Abstract
The generation of nitric oxide (NO) in penile erectile tissue and the subsequent elevation of cyclic GMP (cGMP) levels are important for normal penile erection. Current treatments of erectile dysfunction elevate either cGMP levels by blocking cGMP degrading phosphodiesterase 5 or cyclic AMP (cAMP) levels by intrapenile injection of prostaglandin E1. The molecular target or targets of cGMP in erectile tissue and the role of cAMP for normal penile erection are not known. Herein, we report that mice lacking cGMP-dependent kinase I (cGKI) have a very low ability to reproduce and that their corpora cavernosa fail to relax on activation of the NO/cGMP signaling cascade. Elevation of cAMP by forskolin, however, induces similar relaxation in normal and cGKI-null corpus cavernosum. In addition, sperm derived from cGKI-null mice is normal, can undergo acrosomal reactions, and can efficiently fertilize eggs. Altogether, these data identify cGKI as the downstream target of cGMP in erectile tissue and provide evidence that cAMP signaling cannot compensate for the absence of the cGMP/cGKI signaling cascade in vivo.
Penile erection is produced by an increased blood flow to the corpus cavernosum (CC), made possible by opening of penile resistance vessels (helicine arteries), relaxation of the CC cells, and occlusion of the venous outflow (1). The erectile response in several animal models depends on nitric oxide (NO), produced by nerves as well as vascular endothelium (1, 2–4). NO activates soluble guanylate cyclase, which leads to the production of cyclic GMP (cGMP). cGMP signals via three different receptors in eukaryotic cells, including ion channels, phosphodiesterases, and protein kinases (5). At present, however, the molecular targets that are activated by cGMP and finally execute the relaxation of penile smooth muscle are not known. In addition, two different cGMP-dependent protein kinases (cGKI and cGKII) have been identified in mammals (6, 7). cGKII is expressed in the small intestine, brain, and cartilage (8–10), whereas high levels of cGKI are found in vascular and intestinal smooth muscle, platelets, Purkinje cells of the cerebellum, and CC cells (11, 12). Inactivation of cGKI in mice abolished both NO/cGMP-dependent relaxation of vascular and intestinal smooth muscle and inhibition of platelet aggregation, causing hypertension, intestinal dysmotility, and abnormal hemostasis (13). In the present study, we investigated the function of cGKI in erectile tissue and the capability of cGKI-deficient mice to reproduce. Furthermore, we analyzed whether a cross-talk exists between the cGMP and cyclic AMP (cAMP) signaling cascades in smooth muscle (5, 14), i.e., whether cAMP can cause relaxation via cGKI.
Materials and Methods
Drugs and Chemicals.
The following drugs were used: l-noradrenaline (NA; Aldrich), carbachol, Nω-nitro-l-arginine (L-NNA), forskolin (Sigma), and 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; Tocris Cookson, Bristol, U.K.). NO was freshly prepared for each experiment. An airtight glass beaker containing 20 ml of distilled water was deoxygenated for 1 h with helium gas. The beaker was then bubbled with medical NO gas (purity > 99.5%) for 15 min until saturated solutions were obtained (NO; 3 × 10−3 M).
Animals and Tissues.
Mice (cGKI+/+ and cGKI−/−; ref. 13) were killed by carbon monoxide asphyxia followed by exsanguination. The penis was removed from each animal by cutting the crura CC at the point of adhesion to the lower pubic bone, and the CC were then excised and processed for immunocytochemistry or used for functional experiments as described (15).
Immunohistochemistry.
Tissue sections were preincubated in PBS with 0.2% Triton X-100 for 2 h and then incubated overnight at +4°C with rabbit antisera against protein gene product 9.5 (1:2,000; Ultraclone, Wellow, Isle of Wight, U.K.), cGKI (1:1,000), tyrosine hydroxylase (1:2,000; Pel-Freez Biologicals), or vesicular acetylcholine transporter (VAChT; 1:2,400; Euro-Diagnostica, Malmö, Sweden); or incubated with guinea pig antiserum to vasoactive intestinal polypeptide (VIP, 1:640; Euro-Diagnostica); or incubated with sheep antiserum to neuronal NO synthase (nNOS, 1:6,000; kind gift of P. Emson, Department of Neurobiology, Babraham Institute, Cambridge, U.K.). After rinsing in PBS, the sections were incubated for 90 min with FITC-conjugated swine anti-rabbit IgG (1:80; Dakopatts, Stockholm, Sweden), FITC-conjugated donkey anti-sheep IgG (1:80; Sigma), FITC-conjugated goat anti-guinea pig IgG (1:80; Sigma), Texas Red-conjugated donkey anti-rabbit IgG (1:125; code 711-075-152, Jackson ImmunoResearch), Texas Red-conjugated donkey anti-sheep IgG (1:125; code 713-075-147, Jackson ImmunoResearch), or Texas Red-conjugated donkey anti-guinea pig IgG (1:125; code 706-075-148, Jackson ImmunoResearch). The immunoreactive structures were evaluated as described (15).
Functional Experiments.
The tunica albuginea was carefully opened from its proximal extremity of the CC toward the penile shaft, and the erectile tissue within the CC was microsurgically excised. One preparation (0.3 × 0.3 × 3 mm) was obtained from each CC. All preparations were used immediately after removal. Strip preparations were prepared and mounted in thermostatically controlled organ baths (5 ml; 37°C) containing Krebs solution bubbled with a mixture of 95% O2 and 5% CO2 (pH 7.4). Isometric tension was recorded, and electrical field stimulation (EFS) was performed as described (15).
During an equilibration period of 40 min, tension was adjusted until mean stable tensions of 1.23 ± 0.11 mN (cGKI+/+; n = 11) and 1.21 ± 0.06 mN (cGKI−/−; n = 9) were obtained. To verify the contractile ability of the preparations, a K+ solution (124 mM) was added to the organ baths at the end of the equilibration period. The mean contractile response to K+ amounted to 0.92 ± 0.07 mN (n = 11) and 0.69 ± 0.04 mN (n = 9) in cGKI+/+ and cGKI−/− mice, respectively. The NA concentration used (3 × 10−6 M) corresponded to the approximate EC70 value and produced stable and reproducible contractions. The effects of NO, carbachol, and forskolin were investigated in NA-contracted preparations.
Frequency-response relationships were investigated at supramaximum voltage in all preparations stimulated electrically. Relaxant responses to transmural stimulation of nerves were investigated in NA-contracted preparations. The degree of relaxation was expressed as percentage of the NA-induced contraction. Some preparations were pretreated for 20 min with the NO synthesis inhibitor L-NNA (10−4 M) or the guanylate cyclase inhibitor ODQ (10−6 M). Effects of EFS were then investigated as described above.
A Krebs solution of the following composition was used (in mM): NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 15, NaH2PO4 1.2, and glucose 5.5. In the high K+ solution (124 mM), the NaCl in the normal Krebs solution was replaced by equimolar amounts of KCl.
In Vitro Fertilization (IVF) and Embryo Culture.
The experiments were performed as described (16). Spermatozoa were obtained from the cauda epididymidis of 7- to 8-week-old wild-type and cGKI-deficient male mice. The sperm cells were capacitated in IVF medium (Whittingham's medium supplemented with 30 mg/ml BSA) for 1.5 h at 37°C. At 13.5 h after human chorion gonadotropin, cumulus-enclosed oocytes were inseminated with 106 sperm cells per ml. The mixture of eggs and sperm was incubated in IVF medium for 4 h at 37°C, and thereafter eggs were transferred into pregassed M16 medium supplemented with 4 mg/ml BSA. Fertilization was assessed 24 h after insemination by counting two- and four-cell embryos. Morphologically normal two/four-cell embryos were cultured further in M16 medium until the blastocyst stage.
To estimate sperm motility, a small amount of sperm was removed from IVF medium after 1-h capacitation and investigated under a cover slide by using phase-contrast microscopy. Cells with hyperactivity were considered as progressively motile. Assessment of spontaneous acrosome reactions was done by the Coomassie brilliant blue staining method as described (17).
Calculations.
Student's paired or unpaired two-tailed t tests were used for statistical comparison of two means. ANOVA with Bonferroni correction was used for multiple comparisons. A probability of P < 0.05 was accepted as significant. When appropriate, results are given as mean values ± SEM. n denotes the number of animals and strip preparations. The −log IC50 values (the logarithm of the drug concentrations producing 50% relaxation of the induced response) were determined graphically for each curve by linear interpolation.
Results
There were no obvious differences in sexual behavior between wild-type and cGKI−/− mice. However, when 15 cGKI-null male mice were crossed with wild-type female mice, we observed a single pregnancy resulting in eight offspring. In contrast, wild-type male mice (n = 15; cGKI+/+) produced more than 500 offspring. cGKI-null females were able to reproduce normally when they were mated with wild-type males. These data clearly show that cGKI-null males, in contrast to females, have a reduced fecundity.
Immunohistochemistry.
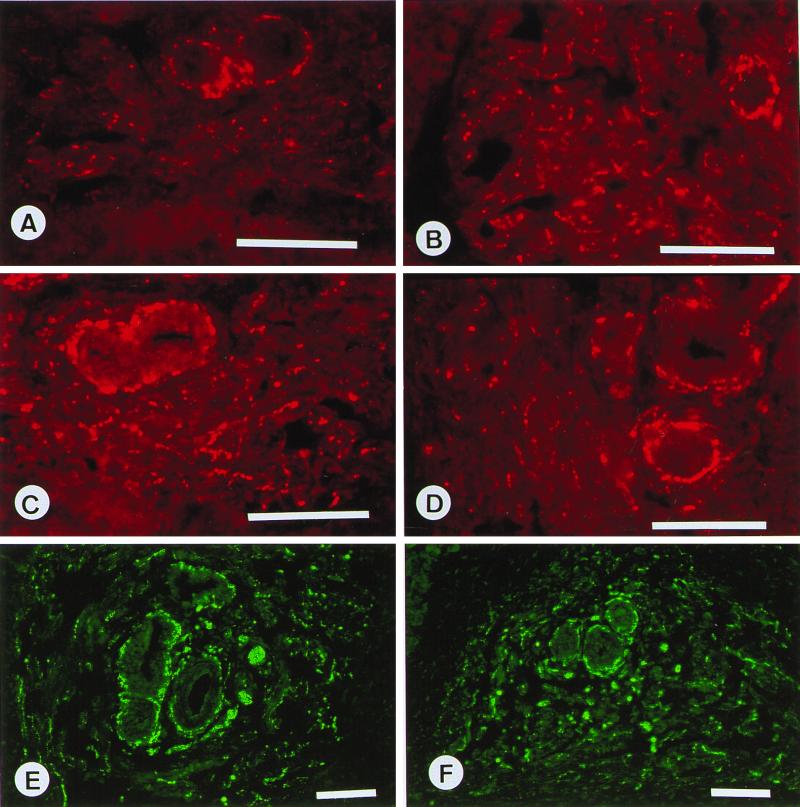
To unravel the cause of the infertility in cGKI-null males, we first tested whether the integrity of and the nerve population in the erectile tissue were altered by the lack of cGKI activity. In normal penile tissue, intense immunoreactivity against cGKI was seen in the smooth muscle of the walls of the central and helicine arteries and in the smooth muscle of the trabecular septa surrounding the cavernous spaces (Fig. 1A). In connective tissue and striated muscle outside the CC tissue, no specific immunoreactivity was observed. In cGKI-deficient mice, both central and helicine arteries as well as trabecular septa are normally developed, and as expected, no cGKI-specific immunoreactivity could be detected in cGKI−/− mice (Fig. 1B). In addition, no difference in number and distribution patterns of nerve populations believed to be of importance for erectile responses could be detected between cGKI+/+ and cGKI−/− mice. In the trabecular tissue surrounding the central and helicine arteries, nerves were observed showing immunoreactivity to protein gene product 9.5 (18), a nonspecific nerve marker (Fig. 1 C and D), tyrosine hydroxylase (Fig. 1 E and F), VAChT (specific for cholinergic nerves; Fig. 2 A and B), VIP (Fig. 2 C and D), and NOS (Fig. 2 E and F). The central and helicine arteries were supplied by nerves rich in protein gene product 9.5-, NOS-, VAChT-, VIP-, and tyrosine hydroxylase-immunoreactive terminals. Double immunolabeling showed that in varicosities and intervaricose segments, VAChT- and VIP-, VAChT- and NOS-, and NOS- and VIP-immunoreactive terminals were colocalized.
Figure 1.
Mouse CC. (A, C, and E) Wild-type mice (cGKI+/+). (B, D, and F) cGKI −/− tissues. (A) Strong cGKI immunoreactivity in smooth muscle bundles and walls of helicinal arteries. (B) Absence of cGKI-immunoreactivity. (C–F) Smooth muscle bundles and/or helicine arteries supplied by varicose terminals expressing immunoreactivities for protein gene product 9.5 (C and D) and tyrosine hydroxylase (E and F). (Bars = 100 μm.)
Figure 2.
Mouse CC. (A, C, and E) Wild-type mice. (B, D, and F) cGKI −/− tissues. (A–F) Smooth muscle bundles and/or helicinal arteries supplied by varicose terminals expressing immunoreactivities for VAChT (A and B), VIP (C and D), and NOS (E and F). (Bars = 100 μm.)
IVF.
Next, we tested whether the reduced fertilization capability of cGKI−/− males is a consequence of sperm dysfunction. Motility, spontaneous acrosome reactivity, and IVF efficiency were compared between sperm derived from normal and cGKI-null mice. After a 1-h capacitation, cGKI+/+ (n = 2) males and cGKI−/− (n = 2) males showed 82 ± 6% and 79 ± 8%, respectively, of progressively motile spermatozoa. The percentages of acrosome-reacted sperm were 27 ± 4% for normal and 29 ± 5% for cGKI−/− mice. IVF of normal mouse oocytes with sperm obtained from wild-type and cGKI−/− males showed no differences in success rate (Table 1).
Table 1.
IVF of mouse oocytes with sperm obtained from wild-type and cGKI−/− males
| Sperm | Number of embryos developed/ number of embryos examined, %
|
|
|---|---|---|
| Two/four-cell | Blastocyst | |
| Wild-type | 38/57 (66.6) | 5/38 (13.1) |
| cGKI−/− | 48/66 (72.2) | 10/48 (20.8) |
Data were collected from two independent experiments.
Smooth Muscle Contraction.
To test whether the distorted fertility of cGKI-null mice is due to erectile dysfunction, we isolated CC tissue from normal and mutant mice and investigated its ability to contract in vitro. Spontaneous contractile activity was observed neither in normal nor in cGKI-deficient CC preparations. The contractile capacity, as tested by NA (10−6 M), was similar in CC tissue from cGKI+/+ and cGKI−/− mice and amounted to 0.45 ± 0.03 mN (n = 11) and 0.39 ± 0.03 mN (n = 9), respectively.
The ability to relax NA-contracted CC preparations through release of endothelial NO was tested by addition of carbachol (10−6 M). Carbachol relaxed preparations from cGKI+/+ mice by 59 ± 6% (n = 11) but had almost no effect (1 ± 1%; n = 8; P < 0.001) in preparations from cGKI−/− mice (Fig. 3). Blockade of NO synthesis in CC tissue from cGKI+/+ mice by pretreatment with L-NNA (10−4 M) reduced the carbachol-induced relaxation to 1 ± 1% (n = 5; P < 0.001; Fig. 3). Inhibition of soluble guanylyl cyclase by addition of ODQ (10−6 M) reduced the carbachol-induced relaxation to 2 ± 1% (n = 6; P < 0.001; Fig. 3).
Figure 3.
Relaxant effect of carbachol (n = 7) in 3 × 10−6 M NA-contracted preparations of CC from cGKI−/− mice (n = 5) and wild-type controls (n = 5). Values are given as means ± SEM. Relaxation in cGKI+/+ mice is practically abolished by L-NNA and ODQ.
Addition of NO induced concentration-dependent and rapid relaxations in NA-contracted preparations from cGKI+/+ mice (n = 10; Fig. 4). The −log IC50 value was 5.85 ± 0.07, and complete relaxation was obtained at NO concentrations of 3 × 10−5 to 10−4 M. Pretreatment of the CC tissues with ODQ markedly inhibited the NO-induced relaxations, shifting the concentration-response curve for NO to the right (−log IC50 = 4.88 ± 0.09). The NO concentration-response curve of normal tissues in the presence of ODQ (10−4 M) was almost identical to the NO concentration-response curve obtained in CC from cGKI-null mice (−log IC50 = 4.66 ± 0.11), indicating that cGKI mediates the cGMP effects in erectile tissue. However, at high concentrations, NO induced relaxations independently of cGKI.
Figure 4.
Relaxant effect of NO in 3 × 10−6 M NA-contracted preparations of CC from cGKI−/− (n = 5) and cGKI+/+ (n = 5) mice. Values are given as means ± SEM. The relaxant response in cGKI+/+ is reduced by ODQ.
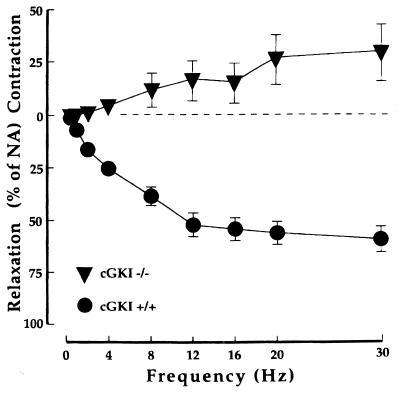
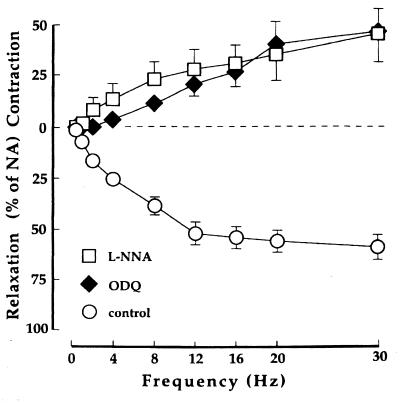
EFS is a way to stimulate the nerves innervating the smooth muscle cells of the CC (1). In NA-contracted preparations from cGKI+/+ mice (n = 10), EFS generated frequency-dependent and tetrodotoxin-sensitive relaxant responses. No relaxation in response to EFS was observed in NA-contracted CC preparations from cGKI-null mice (n = 6). Instead, contractions similar to those found in preparations from cGKI+/+ mice pretreated with 10−4 M L-NNA (n = 5) or 10−6 M ODQ (n = 5) were observed at frequencies above 2 Hz (Figs. 5 and 6).
Figure 5.
Effect of EFS in 3 × 10−6 M NA-contracted preparations of CC from cGKI−/− mice (n = 5) and cGKI+/+ controls (n = 5). Values are given as means ± SEM.
Figure 6.
Effect of EFS in 3 × 10−6 M NA-contracted preparations of CC from cGKI+/+ mice (n = 5). Values are given as means ± SEM. The relaxant response is converted to contraction by L-NNA and ODQ.
Forskolin, stimulating adenylyl cyclase and increasing the concentration of cAMP, produced concentration-dependent relaxations with similar potency (difference not significant) in NA-contracted preparations from cGKI+/+ (n = 7; −log IC50 = 7.04 ± 0.11) and cGKI−/− mice (n = 6; −log IC50 = 6.73 ± 0.11), demonstrating that the cAMP signaling is not affected by the lack of cGKI (Fig. 7). However, at 10−6 M, the highest forskolin concentration used, a statistically significant (P < 0.05) difference in relaxation amplitude was found (72 ± 5% for cGKI−/− and 90 ± 2% for cGKI+/+ mice).
Figure 7.
Relaxant effect of forskolin in 3 × 10−6 M NA-contracted preparations of CC from cGKI−/− mice (n = 5) and cGKI+/+ controls (n = 5). Values are given as means ± SEM. At 10−6 M forskolin, the difference in relaxation is statistically significant (P < 0.05).
Discussion
An important role for neuronally and endothelially produced NO and for the NO/cGMP pathway in penile erection is widely accepted (1, 4). Initially, it was thought that nNOS is the major source for NO in CC tissue (2, 3). A recent report, however, showed that nNOS-deficient mice have normal mating behavior and a normal erectile response to electrical stimulation of the cavernous nerves (19, 20). In addition, isolated CC tissue has normal relaxant responses to electrical nerve stimulation (20), and it was suggested that endothelial NOS is essential for erection, not only in nNOS-deleted mice, but also in normal mice. At present, the role of nNOS in this setting is still debated, because thorough analyses revealed that an alternatively spliced mRNA of the nNOS gene is still expressed in nNOS mutant mice (21). The majority of NO effects are mediated by cGMP (22), and a number of reports have underlined the importance of cGMP in erectile tissue (1–4, 23). Damage to the penile nerves and/or endothelium, as can be found in, e.g., diabetes mellitus, can lead to a decreased production of cGMP in the CC tissue and consequently to erectile dysfunction. Additional support for a central role of cGMP in erection comes from the impressive clinical results with sildenafil (24), which selectively inhibits the activity of phosphodiesterase 5 (which breaks down cGMP) and consequently increases intracellular cGMP levels. A few patients with erectile dysfunction, however, do not respond to sildenafil, suggesting that they may have a functional disturbance of the NO/cGMP signaling cascade distal to the production/breakdown of cGMP.
There is presently no consensus as to whether transmitters other than NO play an important role in the erectile process. The murine penile tissue is well supplied with nerves containing VIP, which has also been shown for humans and other species (1, 25). VIP stimulates adenylyl cyclase and subsequently elevates intracellular concentration of cAMP, which in turn activates cAMP-dependent protein kinase. VIP has a well documented, pronounced, inhibitory, and relaxation-producing effect on strips of CC tissue and cavernosal vessels in vitro (1). Therefore, it has been speculated that the cAMP pathway should be involved in the control of penile smooth muscle tone (26) and in normal erection. The most compelling evidence comes from the effective treatment of erectile dysfunction in humans by intracavernosal injection of prostaglandin E1, which acts through the adenyl cyclase/cAMP signaling pathway (27). We also show in this study that forskolin can activate the adenylyl cyclase and elevate cAMP levels both in normal and cGKI-deficient CC tissue. Nevertheless, cGKI-null mice have a very low ability to reproduce, and their CC tissue shows no relaxation in response to nerve stimulation, suggesting that putative transmitters released from nerves and acting through the cAMP pathway, such as VIP and VIP-related peptides (25), play a minor role in normal erection. Furthermore, under normal physiological situations, signaling via cGKI seems critical in obtaining penile erections. The relaxing effects of NO at high concentrations in the absence of cGKI could be due to a cGMP-independent action of NO (e.g., activation of large conductance calcium-activated potassium channels) and/or an increase of cellular cGMP to such high concentrations that the cAMP-dependent protein kinase is activated. Analyses of isolated cGKI-deficient vascular smooth muscle clearly favor the latter notion (unpublished work).
Our results provide convincing evidence that the highly reduced fecundity of mice lacking cGKI is due to erectile dysfunction and show that NO/cGMP mediates its effect through cGKI. These mice will be a useful model for testing drugs that act downstream of cGKI and stimulate penile erection.
Acknowledgments
This work was supported by Swedish Medical Research Council Grants 6837, 11205, and 12531 and the Medical Faculty of Lund University.
Abbreviations
- cGMP
cyclic GMP
- cAMP
cyclic AMP
- cGKI and cGKII
cGMP-dependent kinase I and II
- CC
corpus cavernosum
- NA
l-noradrenaline
- L-NNA
Nω-nitro-l-arginine
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- VAChT
vesicular acetylcholine transporter
- VIP
vasoactive intestinal polypeptide
- NOS
nitric-oxide synthase
- nNOS
neuronal NOS
- EFS
electrical field stimulation
- IVF
in vitro fertilization
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030419997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030419997
References
- 1.Andersson K-E, Wagner G. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 2.Holmquist F, Stief C G, Jonas U, Andersson K-E. Acta Physiol Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- 3.Burnett A L, Lowenstein C J, Bredt D S, Chang T S K, Snyder S H. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 4.Burnett A L. J Urol. 1997;157:320–324. [PubMed] [Google Scholar]
- 5.Lincoln T M, Cornwell T L. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- 6.Lohmann S M, Vaandrager A B, Smolenski A, Walter U, De Jonge R. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer A, Ruth P, Dostmann W, Sausbier M, Klatt P, Hofmann F. Rev Physiol Biochem Pharmacol. 1998;150:105–149. doi: 10.1007/BFb0033671. [DOI] [PubMed] [Google Scholar]
- 8.Uhler M D. J Biol Chem. 1993;268:13586–13591. [PubMed] [Google Scholar]
- 9.Jarchau T, Hausler C, Markert T, Pohler D, Vanderkerckhove J, De Jonge H R, Lohmann S M, Walter U. Proc Natl Acad Sci USA. 1994;91:9426–9430. doi: 10.1073/pnas.91.20.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeifer A, Aszódi A, Seidler U, Ruth P, Hofmann F, Fässler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann R, Bauer S, Gobel C, Hofmann F, Jakobs K H, Walter U. Eur J Biochem. 1986;158:203–210. doi: 10.1111/j.1432-1033.1986.tb09739.x. [DOI] [PubMed] [Google Scholar]
- 12.Keilbach A, Ruth P, Hofmann F. Eur J Biochem. 1992;208:467–473. doi: 10.1111/j.1432-1033.1992.tb17209.x. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang G, Korth M, Aszódi A, Andersson K-E, et al. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lincoln T M, Komalavilas P, Boerth N J, MacMillan-Crow L A, Cornwell T L. Adv Pharmacol. 1995;34:305–322. doi: 10.1016/s1054-3589(08)61094-7. [DOI] [PubMed] [Google Scholar]
- 15.Hedlund P, Alm P, Andersson K-E. Br J Pharmacol. 1999;127:349–360. doi: 10.1038/sj.bjp.0702556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 17.Moller C C, Bleil J D, Kinloch R A, Wassarman P M. Dev Biol. 1990;137:276–286. doi: 10.1016/0012-1606(90)90254-g. [DOI] [PubMed] [Google Scholar]
- 18.Gulbenkian S, Wharton J, Polak J M. J Auton Nerv Syst. 1987;18:235–247. doi: 10.1016/0165-1838(87)90122-6. [DOI] [PubMed] [Google Scholar]
- 19.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 20.Burnett A L, Nelson R J, Calvin D C, Liu J-X, Demas G E, Klein S L, Kriegsfeld L J, Dawson V L, Dawson T M, Snyder S H. Mol Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- 21.Eliasson M J L, Blackshaw S, Schell M J, Snyder S H. Proc Natl Acad Sci USA. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt H H, Lohmann S M, Walter U. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 23.Holmquist F, Hedlund H, Andersson K-E. J Physiol. 1992;449:295–311. doi: 10.1113/jphysiol.1992.sp019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein I, Lue T F, Padma-Nathan H, Rosen R C, Steers W D, Wicker P A. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 25.Hedlund P, Alm P, Ekström P, Fahrenkrug J, Hannibal J, Hedlund H, Larsson B, Andersson K-E. Br J Pharmacol. 1995;116:2258–2266. doi: 10.1111/j.1476-5381.1995.tb15062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparwasser C, Drescher P, Will J A, Madsen P O. J Urol. 1994;152:2159–2163. doi: 10.1016/s0022-5347(17)32343-1. [DOI] [PubMed] [Google Scholar]
- 27.Andersson K-E, Steers W D. In: Erectile Dysfunction: Issues in Current Pharmacotherapy. Morales A, editor. London: Martin Dunitz; 1998. pp. 97–124. [Google Scholar]