Abstract
We have developed a thin-slice preparation of whole rat carotid body that allows us to perform patch-clamp recording of membrane ionic currents and to monitor catecholamine secretion by amperometry in single glomus cells under direct visual control. In normoxic conditions (PO2 ≈ 140 mmHg; 1 mmHg = 133 Pa), most glomus cells did not have measurable secretory activity, but exposure to hypoxia (PO2 ≈ 20 mmHg) elicited the appearance of a large number of spike-like exocytotic events. This neurosecretory response to hypoxia was fully reversible and required extracellular Ca2+ influx. The average charge of single quantal events was 46 ± 25 fC (n = 218), which yields an estimate of ≈140,000 catecholamine molecules per vesicle. Addition of tetraethylammonium (TEA; 2–5 mM) to the extracellular solution induced in most (>95%) cells tested (n = 32) a secretory response similar to that elicited by low PO2. Cells nonresponsive to hypoxia but activated by exposure to high external K+ were also stimulated by TEA. A secretory response similar to the responses to hypoxia and TEA was also observed after treatment of the cells with iberiotoxin to block selectively Ca2+- and voltage-activated maxi-K+ channels. Our data further show that membrane ion channels are critically involved in sensory transduction in the carotid body. We also show that in intact glomus cells inhibition of voltage-dependent K+ channels can contribute to initiation of the secretory response to low PO2.
Keywords: carotid-body slice, O2 sensing, glomus cell secretion
A decade has passed since patch-clamp experiments on dispersed glomus cells from rabbit and rat carotid bodies showed that they were electrically excitable and contained O2-sensitive voltage-dependent K+ channels (1–6). Although some of the electrophysiological properties and the kind of O2-sensitive K+ channel of glomus cells varied among animal species, these observations gave strong support to a unified membrane model of chemotransduction based on the capability of glomus cells to sense reductions of O2 tension (PO2) through the closure of K+ channels, which in turn lead to Ca2+ influx, transmitter release, and activation of the afferent fibers of the sinus nerve (see refs. 7 and 8 for review). Direct evidence that individual glomus cells worked as O2-sensitive neurosecretory elements was obtained by monitoring cytosolic [Ca2+] and quantal catecholamine secretion in single Fura 2-loaded cells (7, 9–11). Numerous investigators have found O2-sensitive voltage-dependent channels in other tissues (in addition to the carotid body), where these channels participate in a variety of cellular electrical, mechanical, or secretory responses to acute hypoxia (see refs. 12 and 13 for review).
The precise role of voltage-dependent K+ channels in the chemoreceptive properties of the carotid body remains, however, controversial, because there are researchers who believe that the O2-sensitive membrane electrical events in glomus cells are not directly involved in sensory transduction. A major argument supporting this notion is that pharmacological agents, such as tetraethylammonium (TEA), 4-aminopyridine (4-AP), or charybdotoxin (CTX), which can inhibit the O2-sensitive K+ currents in patch-clamped glomus cells, do not elicit neural discharges (or secretion) in whole carotid-body preparations (14–16). Although it has been reported that application of CTX can depolarize glomus cells (17), it has also been shown that TEA and 4-AP fail to depolarize or to increase cytosolic [Ca2+] in dispersed rat glomus cells (18). This last work (18) described an O2-sensitive, K+-selective leak conductance, resistant to TEA and 4-AP, which seemed to contribute to the depolarization of the cells in response to low PO2. In consequence, it was proposed that closure of the leak K+ channels is an obligatory initial event in low PO2-induced excitation of rat glomus cells.
Despite the considerable amount of information on carotid-body function already available, the understanding of the O2-sensing mechanisms is progressing at a relatively slow pace. Research on the carotid body is hampered by its small size, abundance of connective tissue, and profuse vascularization, all of which make it difficult to obtain a viable preparation of enzymatically dispersed cells with consistent physiological properties. These limitations are particularly important when studying the cellular responses to hypoxia, because sensitivity to O2 is a labile process that seems to be easily destroyed by uncontrolled experimental variables (13). In search of an optimal approach to investigate carotid-body physiology, we have developed a thin-slice preparation of the whole organ that does not require enzymatic treatment or mechanical disruption of the tissue. Herein, we show that the carotid-body slice preparation used in combination with single-cell amperometry allows the study of the electrophysiology and secretory activity of intact cells in almost optimal conditions. Our study, which uses this technique, further supports the view that voltage-dependent membrane ion channels participate critically in chemosensory transduction.
Methods
Carotid-Body Slicing.
Experiments were performed on carotid bodies isolated from Wistar rats of ages between postnatal day 5 and 20. Animals were anesthetized by injection of 0.2 mg/kg sodium pentobarbital (Euta-Lender, Madrid). Complete carotid bifurcations were quickly removed and placed on ice-cooled and O2-saturated modified Tyrode's solution (in mM: 148 NaCl/2 KCl/3 MgCl2/10 Hepes/10 glucose, pH 7.4). The carotid body was dissected from the adjacent artery and then included in 3% (wt/vol) low-melting-point agarose (FMC). After quick cooling on ice, the agarose block was glued with cyanoacrylate to the stage of a vibratome chamber and covered by the same cold, O2-saturated Tyrode's solution. Slices 100- to 150-μm-thick were cut with standard razor blades. The resulting slices (usually four) were washed twice with cold sterile PBS and placed on 35-mm Petri dishes with DMEM (GIBCO/BRL) supplemented with 1% (vol/vol) penicillin/streptomycin (GIBCO/BRL) and 10% (vol/vol) FBS (BioWhittaker). The slices were maintained at 37°C in a 5% CO2 incubator 48–96 h before the experiments. At this time, maturation of the O2-sensitive mechanisms, which occurs around postnatal day 10 (19, 20) were almost complete. Sensitivity of the glomus cells to changes in PO2 did not seem to depend on the age of the animals used.
Experimental Setup, Patch-Clamp Recording, and Amperometric Measurement of Secretion.
In most aspects, carotid-body slices were managed following the procedures described for thin brain slices (21, 22). For the experiments, a slice was transferred to a recording chamber (≈200-μl volume) placed on the stage of an upright Zeiss microscope (Axioskop) equipped with long distance water immersion objectives. Once in the chamber, the carotid-body slice was continuously perfused by gravity (flow 1–2 ml/minute) with a solution containing (in mM) 147 NaCl, 2.7 or 4.5 KCl, 23 NaHCO3, 1 MgCl2, 2.5 CaCl2, and 10 glucose. A lyra made of silver wire with glued fine Nylon threads was used to hold the slice to the bottom of the chamber. The standard (normoxic) solution was bubbled with a gas mixture of 5% CO2, 20% O2, and 75% N2 (PO2 ≈ 140 mmHg). Hypoxia was obtained by continuously bubbling the solution in one of the reservoirs with 5% CO2 and 95% N2. The pH of the normoxic and hypoxic solutions was 7.4. The time course of PO2 changes in the chamber was determined with a carbon-fiber electrode that was negatively polarized and worked as an O2 electrode. After switching from normoxia to hypoxia, complete equilibration of the new solution in the chamber required between 1 and 2 minutes. PO2 in the chamber during exposure to hypoxia reached a value of ≈20 mmHg. All pharmacological agents used (ion channel blockers and ion chelators) were obtained from Sigma and were added to the bath solution. The osmolality of the solution (≈300 milliosmol/kg) was maintained by reducing the concentration of NaCl. In the high K+ solutions, KCl replaced NaCl equimolarly. In all the experiments, the temperature of the solutions and the chamber was between 34 and 37°C. Membrane currents were recorded by using the whole-cell configuration of the patch-clamp technique as adapted in our laboratory (23, 24). We used low-resistance pipettes (1–3 MΩ), capacity compensation, and subtraction of linear leakage and capacitive currents. Series resistance compensation was between 40% and 50%. Pipette solution contained (in mM) 125 KCl, 4 MgCl2, 4 MgATP, 10 Hepes, and 10 EGTA (pH 7.2 and osmolality of 285–290 milliosmol/kg). Ionic currents were recorded with an EPC-8 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany), filtered at 3 kHz, digitized, and stored on a computer. Secretory events were recorded with a 10-μm carbon-fiber electrode connected to the input of a high-gain current to voltage converter (10, 25). The electrode was polarized to +750 mV, a value more positive than the oxidation potential of dopamine and the other catecholamines in glomus cells (10), and positioned near a cell under visual control by using a piezoelectric manipulator. Amperometric currents were also recorded with the EPC-8 patch-clamp amplifier. Currents were filtered at 100 Hz and digitized at 250 Hz before storage on computer. Data acquisition and analysis of ionic and amperometric currents were done with an ITC-16 interface (Instrutech, Great Neck, NY) and pulse/pulsefit software (version 8.11, HEKA Electronics).
Tyrosine Hydroxylase (TH) Immunocytochemistry.
Carotid-body slices similar to those used for the experiments described above were fixed overnight at 4°C with PBS/4% (vol/vol) paraformaldehyde. After washing in PBS/0.1% Triton X-100 (PBTx), sections were incubated with PBS/0.3% H2O2 for 2 h and then with PBTx/10% (vol/vol) FCS/1 mg/ml BSA for another 2 h to block nonspecific sites. After incubation, a polyclonal anti-TH antibody (1:1,000; Chemicon) was added overnight. After washing in PBTx, sections were incubated again with biotinylated anti-rabbit antibody (1:200; Pierce) for 12 h. Thereafter, sections were incubated with the ABC kit (Pierce) for 2 h, and specifically bound antibody was revealed by using 3,3′-diaminobenzidine (Sigma) as chromogen. After washing in PBS, sections were mounted on slides and coverslipped.
Results
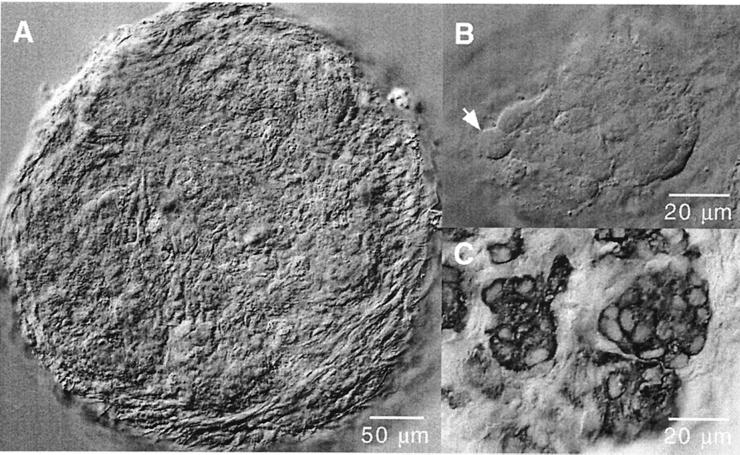
Fig. 1A illustrates the typical appearance of a rat carotid-body slice of round shape (≈400 μm in diameter) surrounded by the connective capsule and attached tissue. During the few hours after slicing, the histological appearance of the sections was rather uniform, without clear separation between the glandular, connective, and vascular tissues. As incubation time progressed, the slice flattened, and the texture became less uniform, with the appearance of cell aggregates clearly separated from the surrounding tissue and with numerous spherical or ovoid cells 8–12 μm in diameter (Fig. 1B). These cell aggregates, similar to the glomeruli described in histological preparations of the carotid body (26), were nicely observed in slices immunostained with anti-TH antibodies showing typical clusters of TH-positive glomus cells (Fig. 1C). We did most of our experiments on slices incubated for at least 48 h, because these slices had better defined glomeruli that seemed to contain a larger number of cells responding to changes in PO2 (see below). For the measurement of whole-cell currents or secretory activity, the patch-clamp and carbon-fiber electrodes, respectively, were placed adjacent to a well identified cell within a cluster (e.g., the cell near the arrow in Fig. 1B). We were able to maintain carotid-body slices in good condition for up to 5 days. The longevity of the slices was not studied systematically, although it seems that, with special care, long-lasting organotypic cultures might easily be achieved.
Figure 1.
Morphological appearance of cells in thin slices of rat carotid body. (A) Low-magnification view of a slice a few hours after cutting. (B) Typical cell aggregate (glomerulus) in a carotid-body slice maintained for 48 h in a CO2 incubator. Individual cells, like the one indicated by the arrow, can be clearly differentiated. (C) Carotid-body slice stained with antibodies anti-tyrosine hydroxylase. Note the typical appearance of glomus cells with large nuclei and the organization of the carotid body in glomeruli.
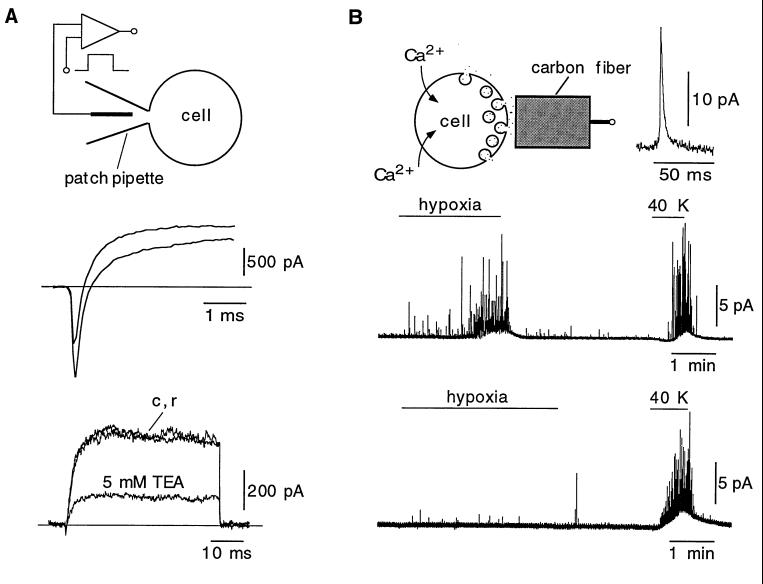
After setting up the slice preparation, our main goal was to study the secretory response to hypoxia of intact cells in the slice (see below); however, we also tested whether cells in the glomeruli could be patch-clamped and therefore were amenable for electrophysiological experiments. Stable whole-cell recordings were easily obtained from glomus cells that had inward and outward voltage-dependent currents qualitatively similar to the currents seen in enzymatically dispersed carotid-body cells (Fig. 2A Middle and Bottom). Net peak inward current at +20 mV was, in most cells, between 50 and 200 pA; however, in some cases, as in the example shown in Fig. 2A Middle, we recorded unusually large inward currents. Detailed electrophysiological characterization of glomus cells in the slices was not attempted in this work, but the large inward current observed in this study is similar to the Na+ current described in a small population of dispersed cells (27). In our experimental conditions, input resistance was between 1 and 3 GΩ, within the range of those reported in isolated rat and rabbit glomus cells (18, 24). This finding suggests that, unless whole-cell recording induced the patch-clamped cell to uncouple, the degree of electrical coupling between neighboring glomus cells is not high. As shown before in dispersed cells (4, 27), the macroscopic K+ current was reversibly reduced by exposure to relatively low concentrations of TEA (Fig. 2A Bottom).
Figure 2.
Electrophysiological and amperometric recordings from individual glomus cells in the slice. (A Top) Macroscopic currents recorded from a glomus cell by using the whole-cell configuration of the patch-clamp technique. (A Middle) Recordings of inward and outward currents from a cell that had particularly large fast inward current. Recordings elicited by depolarizations to +20 and +40 mV from the holding potential of −80 mV are superimposed. (A Bottom) Superimposed recordings from a different glomus cell elicited by depolarizing pulses from −80 mV to +20 mV illustrating the reversible reduction of the current by TEA. c and r are the control and recovery traces, respectively. (B Top) Measurement of secretory activity from glomus cells by amperometry. A large spike-like exocytotic event is shown at an expanded time base. (B Middle) Secretory response of a glomus cell to hypoxia (PO2 ≈ 20 mmHg) and to high extracellular potassium. (B Bottom) Example of a cell that was insensitive to changes of PO2 (nonresponsive cell) but that maintained the typical secretory response to high extracellular potassium.
The secretory activity of individual cells was studied by measuring the current resulting from the oxidation of released catecholamine molecules (Fig. 2B Top). Single exocytotic events appeared as spike-like signals representing the fusion of individual catecholaminergic vesicles. An example of a large secretory spike is shown in Fig. 2B Top Inset. Under normoxic conditions, most cells did not show any measurable secretory activity or had some occasional exocytotic events at a frequency of less than one per minute. Some cells were, however, more active with a spontaneous secretory event frequency of 10 per minute or higher. After switching to the hypoxic solution, cells responded with a progressive increase in the frequency and amplitude of the spikes that partially fused into a broad concentration envelope (Fig. 2B Middle). Reproducible responses to hypoxia were observed three or four times in the cells if several minutes of rest were allowed for recovery between successive stimuli. However, we have not studied in detail other factors regulating the depletion or replenishment of the releasable vesicle pool in our preparation. Spike frequency at the peak of the response to hypoxia (measured in the minute after the initial 90 s of exposure to hypoxia) was 51.8 ± 22 spikes per minute (n = 6; mean ± SD). Given the proximity of the amperometric electrodes to the cell under study, we think that most of the spikes recorded were due to exocytotic events from the same cell; however, we cannot exclude the possibility that some small spikes appearing in the recordings were due to secretory events occurring in neighboring cells. The progressive increase in spike amplitude during exposure to hypoxia was a phenomenon observed repetitively in different experiments, which probably resulted from the intracytoplasmic fusion of vesicles occurring before release (compound exocytosis; ref. 11). After switching back to the normoxic solution, recovery was fast, and cells normally returned to control conditions in less than 30 s.
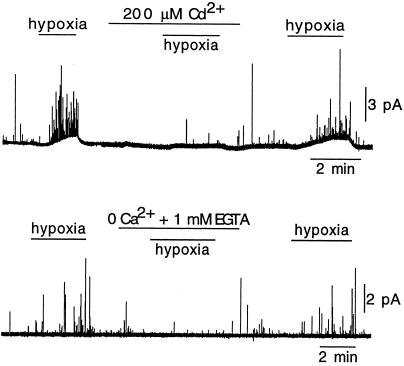
As expected from electrically excitable cells having voltage-dependent Ca2+ channels, all glomus cells made a vigorous secretory response when exposed to high extracellular K+ (see Fig. 2B Middle). Average quantal charge of secretory events elicited during exposure to hypoxia was 46 ± 25 fC (mean ± SD; n = 218 events in six cells; see also Fig. 4A). This value was obtained from the time integral of selected spikes with the fast rising phase and slower decay (Fig. 2B Top Inset) typical of secretory events occurring in the membrane facing the amperometric electrode (10, 25). Assuming that two electronic charges are transferred in the oxidation of a catecholamine molecule, the average number of molecules per vesicle is ≈140,000 ± 75,000 (mean ± SD), a value similar to our estimate in dispersed rabbit glomus cells (10). Besides the cells with characteristic secretory response to hypoxia described above, we also observed nonresponsive cells. Some of these recordings could have been from type II cells, which are nonexcitable (24) and appear intermingled within the glomerulus with glomus (type I) cells. However, most of the nonresponsive cells had the typical appearance of glomus cells and showed a powerful secretory response to high extracellular K+ (Fig. 2B Bottom). In the O2-sensitive glomus cells, the neurosecretory response to hypoxia was almost completely and reversibly abolished by the addition of Cd2+ to the extracellular solution (Fig. 3 Upper) or after removal of extracellular Ca2+ (Fig. 3 Lower). These observations are similar to those reported in rabbit glomus cells (10, 11) and further show that transmitter release elicited by exposure to physiological levels of hypoxia (≈20 mmHg or higher) depends on the influx of extracellular Ca2+.
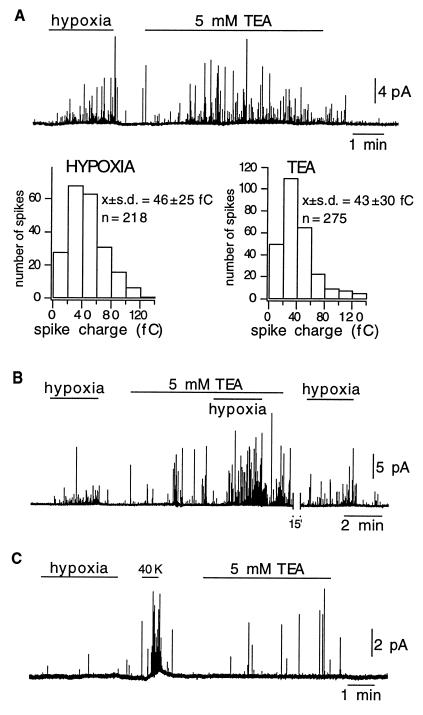
Figure 4.
Secretory response of intact glomus cells to TEA. (A Upper) In a cell that responded to hypoxia, application of TEA to the bath elicited a similar reversible secretory response. (A Lower) Frequency distribution of the charge of secretory events elicited by hypoxia and 5 mM TEA in six different cells. Note that the parameters of the distribution (means ± SD) are the same in the two experimental conditions. (B) Potentiation of the effect of hypoxia by TEA. (C) Secretory response to TEA in a cell that was not responsive to hypoxia.
Figure 3.
The secretory response to hypoxia of glomus cells depends on extracellular Ca2+ influx. (Upper) Reversible abolishment of the response to hypoxia during the blockade of Ca2+ channels by addition of 0.2 mM CdCl2 to the extracellular solution. (Lower) Reversible abolishment of the response to hypoxia by the removal and chelation of Ca2+ from the extracellular solution.
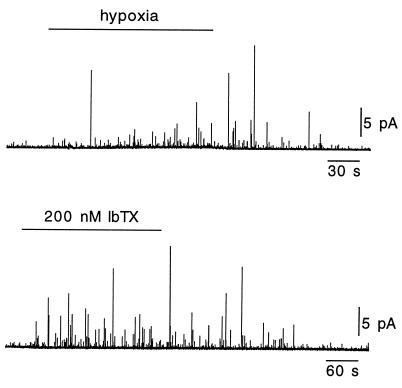
In neonatal and adult cells, the major contributors to the O2-sensitive macroscopic K+ current are voltage- and Ca2+-dependent K+ channels, which are blocked by external TEA (4, 17, 20, 27). For this reason, we tested to see whether application of TEA elicited a secretory response similar to that triggered by hypoxia. In most cells studied (31 of 32), TEA at concentrations of 2 or 5 mM, both of which produce blockade of a large proportion of K+ channels (refs. 4, 17, and 27; Fig. 2A), induced a clear secretory response (Fig. 4A Upper). This effect of TEA was observed even in quiescent cells (without measurable spontaneous quantal release) and regardless of whether extracellular K+ was 2.7 or 4.5 mM. Spike frequency at the peak of the response to 5 mM TEA (measured in the minute after the initial 90 s of exposure to the blocker) was 42 ± 17 spikes per minute (mean ± SD; n = 6), and average quantal charge was 43 ± 30 fC (mean ± SD; n = 275 events in six cells). These values, as well as the distribution of quantal events (Fig. 4A Lower), are similar to those estimated with events elicited by hypoxia, suggesting that both TEA and exposure to low PO2 can trigger the release of the same vesicle pool. As shown in Fig. 4B, TEA also potentiated the secretory response induced by hypoxia. We have not tested whether this additive effect is still present when high (saturating) concentrations of TEA are used; however, we have recorded one cell that responded to hypoxia but was insensitive to TEA alone. As mentioned before, TEA could elicit catecholamine release even from cells that were not responsive to changes in PO2 (Fig. 4C). Thus, these data indicate that direct blockade of the O2-sensitive K+ channels with TEA can elicit a vigorous secretory response from intact glomus cells. Further support for this notion came from experiments with iberiotoxin (IbTX), a selective blocker of the Ca2+- and voltage-activated maxi K+ channels that can induce catecholamine secretion in clusters of carotid-body cells (28, 29). Fig. 5 illustrates that IbTX elicited a secretory response from individual glomus cells similar to that evoked by hypoxia or TEA; washout of IbTX was, however, slower than recovery from the other stimuli.
Figure 5.
Secretory response of intact glomus cells to IbTX. In a cell that responded to hypoxia (Upper), application of IbTX (200 nM) to the bath elicited a similar reversible secretory response (Lower). Note that recovery from IbTX is relatively slow probably because of the high affinity of toxin binding to the channel.
Discussion
In this report, we describe the carotid-body slice preparation and show that this technique allows us to study the electrophysiology and cellular responses of single glomus cells to hypoxia without the need of enzymatic treatment or mechanical disruption of the tissue. We have consistently recorded secretory activity elicited by hypoxia in single rat glomus cells by measuring catecholamine release with a carbon-fiber amperometric electrode. The neurosecretory response to hypoxia in single rat glomus cells is qualitatively similar to the response that we described before in dispersed rabbit glomus cells (10, 11). In both cases, catecholamine release evoked by physiologic low PO2 levels (≈20 mmHg or higher) depends absolutely on extracellular Ca2+ influx. Thus, our data give further support to the view that glomus cells behave as O2-sensitive presynaptic-like elements, in which Ca2+ influx triggered by hypoxia-induced depolarization is the fundamental event leading to transmitter release and activation of the afferent sensory fibers with which the glomus cells form synapses (7–11).
Interestingly, we observed that some cells that had an appearance typical of glomus cells and that responded with exocytotic catecholamine secretion to high extracellular K+ were insensitive to changes in PO2. The insensitivity of these cells to hypoxia was not related to animal age and could be explained either by the need of lower PO2 levels to become depolarized or by the fact that, in this subset of glomus cells, the O2-sensitive machinery is inactivated or uncoupled from the K+ channels. We believe that the last possibility is more likely for two reasons. First, like the hypoxia-responsive cells, the nonresponsive ones were stimulated by TEA. Second and more importantly, when we observed a large number of nonresponsive cells in a particular experiment, the lack of O2-sensitivity tended to be maintained in all the slices of the preparation. Therefore, it seems that insensitivity to hypoxia depends on uncontrolled variables, some of them introduced by the experimental protocol, which may modify the chemical status of the O2-sensing molecule or alter its association with the ion channels. In fact, we realized long ago that the O2-sensitivity of ion channels is a labile phenomenon that can be easily altered by cell dissociation procedures (13).
Another important observation in this study is that external application of TEA and IbTX consistently elicited a secretory response that in most aspects was similar to the response evoked by hypoxia. This similarity contrasts with the lack of TEA effect on dispersed rat glomus cells (18) and indicates that direct blockade of O2-sensitive voltage-dependent K+ channels, which in these cells are those blocked by TEA or IbTX (4, 17, 27, 28), can lead to secretion. A corollary of these data is that inhibition of voltage-dependent K+ channels by hypoxia can contribute to initiation of the secretory response elicited by low PO2. A TEA-resistant leak K+ current inhibited preferentially by anoxia or extreme low PO2 levels has been described in dispersed rat glomus cells (18). Whether the leak and the TEA-sensitive channels are present in the same rat glomus cell type and whether their functions overlap the same range of PO2 values are questions that should be addressed in future experimental work. Based on our present data, it is difficult to understand the insensitivity to TEA (or to CTX and 4-AP) of whole carotid-body preparations reported by some authors (14–16). Although the molecular identity and pharmacology of the K+ channels in the glomus cell-afferent fiber synapse are unknown (see, however, refs. 4, 5, 17, 27, and 30), it is logical to postulate that, regardless of the mechanisms involved in the transduction of the hypoxic stimulus, there must be, at the synapse itself and along the afferent nerve fiber, several types of voltage-dependent K+ channels whose blockade should alter transmitter release as well as the frequency and shape of the propagated action potentials. Because TEA as well as CTX and 4-AP are relatively large, poorly lipid-soluble molecules, a possible explanation could be that the blockers do not diffuse at the appropriate concentration into the extracellular space of carotid bodies either superfused (with the surrounding connective capsule intact) or perfused through the carotid artery.
Acknowledgments
We wish to thank Dr. Juanjo Toledo-Aral for help in the TH immunostaining of carotid-body slices. R.P. is a predoctoral fellow of the Spanish Formación del Personal Investigator program. U.L. was recipient of a postdoctoral fellowship of the European Molecular Biology Organization. Research was supported by the Spanish Dirección General de Enseñauza Superior, Fundación La Caixa, and the Andalusian government.
Abbreviations
- TEA
tetraethylammonium
- 4-AP
4-aminopyridine
- CTX
charybdotoxin
- IbTX
iberiotoxin
- TH
tyrosine hydroxylase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030522297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030522297
References
- 1.Duchen M R, Caddy K W T, Kirby G C, Patterson D L, Ponte J, Biscoe T J. Neuroscience. 1988;26:291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- 2.López-Barneo J, López-López J R, Ureña J, González C. Science. 1988;242:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 3.Delpiano M A, Hescheler J. FEBS Lett. 1989;249:195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- 4.Peers C. Neurosci Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- 5.Ganfornina M D, López-Barneo J. Proc Natl Acad Sci USA. 1991;88:2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stea A, Nurse C A. Pflügers Arch. 1991;418:93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- 7.López-Barneo J, Benot A R, Ureña J. News Physiol Sci. 1993;8:191–195. [Google Scholar]
- 8.González C, Almaraz L, Obeso A. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 9.Buckler K J, Vaughan-Jones R D. J Physiol (London) 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ureña J, Fernández-Chacón R, Benot A R, Alvarez de Toledo G, López-Barneo J. Proc Natl Acad Sci USA. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montoro R J, Ureña J, Fernández-Chacón R, Alvarez de Toledo G, López-Barneo J. J Gen Physiol. 1996;107:133–143. doi: 10.1085/jgp.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Barneo J. Trends Neurosci. 1996;19:435–440. doi: 10.1016/0166-2236(96)10050-3. [DOI] [PubMed] [Google Scholar]
- 13.López-Barneo J, Montoro R J, Ortega-Sáenz P, Ureña J. In: Oxygen Regulation of Ion Channels and Gene Expression. López-Barneo J, Weir K S, editors. New York: Futura; 1998. pp. 127–144. [Google Scholar]
- 14.Doyle T P, Donnelly D F. J Appl Physiol. 1994;77:2606–2611. doi: 10.1152/jappl.1994.77.6.2606. [DOI] [PubMed] [Google Scholar]
- 15.Osanai S, Buerk D G, Mokashi A, Chugh D K, Lahiri S. Brain Res. 1997;747:324–327. doi: 10.1016/s0006-8993(96)01313-3. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri S, Roy A, Rozanov C, Mokashi A. Brain Res. 1998;794:162–165. doi: 10.1016/s0006-8993(98)00276-5. [DOI] [PubMed] [Google Scholar]
- 17.Wyatt C N, Peers C. J Physiol (London) 1995;483:559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckler K J. J Physiol (London) 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnelly D F, Doyle T P. J Physiol (London) 1994;475:267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatton C J, Carpenter E, Pepper D R, Kumar P, Peers C. J Physiol (London) 1997;501:49–58. doi: 10.1111/j.1469-7793.1997.049bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards F A, Konnerth A, Sakmann B, Takahashi T. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 22.Schneggenburger R, López-Barneo J, Konnerth A. J Physiol (London) 1992;445:261–276. doi: 10.1113/jphysiol.1992.sp018923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 24.Ureña J, López-López J R, González C, López-Barneo J. J Gen Physiol. 1989;93:979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow R H, von Ruden L. In: Single Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 245–275. [Google Scholar]
- 26.Fidone S J, González C. In: The Respiratory System II: Handbook of Physiology. Fishman A P, editor. Bethesda: Am. Physiol. Soc.; 1986. pp. 557–621. [Google Scholar]
- 27.López-López J R, González C, Pérez-García M T. J Physiol (London) 1997;499:429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvez A, Gimenez-Gallego G, Reuben J P, Roy-Contancin L, Feigenbaum P, Kaczorovski G J, Garcia M L. J Biol Chem. 1990;264:11083–11090. [PubMed] [Google Scholar]
- 29.Jackson A, Nurse C. J Neurochem. 1997;69:645–654. doi: 10.1046/j.1471-4159.1997.69020645.x. [DOI] [PubMed] [Google Scholar]
- 30.Ganfornina M D, López-Barneo J. J Gen Physiol. 1992;100:401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]