, 28), the cerebellum integrates the context and behavioral information underlying spatial procedural learning. The specificity of the cerebellar role in learning is corroborated further by evidence that, once acquired, spatial procedures can be performed efficiently in the presence of cerebellar damage, in agreement with the neuroimaging evidence of low cerebellar activation after prolonged practice.
Two brain regions in monkeys were described experimentally as implicated in encoding aspects of action. Neurons selective for the sight of actions are reported to be located in the superior temporal sulcus (32), and neurons activated when the monkey either performs or observes the experimenter performing the same meaningful action were described in the inferior premotor cortex (33). This supports the existence of representational neurons and of a common substrate for motor preparation and perception of movement (17). This coincidence of activation was suggested to be the neural basis for understanding the meaning of actions as well as for learning by observation (34, 35). The present data extend the observation/execution matching mechanism proposed for cortical areas to the cerebellar structures.
What is learned through observation in our paradigm? It can be argued that the animals learned the platform location by observation. In fact, in the protocol used the platform was only in two different pool positions and was completely visible in the cue trials. Furthermore, observers saw demonstrator animals standing on the platform for 30 sec at the end of every trial, thus attracting attention to the platform presence, an effect known as “local enhancement” (36, 37). However, this localization learning hypothesis can be discarded by taking into account the MWM performances of observer animals. If animals had acquired a cognitive map of the two platform locations by observation, their search would have been restricted to these two locations. No animal showed a bias toward the quadrants that contained or had contained the platform. Not even the O+H group, which greatly benefited from observation, displayed the direct reaching of the platform typical of animals that know where the platform is, except in the last sessions. Moreover, the OH+H group, which had observed impaired exploration behaviors in the same MWM paradigm and then with the same localization information, did not display any ameliorative effect of observation, indicating that the platform position is not the relevant factor that allows overcoming cerebellar spatial deficits. Also, some experimental evidence converges in indicating that the animals learn by observation the spatial procedures that others use to solve the spatial problem. During the observation training, the O+H group observed very different swimming trajectories and behaviors, which were linked to different exploration strategies (peripheral swimming, extended searching, restricted searching, direct finding) that individual specimens put into action in different testing phases. Every demonstrator animal swam in the pool, developing an exploration strategy different not only from that of other animals but even different from the one that the animal itself had put into action before or was going to develop in the subsequent trials. Thus, these performances were not a fixed sequence of movements, even if very complex, but were spatial procedures aimed at pool exploration and platform searching. When they actually experienced the task after HCb, observers did not exhibit stereotyped swimming behaviors copied from others, but coherent and elaborate exploration strategies through space. It is worth noting that the animals belonging to this group displayed the correct sequencing of exploration strategies, reproposing the same temporal steps as control animals, demonstrating that they not only had learned the single strategies but also when their use was appropriate. Further support for the procedural learning interpretation derives from the OH+H animals' behavior that reproduced the impaired searching they had observed. In summary, observers appear to learn exploration behaviors that are effective or ineffective in relation to the models they have observed. Thus, by observation, as well as by actual performance, our animals did not learn a “what” or a “where,” but a “how.”
Theoretically, observational learning of a spatial performance requires that observers understand the actions of other individuals in terms of the same neural code they use to produce the same motor behavior themselves (38). The prevalent interpretation of observational learning assumes temporally distinct stages in which the information is extracted from a model, transformed into a representation code, and stored as some template of actions; then, the rehearsal processes enhance this memory representation, inducing learning. By repeated observation the neural commands that evoke the corresponding behavioral strategies are boosted and tuned and the observed behavior is definitely learned (39). Within this theoretical framework, in agreement with recent clinical (40) and neuroimaging data (41) in humans, the present data indicate that the cerebellar contribution may be to sustain the rehearsal of cortical processes. Although this is still a working hypothesis, it is particularly appealing in light of the increasing interest in the cerebellar involvement in cognition.
Acknowledgments
We thank Dr. R. Filippetti and his staff for technical assistance. This research was supported by Consiglio Nazionale delle Ricerche and Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica grants to M.M. and L.P.
Abbreviations
- MWM
Morris water maze
- HCb
hemicerebellectomy
- HCbed
hemicerebellectomized
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040554297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040554297
References
- 1.Joyal C C, Meyer C, Jacquart G, Mahler P, Caston J, Lalonde R. Brain Res. 1996;739:1–11. doi: 10.1016/s0006-8993(96)00333-2. [DOI] [PubMed] [Google Scholar]
- 2.Lalonde R. In: The Cerebellum and Cognition. Schmahmann J D, editor. San Diego: Academic; 1997. pp. 191–215. [Google Scholar]
- 3.Petrosini L, Molinari M, Dell'Anna M E. Eur J Neurosci. 1996;8:1882–1896. doi: 10.1111/j.1460-9568.1996.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 4.Molinari M, Petrosini L, Grammaldo L G. In: The Cerebellum and Cognition. Schmahmann J D, editor. San Diego: Academic; 1997. pp. 217–230. [Google Scholar]
- 5.Morris R G M, Garrud P M, Rawlins J N P, O'Keefe J. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 6.Eichenbaum H, Stewart C, Morris R G M. Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- 7.Nadel L. Hippocampus. 1991;1:221–229. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- 8.Kolb B, Sutherland R J, Whishaw I Q. Behav Neurosci. 1983;97:13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- 9.Petrosini L, Leggio M G, Molinari M. Prog Neurobiol. 1998;56:191–210. doi: 10.1016/s0301-0082(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 10.White A, Hardy L. Brit J Psychol. 1995;86:169–180. doi: 10.1111/j.2044-8295.1995.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 11.Yágüez L, Nagel D, Hoffman H, Canavan A G M, Wist E, Hömberg V. Behav Brain Res. 1998;90:95–106. doi: 10.1016/s0166-4328(97)00087-9. [DOI] [PubMed] [Google Scholar]
- 12.Byrne R W, Russon A E. Behav Brain Sci. 1998;21:667–721. doi: 10.1017/s0140525x98001745. [DOI] [PubMed] [Google Scholar]
- 13.Molinari M, Leggio M G, Solida A, Ciorra R, Misciagna S, Silveri M C, Petrosini L. Brain. 1997;120:1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- 14.Petrosini L, Molinari M, Gremoli T. Exp Brain Res. 1990;82:472–482. doi: 10.1007/BF00228789. [DOI] [PubMed] [Google Scholar]
- 15.Molinari M, Petrosini L, Gremoli T. Exp Brain Res. 1990;82:483–492. doi: 10.1007/BF00228790. [DOI] [PubMed] [Google Scholar]
- 16.Leggio M G, Neri P, Graziano A, Mandolesi L, Molinari M, Petrosini L. Exp Brain Res. 1999;127:1–11. doi: 10.1007/s002210050768. [DOI] [PubMed] [Google Scholar]
- 17.Jeannerod M. Behav Brain Sci. 1994;17:187–245. [Google Scholar]
- 18.Etienne A S, Maurer R, Seguinot V. J Exp Biol. 1996;199:201–209. doi: 10.1242/jeb.199.1.201. [DOI] [PubMed] [Google Scholar]
- 19.Whishaw I Q, McKenna J, Maaswinkel H. Curr Opin Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- 20.Whishaw I Q, Maaswinkel H. J Neurosci. 1998;18:3050–3058. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta J C, Fazio F. Nature (London) 1994;371:600–602. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- 22.Decety J. Cognit Brain Res. 1996;3:87–93. doi: 10.1016/0926-6410(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 23.Decety J, Grézes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- 24.Stephan K M, Fink G R, Passingham R E, Silbersweig A O, Ceballos-Baumann A O, Frith C D, Frackowiak R S J. J Neurophysiol. 1993;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- 25.Tyszka J M, Grafton S T, Chew W, Woods R P, Colletti P M. Ann Neurol. 1994;35:746–749. doi: 10.1002/ana.410350617. [DOI] [PubMed] [Google Scholar]
- 26.Parson L M, Fox P T, Downs J H, Glass T, Hirsch T B, Martin C C, Jerabek P A, Lancaster J L. Nature (London) 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 27.Thach W T. Behav Brain Sci. 1996;19:411–431. [Google Scholar]
- 28.Thach W T. Trends Cognit Sci. 1998;2:331–337. doi: 10.1016/s1364-6613(98)01223-6. [DOI] [PubMed] [Google Scholar]
- 29.Flament D, Ellerman J M, Kim S-G, Ugurbil K, Ebner T J. Human Brain Map. 1996;4:210–226. doi: 10.1002/hbm.460040302. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins I H, Brooks D J, Nixon P D, Frackowiak R S J, Passingham R E. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen S E, van Mier H, Fiez J A, Raichle M E. Proc Natl Acad Sci USA. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrett D I, Harris M H, Bevan R, Thomas S, Bensons P J, Mistlin A J, Chitty A J, Hietanen J K, Ortega J E. J Exp Biol. 1989;146:87–113. doi: 10.1242/jeb.146.1.87. [DOI] [PubMed] [Google Scholar]
- 33.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 34.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 35.Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Exp Brain Res. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- 36.Heyes C M, Dawson G R, Nokes T. Q J Exp Psychol. 1992;45:229–240. [Google Scholar]
- 37.Heyes C M, Jaldow E, Nokes T, Dawson G R. Behav Proc. 1994;32:173–182. doi: 10.1016/0376-6357(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 38.Decety J. Trends Cognit Sci. 1999;3:172–178. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- 39.Carroll W R, Bandura A. J Mot Behav. 1985;17:269–281. doi: 10.1080/00222895.1985.10735349. [DOI] [PubMed] [Google Scholar]
- 40.Silveri M C, Di Betta A M, Filippini V, Leggio M G, Molinari M. Brain. 1998;121:2175–2187. doi: 10.1093/brain/121.11.2175. [DOI] [PubMed] [Google Scholar]
- 41.Paulesu E, Frith C D, Frackowiak R S. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]