Abstract
Two forms of gonadotropin-releasing hormone (GnRH) were isolated from the gonads of the tunicate, Ciona intestinalis. The primary structure of the purified peptides was determined by MS and chemical sequence analysis. Both GnRH forms have blocked NH2 and COOH termini, and their primary structures are identical to mammalian (mGnRH) and chicken I (cGnRH-I) forms reported previously in vertebrates. A total of 1.2 mg of purified cGnRH-I and 0.98 mg of mGnRH was obtained from 100 g of Ciona gonads. The physiological effects of native GnRHs included the induction of synthesis and secretion of sex steroids from ciona gonads and the secretion of luteinizing hormone from rat pituitary. These results suggest that the primary structure and functional roles of mGnRH and cGnRH-I have been highly conserved throughout evolution of chordates.
Keywords: neuropeptides, evolution, reproduction
Gonadotropin-releasing hormone (GnRH) was first isolated as a hypothalamic decapeptide in mammals (1, 2). Its primary role is related to the regulation of pituitary gonadotropin release and synthesis (3–6). There is evidence for the presence of structural variants of GnRH in chordates. A number of reviews have surveyed the literature on the presence, distribution, characterization, and functions of GnRHs in vertebrates and invertebrates (3–8). Indeed, the presence of GnRH in chordates spans the entire genealogy of this phylum, from tunicates and cephalochordates to all classes of vertebrates. The majority of studies on vertebrates involved immunological identification, ontogenetic pattern, immunohistochemical localization, and characterization of GnRH/GnRH-like peptides in the central nervous system. However, there is evidence that GnRH/GnRH-like peptides are present in nonneural tissues as well, such as the gonads in mammals, amphibians, and fish, human placenta, mammary gland, and mammalian pancreas (see refs. 7 and 9–14). Despite a number of immunological studies indicating the presence of GnRH-like peptides in the gonads of several species of vertebrates, the primary structure of gonadal GnRH(s) has been determined only in the ovary of the goldfish (14).
Among the invertebrate chordates, immunohistochemical localization of GnRH-like peptides was described in the cerebral ganglion and the neural gland, as well as along the inner wall of the dorsal blood sinus and on the anterior surface of the ovary of two protochordates (Tunicata), Ciona intestinalis and Chelyosoma productum (15–19). Recently, two novel GnRH variants were isolated and purified from the ascidian, C. productum, and named as tunicate GnRH-I and GnRH-II (18). Powell et al. (18) used only the dorsal portion of the body that does not contain gonads. To our knowledge, there is no report of the presence of GnRH/GnRH-like peptides in the gonads of any invertebrate chordate. We have isolated, purified, and determined the primary structure of two GnRH peptides from the gonads of a tunicate protochordate, C. intestinalis. In addition, we have obtained in vitro evidence of their biological activity.
Materials and Methods
Tissue Collection.
Five hundred sexually mature C. intestinalis were collected in the Bay of Naples during the period of April 1996 to June 1998. Gonads were frozen immediately on dry ice and stored at −80°C until used.
Extraction and Purification.
Except for RP-HPLC analysis at room temperature, all other steps of extraction and purification were conducted at 2–4°C. Step 1: Frozen gonads (100 g) were defrosted in 500 ml of 0.2 M perchloric acid and homogenized by using Ultra-Turrax T25 (Janke & Kunkel Ika Labortechnik, Staufen, Germany). The homogenate was centrifuged for 1 hr at 30,000 × g, and the supernatant was brought to neutral pH with 1 M KOH. To obtain maximum insolubilization of potassium perchlorate, the supernatant was left overnight at 4°C. Step 2: After centrifugation, the supernatant was divided into four aliquots and each aliquot was applied to a C-18 extraction column (1.8 × 4.0 cm) containing 10 g of resin octadecyl silane (Waters Sep-Pak cartridge; Millipore, or Varian Mega Bond Elut), previously activated in 100% methanol and equilibrated in distilled water. The columns were eluted by an appropriate water vacuum system under moderate aspiration. After adsorption of the sample, the columns were washed with 100 ml of distilled water followed by 10% methanol (100 ml/column). Subsequently, each column was eluted with 50 ml of 60% methanol. This last eluant was used for the next step of purification. Step 3: The fluent was diluted 1:1 with distilled water and filtered on a 10,000-Da molecular mass cut-off membrane applied to a 200-ml ultrafiltration cell (model 8200; Amicon) under nitrogen pressure of 4 bars. When the sample volume was reduced to about 3–4 ml, 20 ml of distilled water was added to the cell and refiltered as above. Step 4: Filtrates were combined and refiltered on a 1,000-Da molecular mass cut-off membrane until a final volume of 5–6 ml. Once again, 20 ml of distilled water was added to the cell, and filtration was continued until a reduction of the volume to 1 ml. Step 5: Aliquots of 100 μl each were injected into a RP-HPLC analytical column (4.6 × 250 mm, 5-μ particle size, 300-Å pore size, supelcosil LC-18 matrix; Supelco) connected with a Beckman HPLC Gold system. The mobile phase was 5% acetonitrile (solvent A) and 90% acetonitrile (solvent B), both in 20 mM sodium acetate buffer at pH 5.5. The concentration of solvent B was increased from 0 to 50% for 50 min, then raised from 50% to 100% over 5 min, and finally held at 100% for 5 min. The elution profile was monitored continuously at 220 nm, and the flow rate was 1.2 ml/min. Two peaks with elution time of 21.5–22.0 min and 29.0–29.5 min, corresponding to the elution time, respectively, of synthetic chicken I (c-I) GnRH (cGnRH-I) and mammalian GnRH (mGnRH), under the same conditions, were collected and dried in a speed vacuum system at cold. All dried residues of the same fraction were dissolved in 200 μl of 0.001 M HCl and used for characterization and determination of biological properties of the isolated peptide. In a subsequent HPLC run, each fraction gave only one sharp peak corresponding to the elution time, respectively, of the cGnRH-I and mGnRH forms.
Peptide Determination.
Total peptide concentration was determined by the bicinchoninic acid method (20) by using the Perstorp Biotec reagent kit manufactured by Pierce with mGnRH as standard (Sigma).
Mammalian GnRH Assay (RIA).
In addition to HPLC, mGnRH also was determined by RIA. In this case the fractions eluted from the HPLC column were used according to a procedure described earlier (21, 22).
Amino Acid Composition Analysis.
An aliquot (5 μg) of each purified peptide was hydrolyzed in 100 μl of 6 M HCl containing 2% thioglycolic acid in a sealed, evacuated tube at 110°C for 16 hr. The hydrolyzed samples were lyophilized, reconstituted in 0.15 M lithium citrate buffer (pH 2.2), and subjected to amino acid analysis (Beckman Instrument model 121 MB). Because this procedure destroys the tryptophan, a second hydrolysis was carried out in which the peptide was incubated with 2 M NaOH for 16 hr at 100°C to detect the amount of this amino acid.
Amino Acid Sequence.
The amino-terminal amino acid sequence was determined by an automated, repetitive Edman degradation by using an Applied Biosystems Procise Sequencer (Perkin–Elmer). The peptides (10-μg aliquots) were incubated previously with 0.1 μg of calf liver pyroglutamate aminopeptidase (Boehringer Mannheim) in 100 μl of 0.1 M sodium phosphate buffer (pH 8.0) containing 10 mM Na2 EDTA, 5 mM dithioerythritol, and 5% glycerol (vol/vol) at 25°C for 4 hr. After incubation, the sample was subjected to the sequencer with off-line phenylthiohydantoin–amino acid identification under gradient elution conditions.
Fast Atom Bombardment MS.
Purified peptides (20 nmol each) were dissolved in 10 μl of trifluoroacetic acid/H2O/acetonitrile (1:740:740, vol/vol/vol). One microliter was delivered with the liquid matrix mixture of DTT and dithioerythritol (23) on a gold probe for analysis from m/z 500 to m/z 1,600. Thirty fast atom bombardment mass spectra were scanned for each peptide/matrix sample introduced directly into the mass spectrometer ion source. Each sample was bombarded with a beam of xenon fast atoms. Mass spectra were recorded with a Kratos MS-50 mass spectrometer fitted with a high-field magnet and equipped with a Kratos DS-55 data system (Kratos Analytical Instruments), previously calibrated against cesium iodide/glycerol and sodium fluoride over the mass range (23). Under similar conditions, mass spectra of corresponding synthetic GnRHs also were recorded.
Immunocytochemistry.
Gonads were fixed in Bouin's fluid or in 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Paraffin and cryostat sections were incubated in polyclonal anti-mGnRH or anti-cGnRH-I (dilution, 1:10,000 or 1:20,000). The succeeding steps consisted of treatment with biotinylated goat anti-rabbit IgG (1:150) and a preformed avidin-biotinylated horseradish peroxidase complex. The immunoreaction product was visualized with diaminobenzidine. Preabsorption controls with homologous synthetic GnRH and controls omitting the primary antibody were carried out.
In Vitro Bioassay (Biological Activity).
GnRH induction of sex steroid release and synthesis in vitro by Ciona gonads.
One gram of fresh gonadal tissue was placed in 20 ml of sea water in a Petri dish and mixed with 0.5 mg of native or synthetic mGnRH or cGnRH-I. Treatments with mGnRH and cGnRH-I were done on different days. Each treatment was done in triplicate, and an additional set without GnRH served as control. The mixture was incubated at 25°C under moderate agitation for 180 min. At 60- and 120-min intervals of incubation, 0.1 mg of GnRH was added to the medium. Incubation liquid was extracted with 100 ml of diethyl ether. The gonadal tissue was homogenized in 20 ml of PBS and then extracted with 200 ml of diethyl ether. In both cases, the ether phase was separated and dried and the residue was suspended in 0.5 ml of PBS containing 1 mg of BSA and centrifuged at 14,000 × g for 10 min. The supernatant was used for RIA of sex steroids. Progesterone, testosterone, and 17β-estradiol were measured by using hormone kits from Biochem Immunosystem Italia (Bologna, Italy).
GnRH induction of luteinizing hormone (LH) release from rat pituitary.
Each rat pituitary was cut (longitudinally) in half. Each piece was incubated in 2 ml of Krebs–Ringer buffer (pH 7.4) containing a mixture of protease inhibitors (Sigma) and 100 nM native or synthetic mGnRH under constant shaking at 37°C. After 10, 20, 30, and 40 min of incubation, 100-μl aliquots of incubation medium were used for LH determination by an RIA kit (Amersham Pharmacia). The same procedure was carried out for another set of pituitary halves, except that no GnRH was added.
Results
GnRH Purification.
The method described above allowed the extraction and purification of two GnRH forms from the gonads of C. intestinalis. The two decapeptides isolated possess chemical and biological characteristics of cGnRH-I and mGnRH. Results obtained on total peptides and GnRH-like peptides through the various steps of purification are summarized in Table 1. Starting with 100 g of gonadal tissue, 1.2 mg of cGnRH-I and 0.98 mg of mGnRH were obtained in the final step of purification. As a major strategy to obtain the maximum recovery of GnRH-like peptides, the gonads were homogenized directly in perchloric acid for an immediate inactivation of the proteases present in the tissue and at the same time to obtain a deproteinized sample containing the total perchloric acid-soluble peptides including those of our interest. The subsequent steps of purification allowed us to obtain the maximum recovery of the GnRH-like peptides (Table 1). Using cGnRH-I and mGnRH standards, during each step of purification the recovery was approximately of 80–90%, and after the last step of purification the total recovery was around 50%. We conclude that the total amount of cGnRH-I and mGnRH in 100-g gonads would be around 2.4 mg and 1.96 mg, respectively.
Table 1.
Purification of GnRH peptides from the gonads of C. intestinalis
| Volume, ml | Peptides, mg/ml | Peptides, total mg | Total peptide recovery, % | |
|---|---|---|---|---|
| Step 1: PCA homogenization | 520.0 | 2.41 | 1,253.2 | 100 |
| Step 2: C-18 extraction column | 200.2 | 0.57 | 115.8 | 9.24 |
| Step 3: Filtration at 10,000 Dal | 210.0 | 0.064 | 13.4 | 1.07 |
| Step 4: Filtration at 1,000 Dal | 1.0 | 4.98 | 4.98 | 0.39 |
| Step 5: HPLC chromatography | ||||
| cGnRH-I-like peak | 0.2 | 6.02 | 1.20 | 0.096 |
| mGnRH-like peak | 0.2 | 4.91 | 0.98 | 0.078 |
Results obtained from 100-g gonads.
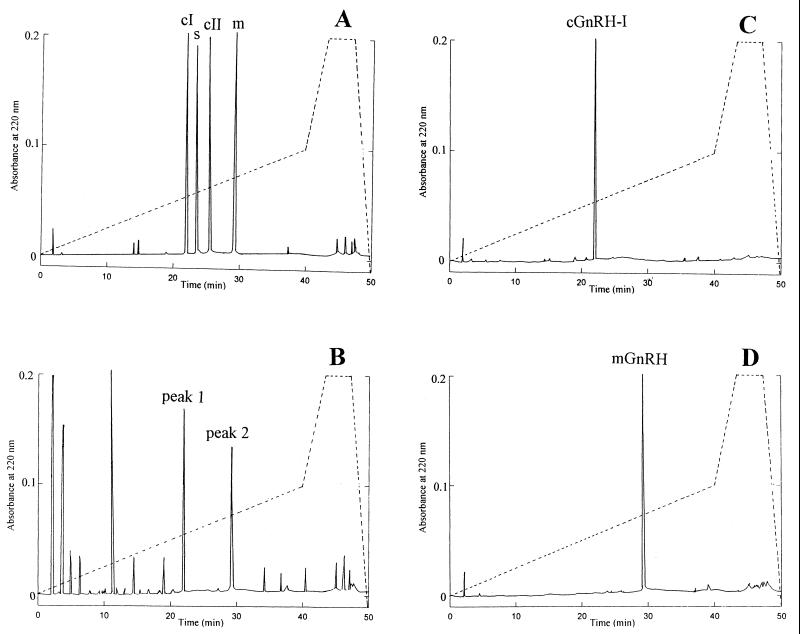
Fig. 1A shows the HPLC elution profile of peptides contained in a 100-μl sample obtained after step 4 (see Table 1) of purification. Only two of the major peaks, 1 and 2, showed significant GnRH immunoreactivity. These two peaks eluted at the retention time of 21.9 and 29.1 min, respectively. From separate HPLC runs, conducted under similar conditions, on four synthetic GnRH variants, namely, c-I, c-II, salmon, and mGnRH (Fig. 1B), it was confirmed that peaks 1 and 2 matched exactly the elution time of that of synthetic cGnRH-I and mGnRH, respectively. Material related to peaks 1 and 2 then was collected separately and subjected again to an HPLC run as before. The entire procedure was repeated several times, and the early- and late-eluting peptide each yielded only one sharp peak, always with the same retention times as that of synthetic cGnRH-I and mGnRH, respectively (Fig. 1 C and D). The peptide contained in each peak, peaks 1 and 2, appeared homogenous.
Figure 1.
RP-HPLC of synthetic and native GnRHs. (A) Elution of four synthetic GnRH molecules mixed in buffer. Dashed line represents the stepwise gradient (see Materials and Methods for details). Five micrograms of each peptide was added in combination. Peaks were identified from chromatographs of individual peptides run separately or combined under similar conditions. Elution time for chicken I (c-I), salmon (s), chicken II (c-II), and mammalian (m) GnRHs was 21.9 min, 23.5 min, 25.1 min, and 29.1 min, respectively. (B) Elution of gonadal extract from C. intestinalis after step 4 of purification (see Table 1) under similar conditions was as in A. Peaks 1 and 2 were GnRH-immunoreactive and were subjected to further purification and structural characterization. (C) Elution of purified peak 1. Note the exact correspondence of the retention time with that of synthetic cGnRH-I as in A. (D) Elution of purified peak 2. Note the correspondence of the retention time with that of synthetic mGnRH as in A. In each graph, one of several HPLC runs is shown; all column runs of each graph generated similar UV absorbance peaks.
Immunoreactivity.
The immunoreactivity of GnRH-like peptides purified from the gonads of C. intestinalis was tested in standard RIA by using polyclonal antisera raised against synthetic mGnRH, which were cross-reactive with other known GnRH forms. The displacement curves obtained by serially diluted native peptides at elution time 29.1 (Fig. 1 B and D) indicated the presence of peptide molecules in the gonadal extract immunologically similar to the known form of mGnRH.
Amino Acid Composition.
After the final step of purification, each of the two native peptides from Ciona gonads was analyzed for the amino acid composition according to the conventional technique of chemical hydrolysis (see Materials and Methods). The amino acid composition of these two peptides is shown in Table 2. The accuracy of the amino acid composition analysis was verified with values obtained for the synthetic cGnRH-I and mGnRH.
Table 2.
Amino acid composition of acid hydrolysate of peptides purified from early- and late-eluting HPLC peaks (peaks 1 and 2) of C. intestinalis gonadal extract
| Amino acid | nmol/μg | Amino acid ratio | Integral residues |
|---|---|---|---|
| Peak 1 peptide (cGnRH-I-like) | |||
| Glutamic acid | 2.01 | 2.20 | 2 |
| Hystidine | 0.97 | 1.06 | 1 |
| Tryptophan | 0.89 | 0.97 | 1 |
| Serine | 0.92 | 0.98 | 1 |
| Tyrosine | 1.01 | 1.11 | 1 |
| Glycine | 1.98 | 2.17 | 2 |
| Leucine | 0.91 | 1.00 | 1 |
| Proline | 1.01 | 1.10 | 1 |
| Ammonium ion | 1.88 | 2.06 | 2 |
| Peak 2 peptide (mGnRH-like) | |||
| Glutamic acid | 1.01 | 1.08 | 1 |
| Hystidine | 0.99 | 1.06 | 1 |
| Tryptophan | 0.93 | 1.00 | 1 |
| Serine | 0.90 | 0.96 | 1 |
| Tyrosine | 0.97 | 1.01 | 1 |
| Glycine | 1.96 | 2.10 | 2 |
| Leucine | 0.93 | 1.00 | 1 |
| Arginine | 1.02 | 1.09 | 1 |
| Proline | 1.00 | 1.07 | 1 |
| Ammonium ion | 0.97 | 1.04 | 1 |
Molar ratios of amino acids were normalized to 1.0 for leucine.
Amino Acid Sequence.
All known GnRH forms isolated and purified from animal tissues have one characteristic in common, i.e., the pyroglutamate residues (5-pyrrolidone-2-carboxylic acid) at the amino-terminal position resistant to Edman degradation and the glycine amidate (Gly⋅NH2) in the C-terminal position. Experiments directed to determine the amino acid sequence by using a procedure described in Materials and Methods indicated that both purified native peptides from Ciona gonads were blocked at the N-terminal position. Therefore, the purified peptides first were treated with pyroglutamate aminopeptidase before being subjected to the amino acid sequencing.
As shown in Table 3, the peptide purified from the peak eluting at 21.9 min in the HPLC run presented an identical sequence of amino acids as that of cGnRH-I. Because from the amino acid analysis performed on separate samples after total acid hydrolysis we found two residues of glutamic acid for each molecule of the intact cGnRH-I peptide (Table 2), we deduced that one molecule of glutamic acid was pyroglutamic acid occurring at the first position at the N-terminal of the peptide and the second one at position 8 was glutamine. Furthermore, from total HCl hydrolysis we obtained two ammonium ions, and, hence, it is deduced that one ammonium ion is occupied by the glutamine (eighth amino acid position from the N terminal), whereas the second one is bound to glycine at the C-terminal position to form the glycinamide. We conclude that the sequence of Ciona GnRH-like peptide present in the first HPLC peak is the following: pGlu-His-Trp-Ser-Tyr-Gly-Leu-Gln-Pro-Gly-NH2 (Table 3). Similarly, the purified peptide eluting at 29.1 min in HPLC run presented an amino acid sequence identical to that of mGnRH. Here again, from the amino acid analysis performed on a separate sample after total acid hydrolysis, we found one residue of glutamic acid and one of ammonium ion for each molecule of the intact peptide (Table 3), and we deduced that one molecule of glutamic acid was pyroglutamic acid (not identified in the sequence analysis), which occurs at the N-terminal position of the peptide, and the ammonium residue is bound to Gly to form the glycinamide. The amino acid sequence of the GnRH peptide in the second HPLC peak therefore was as follows: pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2 (Table 3).
Table 3.
Primary structure of GnRH peptides from gonads of C. intestinalis
| Peptide | Composition | Integrated with HCl hydrolysis |
|---|---|---|
| Native cGnRH-I | His-Trp-Ser-Tyr-Gly-Leu-Gln-Pro-Gly | pGlu-His-Trp-Ser-Tyr-Gly-Leu-Gln-Pro-Gly⋅NH2 |
| Native mGnRH | His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly | pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly⋅NH2 |
MS.
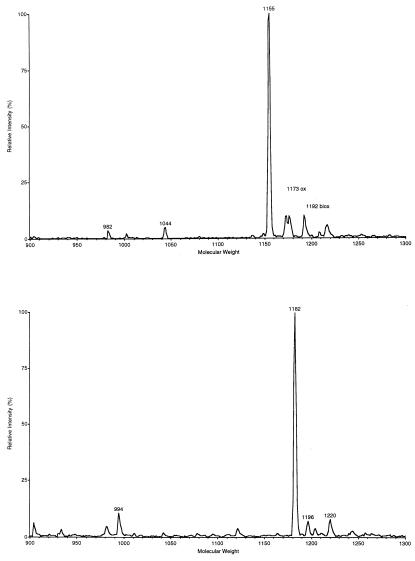
The mass of the intact molecule ([M+H]+) of the early-eluting GnRH peak was measured as m/z 1155.6 (Fig. 2A) and that of the late-eluting peak was measured as m/z 1182.6 (Fig. 2B). Consistently similar values were obtained when synthetic GnRH standards, namely, cGnRH-I and mGnRH, were used in similar conditions (data not shown).
Figure 2.
Mass spectra of C. intestinalis gonadal GnRHs. (Upper) Mass spectrum of purified native cGnRH-I-like peptide. (Lower) Mass spectrum of purified native mGnRH-like peptide. Mass spectra of synthetic mGnRH and cGnRH-I, not shown here, corresponding exactly to those shown here.
Immunocytochemistry.
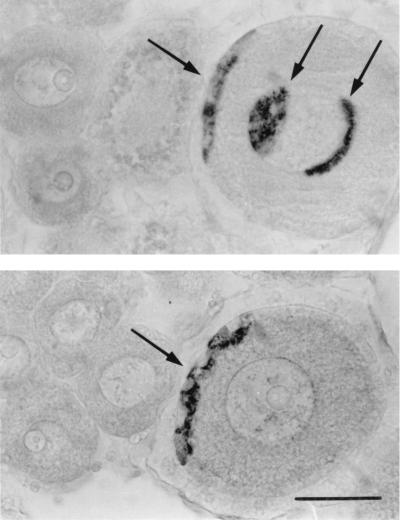
GnRH immunostaining was localized to granulosa cells and to the perinuclear zone of the oocytes exceeding 25 μm in diameter (Fig. 3). No GnRH immunolabeling was observed in smaller oocytes and in the male compartment of the gonad.
Figure 3.
GnRH immunoreactivity in the oocytes from two different samples of C. intestinalis. Arrows indicate immunostaining in granulosa cells and in the perinuclear zone. (Bar = 50 μm.)
Biological Activity.
In vitro treatment of Ciona gonads with native cGnRH-I and mGnRH stimulated the release and synthesis of sex steroids, namely, testosterone, progesterone, and 17β-estradiol (Table 4). A similar treatment with synthetic cGnRH-I and mGnRH also induced a significant increase in sex steroid concentration in the incubation medium and in the gonads (data not shown). The magnitude of increase in sex steroid release and synthesis induced by native and synthetic GnRHs was statistically similar.
Table 4.
In vitro induction of Ciona gonadal release and synthesis of sex steroids by native cGnRH-I and mGnRH
| Sex steroid | Release, ng/g tissue | Synthesis, ng/g tissue | ||
|---|---|---|---|---|
| Control | mGnRH | Control | mGnRH | |
| Testosterone | 0.20 ± 0.04 | 0.70 ± 0.08 | 3.00 ± 0.50 | 8.50 ± 1.00 |
| Progesterone | 0.05 ± 0.01 | 0.15 ± 0.03 | 0.75 ± 0.10 | 1.50 ± 0.30 |
| 17β-estradiol | 0.08 ± 0.01 | 0.20 ± 0.05 | 0.25 ± 0.05 | 0.75 ± 0.20 |
| Control | cGnRH-I | Control | cGnRH-I | |
| Testosterone | 0.15 ± 0.03 | 0.40 ± 0.07 | 2.80 ± 0.60 | 7.75 ± 0.80 |
| Progesterone | 0.10 ± 0.02 | 0.25 ± 0.04 | 0.50 ± 0.10 | 1.00 ± 0.15 |
| 17β-estradiol | 0.05 ± 0.01 | 0.10 ± 0.02 | 0.30 ± 0.05 | 0.50 ± 0.10 |
Results are the mean ± SD obtained from three different experiments (see Materials and Methods). Release represents the hormone concentration assayed in the incubation liquid, and synthesis means the hormone concentration assayed in the gonadal tissue. Student's t test was used to analyze the statistical differences between control and treated results. The difference between GnRH and control was significant, 0.01 < P < 0.05, except for estradiol cGnRH-I vs control, where P > 0.05.
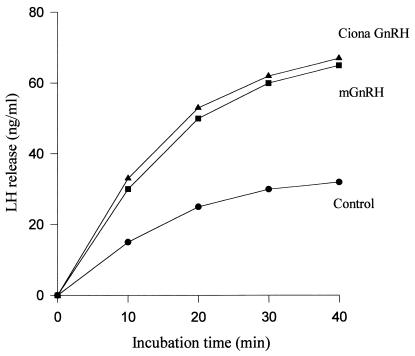
The native mGnRH induced LH release from rat pituitary in vitro, and the magnitude of this release was almost twice that observed in controls (Fig. 4). The LH-releasing activity of the isolated mGnRH agreed well with that of the synthetic preparation. This result is additional confirmation that the mGnRH-like peptide isolated from Ciona gonads is identical to synthetic mGnRH both biochemically and physiologically.
Figure 4.
Rat pituitary LH release response in vitro to synthetic (■) and native (▴) mGnRH. After 40 min, the curves plateaued until 100 min.
Discussion
This study shows that the gonads of a protochordate contain GnRH molecules biochemically and physiologically identical to those already described in vertebrates and that there is more than one molecular form. We have demonstrated conclusively that the protochordate (C. intestinalis) gonad contains two types of GnRH molecules with the structures of pGlu-His-Trp-Ser-Tyr-Gly-Leu-Gln-Pro-Gly⋅NH2 and pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly⋅NH2. These structures are identical to the primary structures of two GnRH peptides purified, respectively, from chicken brain (24, 25) and mammalian brain (1, 2) and known as cGnRH-I and mGnRH. Our conclusions are based on clear evidence demonstrating the HPLC elution profile, amino acid sequence, and GnRH-immunoreactivity in the gonads as well as GnRH-like biological activity of peptides purified from the gonadal extract of C. intestinalis. Both native peptides were found to stimulate sex steroid production in C. intestinalis gonads, and the native mGnRH enhanced LH secretion from rat pituitary halves in vitro. In both bioassay systems the corresponding synthetic GnRH forms yielded identical results. Thus, these two forms of GnRH have been conserved through the evolution of chordates and our view of the evolution of known forms of GnRH across the chordate lineage may need to be reformulated.
Previous investigations had shown that more than one form of GnRH molecule is found in all vertebrate groups, with emphasis on the fact that the cGnRH-II form represents the most conserved molecule across vertebrates (see refs. 4–6). However, the present data demonstrate that cGnRH-I form is not limited to reptiles and birds, among vertebrate chordates, but is present in invertebrate chordates as well, and that mGnRH is most likely the form that spans the entire chordate tree, from protochordates to mammals.
It is striking that a protochordate group contains four different forms of GnRHs. On one hand, it is consistent with the widely held contention that every vertebrate group has more than one form of GnRH, but on the other, it leads us to reconsider more carefully as to when or at which stage of chordate evolution the different (known) molecular forms of GnRH appear. The nervous tissue of the ascidian, C. productum, was shown to contain two GnRH forms different from all nine GnRH forms characterized among vertebrate classes (18, 26). This favored the idea that protochordate GnRH forms would be different than those arising with the evolution of vertebrates. Now it is evident that this is not the case. More species of protochordates and lower vertebrates must be added to the repertoire to verify the presence of novel GnRH forms as well as the distribution of known (classical) GnRH forms.
Acknowledgments
We thank Drs. Paola Cirino and Alfonso Toscano for culture of ascidians. This research was supported financially by the Stazione Zoologica and the Università degli Studi di Napoli Federico II.
Abbreviations
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- mGnRH
mammalian GnRH
- cGnRH-1
chicken I GnRH
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040549097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040549097
References
- 1.Matsuo H, Baba Y, Nair R M G, Arimura A, Schally A V. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 2.Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R. Proc Natl Acad Sci USA. 1972;69:278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastogi R K, Iela L. Zool Sci. 1994;11:363–373. [Google Scholar]
- 4.Sherwood N M, von Schalburg K, Lescheid D W. In: GnRH Neurons Gene to Behavior. Parhar I S, Sakuma Y, editors. Tokyo: Brain Shuppan; 1997. pp. 3–25. [Google Scholar]
- 5.King J A, Millar R P. In: GnRH Neurons Gene to Behavior. Parhar I S, Sakuma Y, editors. Tokyo: Brain Shuppan; 1997. pp. 51–77. [Google Scholar]
- 6.Muske L E. In: GnRH Neurons Gene to Behavior. Parhar I S, Sakuma Y, editors. Tokyo: Brain Shuppan; 1997. pp. 145–180. [Google Scholar]
- 7.Tan L, Rousseau P. Biochem Biophys Res Commun. 1982;109:1061–1071. doi: 10.1016/0006-291x(82)92047-2. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg J I, Garofalo R, Price C J, Chang J P. J Comp Neurol. 1993;336:571–582. doi: 10.1002/cne.903360409. [DOI] [PubMed] [Google Scholar]
- 9.Seeburg P G, Adelman J P. Nature (London) 1984;311:666–668. doi: 10.1038/311666a0. [DOI] [PubMed] [Google Scholar]
- 10.Chieffi G, Pierantoni R, Fasano S. Int Rev Cytol. 1991;127:1–55. doi: 10.1016/s0074-7696(08)60691-9. [DOI] [PubMed] [Google Scholar]
- 11.Palmon A, Aroya N B, Tel-Or S, Burstein Y, Fridkin M, Koch Y. Proc Natl Acad Sci USA. 1994;91:4994–4996. doi: 10.1073/pnas.91.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng C, Fan N C, Ligier M, Vaananen J, Leung P C K. Endocrinology. 1994;135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- 13.Battisti A, Pierantoni R, Vallarino M, Trabucchi M, Carnevali O, Polzonetti-Magni A M, Fasano S. Peptides. 1997;18:1029–1037. doi: 10.1016/s0196-9781(97)00024-7. [DOI] [PubMed] [Google Scholar]
- 14.Pati D, Habibi H R. Endocrinology. 1998;139:2015–2024. doi: 10.1210/endo.139.4.5877. [DOI] [PubMed] [Google Scholar]
- 15.Georges D, Dubois M P. C R Acad Sci Paris. 1980;290:29–31. [PubMed] [Google Scholar]
- 16.Kelsall R, Coe I R, Sherwood N M. Gen Comp Endocrinol. 1990;78:479–494. doi: 10.1016/0016-6480(90)90037-m. [DOI] [PubMed] [Google Scholar]
- 17.Mackie G O. J Mar Biol Assoc UK. 1995;75:141–151. [Google Scholar]
- 18.Powell J F F, Reska-Skinner S M, Prakash M O, Fischer W H, Park M, Rivier J E, Craig A G, Mackie G O, Sherwood N M. Proc Natl Acad Sci USA. 1996;93:10461–10464. doi: 10.1073/pnas.93.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsui H, Yamamoto N, Ito H, Oka Y. Gen Comp Endocrinol. 1998;112:426–432. doi: 10.1006/gcen.1998.7160. [DOI] [PubMed] [Google Scholar]
- 20.Smith P K, Krohn R I, Hermanson A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 21.Moenter S M, Caraty A, Karsch F J. Endocrinology. 1980;127:1375–1384. doi: 10.1210/endo-127-3-1375. [DOI] [PubMed] [Google Scholar]
- 22.Zanisi M, Messi E, Galbiati M. Amino Acids. 1984;6:47–56. doi: 10.1007/BF00808122. [DOI] [PubMed] [Google Scholar]
- 23.Buko A M, Philips L R, Fraser B A. Biomed Mass Spectrom. 1982;10:324–333. doi: 10.1002/bms.1200100504. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto K, Hasegawa Y, Minegishi T, Nomura M, Takahashi Y, Igarashi M, Kanegawa K, Matsuo H. Biochem Biophys Res Commun. 1982;107:820–827. doi: 10.1016/0006-291x(82)90596-4. [DOI] [PubMed] [Google Scholar]
- 25.King J A, Millar R P. J Biol Chem. 1982;257:10729–10732. [PubMed] [Google Scholar]
- 26.Craig A G, Fischer W H, Park M, Rivier J E, Musselman B D, Powell J F F, Reska-Slinner S M, Prakash M O, Mackie G O, Sherwood N M. FEBS Lett. 1997;413:215–225. doi: 10.1016/s0014-5793(97)00840-5. [DOI] [PubMed] [Google Scholar]