Abstract
The role of leptin was investigated in two models of T cell-mediated hepatitis: the administration of Con A or of Pseudomonas aeruginosa exotoxin A (PEA). In both models, leptin-deficient (ob/ob) mice were protected from liver damage and showed lower induction of tumor necrosis factor (TNF) α and IL-18 compared with their lean littermates. Neutralization of TNF-α reduced induction of IL-18 by either Con A (70% reduction) or PEA (40% reduction). Pretreatment of lean mice with either soluble TNF receptors or with an anti-IL-18 antiserum significantly reduced Con A- and PEA-induced liver damage. The simultaneous neutralization of TNF-α and IL-18 fully protected the mice against liver toxicity. However, neutralization of either IL-18 or TNF-α did not inhibit Con A-induced production of IFN-γ. Thymus atrophy and alterations in the number of circulating lymphocytes and monocytes were observed in ob/ob mice. Exogenous leptin replacement restored the responsiveness of ob/ob mice to Con A and normalized their lymphocyte and monocyte populations. These results demonstrate that leptin deficiency leads to reduced production of TNF-α and IL-18 associated with reduced T cell-mediated hepatotoxicity. In addition, both TNF-α and IL-18 appear to be essential mediators of T cell-mediated liver injury.
Leptin, the product of the ob gene, is a 16-kDa protein that plays a critical role in the regulation of body weight by inhibiting food intake and stimulating energy expenditure (1). Defects in leptin production, such as those observed in ob/ob mice, or in the long isoform of the leptin receptor (db/db mice and fa/fa rats), cause severe hereditary obesity in rodents and humans (2–6). In addition to its effects on body weight, leptin has a variety of other functions, including the regulation of hematopoiesis, angiogenesis, wound healing, and the immune and inflammatory response (7–14). Hyperresponsiveness to monocyte/macrophage-activating stimuli, i.e., endotoxin [lipopolysaccharide (LPS)] or tumor necrosis factor (TNF) α, is present in leptin-deficient (ob/ob) mice (9–11). Additionally, decreased circulating leptin levels are associated with reduced lymphocyte cellularity in the thymus and spleen (15–19).
Interactions exist between leptin and cytokines. In fact, proinflammatory cytokines increase leptin levels, whereas leptin regulates the production of several pro- and anti-inflammatory cytokines (9, 13, 20, 21). Furthermore, leptin regulates the balance of T helper (Th)1/Th2 cytokines (22). TNF-α is a proinflammatory cytokine that plays a crucial role in the response to tissue injury, infection, and inflammation (23). TNF-α causes acute inflammatory hepatocellular apoptosis followed by organ failure (24) and is the central mediator in several experimental models of hepatotoxicity (25–27). IL-18, a pleiotropic cytokine produced by activated macrophages, plays an important role in the Th1 response (28). IL-18 synergizes with IL-12 to induce the production of IFN-γ and potentiates Th1 and natural killer cell cytotoxicity by augmenting Fas ligand- and perforin-mediated cytotoxic activity (29, 30). IL-18 also plays a critical role in TNF-α- and Fas ligand-mediated liver injury (31).
In the present study, we investigated the role of leptin as a regulator of T cell-mediated inflammation in vivo. Two experimental models of T cell-mediated hepatotoxicity were studied: the administration of the T cell mitogen Con A (25, 32) or of Pseudomonas aeruginosa exotoxin A (PEA) (27). In each model, T lymphocytes and TNF-α are required for the induction of liver injury (25–27, 33, 34). The present results demonstrate that leptin plays an important role in T cell-mediated liver toxicity in association with a regulatory effect on thymus and peripheral blood cellularity as well as on the production of two proinflammatory cytokines, TNF-α and IL-18.
Materials and Methods
Materials.
Con A (type IV-S) and PEA were from Sigma. The soluble TNF-α receptor p55 (TNFsRp55) (35) and recombinant murine leptin were kind gifts of Amgen Biologicals. The neutralizing anti-murine IL-18 antiserum was produced by immunizing rabbits with recombinant murine mature IL-18, as described (36).
Animal Experimentation.
Animal protocols were approved by the Animal Studies Committee of the San Francisco Veteran Affairs Medical Center and of the University of Colorado Health Sciences Center. Four- to five-week-old female leptin-deficient (C57BL/6Job/ob) mice, their lean littermates (+/?), and C57BL/6J mice were obtained from The Jackson Laboratory. Mice received an i.v. injection in the tail vein of either 200 μg of Con A dissolved in pyrogen-free saline or 300 μg/kg of PEA in pyrogen-free saline containing 0.1% human serum albumin. Control mice received an i.v. injection of the corresponding vehicle. In some experiments, mice received an i.p. injection of either TNFsRp55 (10 mg/kg) (35), anti-IL-18 antiserum (400 μl/mouse) (36), or a combination of the two. A group of mice receiving normal rabbit serum was included as the control for mice receiving the anti-IL-18 antiserum.
For leptin replacement, 4-wk-old ob/ob mice received i.p. injections of either murine leptin (1 μg/g initial body weight, twice daily) or saline for 10 days. A group of +/? also was included in the experiment. Mice were weighed and food intake recorded every 3 days. At the conclusion of leptin treatment, mice received either an i.v. injection of Con A or remained untreated for evaluation of thymus cellularity and differential blood cell counts. At different times after Con A or PEA injection, blood was collected from the retroorbital plexus under alothane anesthesia and serum prepared. Because the kinetics of hepatotoxicity and cytokine production after administration of PEA is slower compared with Con A (27), alanine aminotransferase (ALT), TNF-α, and IL-18 levels were evaluated 16 h after injection of PEA.
FACS Analysis and Differential Blood Cell Counts.
The thymus was excised, teased into single cell suspensions, and placed in Hanks' balanced salt solution (HBSS) with 5% FCS on ice. Red blood cells were lysed by using hemolytic Gey's solution. The number of viable thymocytes was calculated on a hemocytometer. The thymocytes were labeled with anti-CD4 biotinylated (clone GK1.5) and anti-CD8a (clone 53–67)-directly conjugated FITC antibodies (PharMingen). Streptavidin-phycoerythrin (Southern Biotechnology Associates) was used as a second-step reagent. The labeling procedures were carried out at 4°C in HBSS with 5% FCS. Flow cytometric analysis of 10,000 thymocytes was conducted on a FACScalibur (Becton Dickinson) flow cytometer and analyzed by using the cellquest analysis program. Differential blood cell counts were performed by Idexx Laboratories (Westbrook, MA).
Cytokine and Transaminase Measurement.
TNF-α was measured by using a DuoSet ELISA kit from R & D Systems. IFN-γ and IL-12 p70 were measured by using ELISA kits from Endogen (Woburn, MA). IL-18 was measured by using an electrochemiluminescence assay as described (37). Serum ALT was measured by using a kit from Sigma.
Results
Protection from Con A-Induced Liver Toxicity in ob/ob Mice.
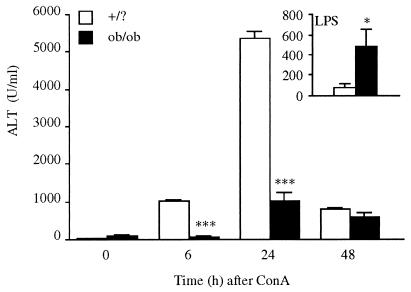
Ob/ob mice and their lean littermates (+/?) were injected with 200 μg/mouse of Con A, and blood was collected at various times thereafter for evaluation of hepatotoxicity by measurement of serum ALT levels. As shown in Fig. 1, Con A significantly increased serum ALT levels in +/? mice, with peak levels observed 24 h after administration. However, in ob/ob mice, Con A-induced serum ALT levels were markedly reduced compared with +/? mice (96% and 81% reduction at 6 and 24 h, respectively, compared with +/? mice). In contrast, LPS-induced hepatotoxicity was significantly increased in ob/ob mice compared with +/? mice (see Inset in Fig. 1).
Figure 1.
Reduced liver damage in ob/ob compared with +/? mice after administration of Con A. +/? and ob/ob mice received an i.v. injection of Con A or saline. Blood was collected at various times thereafter for measurement of ALT levels. Data are mean ± SEM of five mice per group. ***, P < 0.001 vs. +/? mice by unpaired Student's t test. (Inset) +/? and ob/ob mice received an i.p. injection of LPS (0.5 mg/kg). Blood was collected 24 h later for measurement of ALT levels. Data are mean ± SEM of five mice per group. *, P < 0.05 vs. +/? mice by unpaired Student's t test.
Reduced TNF-α and IL-18 Levels in ob/ob Mice Injected with Con A.
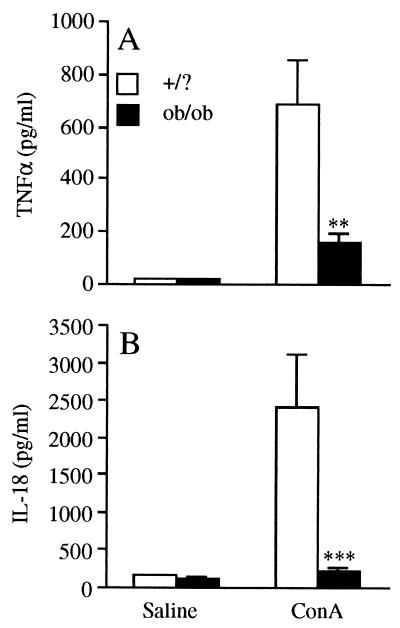
Levels of TNF-α, IL-18, IFN-γ, and IL-12 were evaluated in the serum of both +/? and ob/ob mice after administration of Con A. As shown in Fig. 2A, serum TNF-α levels were markedly increased in +/? mice 1 h after administration of Con A. In contrast, the increase in serum TNF-α was greatly attenuated (77% reduction) in ob/ob compared with +/? mice. Likewise, Con A significantly increased serum IL-18 levels in +/? mice, with peak levels occurring 6 h after injection. In contrast, there was no significant increase in serum IL-18 in Con A-treated ob/ob mice (Fig. 2B). Although Con A stimulated IL-12 and IFN-γ production, the magnitude of the increase was similar in ob/ob and +/? mice (data not shown), indicating that the impairment in production after Con A administration was specific for TNF-α and IL-18.
Figure 2.
Reduced TNF-α and IL-18 in ob/ob compared with +/? mice. +/? and ob/ob mice received an i.v. injection of Con A or saline. (A) Serum TNF-α levels at 1 h. (B) IL-18 levels at 6 h. Data are mean ± SEM of five mice per group. **, P < 0.01; ***, P < 0.001 vs. +/? mice by unpaired Student's t test.
Reduced PEA-Induced Hepatotoxicity and Cytokine Production in ob/ob Mice.
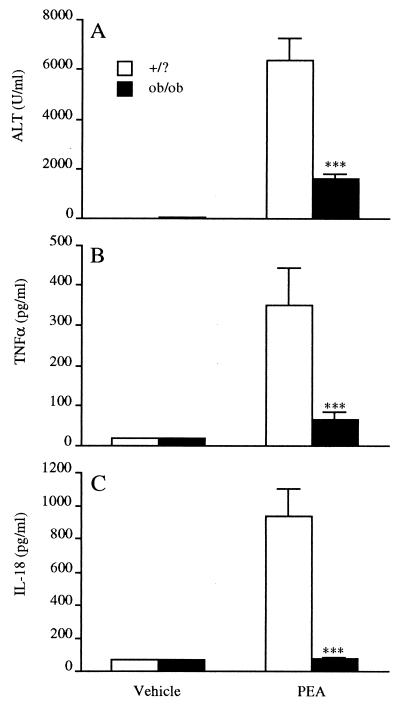
We next investigated whether ob/ob mice are also resistant to PEA-induced hepatotoxicity, which is also mediated by TNF-α and dependent on T cells (27). As shown in Fig. 3A, ob/ob mice were significantly protected from PEA-induced liver injury (serum ALT levels at 16 h were 75% lower in ob/ob compared with +/? mice). Furthermore, circulating levels of TNF-α (Fig. 3B) and IL-18 (Fig. 3C) were significantly reduced in ob/ob mice compared with +/? mice injected with PEA (82% and 92% reduction for TNF-α and IL-18, respectively).
Figure 3.
Reduced PEA-induced hepatotoxicity and cytokine production in ob/ob mice. Ob/ob and +/? mice received an i.v. injection of either PEA or vehicle. Blood was collected at 16 h for measurement of serum ALT (A), TNF-α (B), or IL-18 (C) levels. Data are mean ± SEM of five mice per group. ***, P < 0.001 vs. +/? mice by unpaired Student's t test.
Role of Endogenous TNF-α in the Induction of IL-18 by Con A and PEA.
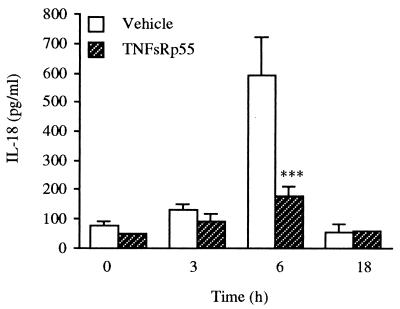
Because ob/ob mice exhibit reduced circulating levels of both TNF-α and IL-18 after administration of Con A, and because production of TNF-α preceded the increase in serum IL-18, the role of endogenous TNF-α in mediating Con A-induced IL-18 was evaluated. Wild-type C57BL/6J mice were injected with TNFsRp55 immediately before administration of Con A, and serum IL-18 levels were measured. As shown in Fig. 4, neutralization of TNF-α activity significantly blunted the induction of IL-18 by Con A. In mice injected with PEA, neutralization of endogenous TNF-α resulted in 40% inhibition of serum IL-18 levels (data not shown). However, even in the presence of TNFsRp55, Con A- or PEA-induced IL-18 levels were significantly elevated compared with those observed in saline-injected mice.
Figure 4.
TNF-α neutralization inhibits induction of IL-18 by Con A. C57BL/6J mice received an i.p. injection of TNFsRp55 or saline, immediately followed by the i.v. administration of either Con A or saline. Blood was collected at 3, 6, or 18 h for evaluation of IL-18 levels. Data are mean ± SEM of five mice per group. ***, P < 0.001 vs. corresponding vehicle by unpaired Student's t test.
TNF-α and IL-18 Mediate Con A- and PEA-Induced Liver Toxicity.
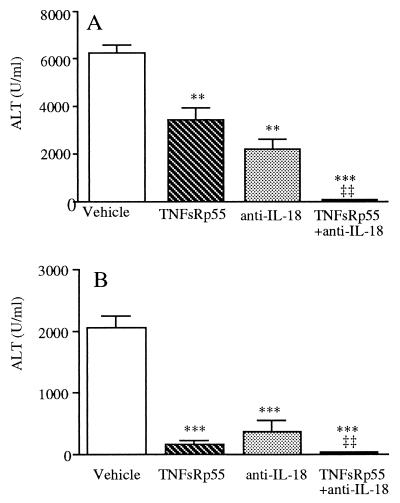
To evaluate the role of TNF-α and IL-18 in mediating Con A- and PEA-induced hepatotoxicity, C57BL/6J mice were pretreated with TNFsRp55, with a neutralizing anti-IL-18 antiserum, or with the combination of the two. As shown in Fig. 5A, TNFsRp55 or the anti-IL-18 antiserum each significantly reduced Con A-induced serum ALT levels (approximately 50–60% reduction compared with vehicle). However, when mice were injected with TNFsRp55 plus anti-IL-18, a complete inhibition of Con A-induced ALT was observed. Neither TNFsRp55 nor the anti-IL-18 antiserum reduced Con A-induced IFN-γ (data not shown).
Figure 5.
Blockade of TNF-α and IL-18 protects mice from Con A- and PEA-induced hepatotoxicity. C57BL/6J mice received an i.p. injection of either TNFsRp55, anti-IL-18 antiserum, a combination of TNFsRp55 and anti-IL-18 antiserum or vehicle, immediately followed by the i.v. administration of Con A (A) or PEA (B). Data are mean ± SEM of five mice per group. **, P < 0.01; ***, P < 0.001 vs. Con A or PEA alone; ‡‡, P < 0.01 vs. either TNFsRp55 or anti-IL-18 by factorial ANOVA.
As shown in Fig. 5B, in PEA-injected mice, neutralization of either TNF-α or IL-18 resulted in 93% and 83% inhibition of serum ALT levels, respectively. The combined blockade of TNF-α and IL-18 resulted in 99% protection.
Leptin Replacement Restores the Sensitivity to Con A-Induced Hepatitis in ob/ob Mice.
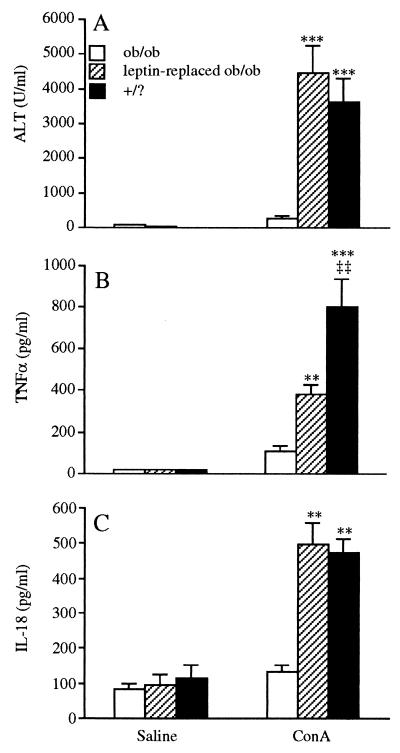
We evaluated whether exogenous leptin replacement could restore the ability of ob/ob mice to respond to Con A. Leptin was administered to ob/ob mice employing a treatment regime known to correct thymic atrophy in these mice (19). Ob/ob mice received two daily injections of leptin or saline for 10 days, after which time the response to Con A was evaluated. As shown in Fig. 6A, leptin replacement restored the ability of Con A to induce hepatotoxicity in ob/ob mice. In fact, no significant differences in Con A-induced serum ALT levels were observed between leptin-replaced ob/ob mice and +/? mice. Accordingly, leptin replacement also restored, in part, the ability of ob/ob mice to produce TNF-α in response to Con A and completely restored Con A-induced serum IL-18 levels (Fig. 6 B and C). Interestingly, a shorter schedule (2.5 days) of leptin replacement, previously shown to restore normal responsiveness to LPS in ob/ob mice (9), did not result in restoration of the response of ob/ob mice to Con A (data not shown), suggesting that the unresponsiveness of ob/ob to Con A and the hyperresponsiveness to LPS are mediated by different mechanisms.
Figure 6.
Leptin replacement restores responsiveness to Con A in ob/ob mice. Ob/ob mice received two daily injections of either saline (ob/ob) or leptin (leptin-replaced ob/ob) for 10 days. +/? mice remained untreated. After treatment, mice received an i.v. injection of either saline or Con A. (A) Serum ALT at 24 h. (B) Serum TNF-α at 1 h. (C) Serum IL-18 at 6 h. Data are mean ± SEM of seven mice per group. **, P < 0.01; ***, P < 0.001 vs. ob/ob mice; ‡‡, P < 0.01 vs. leptin-replaced ob/ob mice by factorial ANOVA.
Effect of Exogenous Leptin on Leukocyte Populations in ob/ob Mice.
When cells from the thymus were analyzed (Table 1), we confirmed that ob/ob mice have reduced thymic cellularity (54% reduction in total lymphocyte cellularity in ob/ob mice compared with +/? mice) (16–19). In particular, CD4+ CD8+ were reduced by 59%, CD4+ CD8− by 54%, CD4− CD8+ by 36%, and CD4− CD8− by 31% in ob/ob mice compared with +/? mice. However, the percentage of CD4+ CD8+, CD4+ CD8−, CD4− CD8+, and CD4− CD8− cells did not differ between ob/ob and +/? mice (data not shown). Leptin replacement resulted in a 3-fold increase in the total thymic cellularity of ob/ob mice. The absolute number of CD4+ CD8+, CD4+ CD8−, and CD4− CD8− cells significantly increased after leptin replacement, whereas CD4− CD8+ cells were not significantly affected. Interestingly, leptin-replaced ob/ob mice had significantly higher thymic cellularity than +/? mice, suggesting that supraphysiologic amounts of leptin may influence lymphocyte cellularity in the thymus. In addition, as previously demonstrated (15–19), spleen weight was significantly reduced in ob/ob mice compared with +/? mice, and leptin-replacement normalized spleen weight (spleen weight was 40.00 ± 8.52, 65.00 ± 2.89, and 63.70 ± 3.75 mg in ob/ob, leptin-replaced ob/ob, and +/? mice, respectively; mean ± SEM, n = 4)
Table 1.
Effect of leptin replacement on lymphocyte subpopulations in the thymus
| Thymocyte population | ob/ob | Leptin-replaced ob/ob | +/? |
|---|---|---|---|
| Total | 40.50 ± 13.00** | 134.50 ± 24.39‡ | 87.50 ± 12.81 |
| CD4+ CD8+ | 22.65 ± 8.20** | 84.79 ± 18.76‡ | 55.13 ± 9.80 |
| CD4+ CD8− | 2.99 ± 1.05** | 12.23 ± 2.17‡ | 6.44 ± 1.86 |
| CD4− CD8+ | 2.22 ± 1.13 | 3.73 ± 1.38 | 3.48 ± 1.21 |
| CD4− CD8− | 1.13 ± 0.24** | 2.86 ± 0.45‡ | 1.64 ± 0.20 |
ob/ob mice received two daily injections of either saline (ob/ob) or leptin (leptin-replaced ob/ob) for 10 days. +/? mice were left untreated. After treatment, the thymus was harvested and processed for FACS analysis as indicated in Materials and Methods. Data are mean ± SEM of four mice per group. **, P < 0.01 vs. either leptin-replaced ob/ob or +/? mice; ‡, P < 0.05 vs. +/? mice by factorial ANOVA.
The thymus and the spleen were not the sole compartments affected by leptin deficiency. As indicated in Table 2, the absolute number of circulating leukocytes was significantly reduced in ob/ob mice compared with +/? mice. However, leukocyte subsets were differentially affected by the lack of leptin. Circulating lymphocytes were reduced by 54%, whereas there was a 4-fold increase in the number of monocytes and a nonsignificant trend to an increase in the number of neutrophils in ob/ob mice. As indicated in Table 2, leptin replacement restored circulating leukocyte populations to normal levels.
Table 2.
Effect of leptin replacement on numbers of circulating leukocytes and erythrocytes
| ob/ob | Leptin-replaced ob/ob | +/? | |
|---|---|---|---|
| RBC (106/μl) | 10.80 ± 0.22 | 10.18 ± 0.43 | 10.28 ± 0.23 |
| WBC (103/μl) | 4.87 ± 0.36 | 8.72 ± 0.07** | 7.15 ± 1.14* |
| Lymphocytes/μl | 3720.75 ± 55.40 | 7941.25 ± 146.43** | 6829.25 ± 854.91** |
| Monocytes/μl | 284.50 ± 64.65 | 56.25 ± 18.05** | 67.75 ± 19.62** |
| Neutrophils/μl | 843.75 ± 270.02 | 522.50 ± 59.05 | 400.25 ± 125.52 |
ob/ob mice received two daily injections of either saline (ob/ob) or leptin (leptin-replaced ob/ob). +/? mice were left untreated. Data are mean ± SEM of four mice per group. *, P < 0.05; **, P < 0.01 vs. ob/ob mice by factorial ANOVA.
Discussion
Using two distinct models, we demonstrate that leptin-deficient ob/ob mice are protected from T cell-mediated liver toxicity. The reduced susceptibility of ob/ob mice to Con A- and PEA-induced hepatotoxicity is likely the result of defective production of TNF-α and IL-18. In fact, inhibition of TNF-α and/or IL-18 protects wild-type mice from T cell-mediated hepatotoxicity. The role of TNF-α in mediating the toxic effects of Con A and PEA has been reported (25–27, 33). IL-18 has been implicated in LPS- or Fas-induced liver damage (31, 38). In the present study, we demonstrate a critical role for IL-18 in Con A- and PEA-induced, T cell-mediated, liver toxicity. In line with the present data are studies showing that caspase-1-deficient mice, which cannot produce bioactive IL-18 because of a failure to process pro-IL-18 (39), are protected from Con A-induced liver damage (26). It is worth noting that simultaneous neutralization of TNF-α and IL-18 afforded complete protection from T cell-mediated liver toxicity, indicating that TNF-α and IL-18 act together in mediating liver damage. IFN-γ did not appear to play a significant role in hepatotoxicity in the Con A model.
Leptin directly regulates IFN-γ production from T cells (22). However, despite the presence of lymphoid atrophy, we did not observe reduced production of IFN-γ in ob/ob mice injected with Con A, suggesting that T cells are not the major source of IFN-γ in this in vivo model. Although it has been suggested that IFN-γ plays an important role in Con A-induced liver damage (40), our data point to a major role for TNF-α and IL-18, but not IFN-γ, in Con A-induced hepatotoxicity. Two independent observations lead to this conclusion: (i) neutralization of TNF-α or IL-18 protected mice from Con A-induced hepatotoxicity without reducing IFN-γ levels; (ii) ob/ob mice are protected from Con A-induced hepatotoxicity despite no reduction in IFN-γ levels. Previous data obtained in hypothyroid mice support our observations (41): in the presence of hypothyroidism, protection from Con A-induced hepatotoxicity is associated with reduced levels of TNF-α but not of IFN-γ.
Although IL-18 is primarily considered an IFN-γ-inducing factor, this cytokine possesses other activities, including induction of proinflammatory cytokines (28). We previously reported that IL-18 is not required for the induction of IFN-γ by Con A in murine splenocytes in vitro (36). In the present study, these data are confirmed in vivo. In fact, ob/ob mice exhibited reduced IL-18, but not IFN-γ levels. More importantly, neutralization of IL-18 activity reduced Con A-induced liver damage without significantly altering IFN-γ levels. Therefore, IL-18 can mediate liver toxicity independently of IFN-γ. Although little is known about the regulation of IL-18 production in the models studied, we demonstrate that endogenous TNF-α partly mediates Con A- and PEA-induced IL-18 production in vivo. However, because interactions between T lymphocytes and dendritic cells can induce IL-18 in vitro (42), it is possible that, in addition to TNF-α, T cells produce an additional factor(s) contributing to induction of IL-18.
Although several studies report the presence of lymphoid atrophy in ob/ob mice (15–19), to our knowledge the present data are the first showing reduced T cell-mediated inflammatory responses in vivo in ob/ob mice. The observation that ob/ob mice are protected from liver damage induced by T cell-activating stimuli such as Con A or PEA is in striking contrast to data demonstrating increased sensitivity of ob/ob mice to proinflammatory monocyte/macrophage-activating stimuli, particularly LPS and TNF-α (9–11). However, in the present report, we show that lymphoid atrophy is not the only hematopoietic abnormality present in ob/ob mice. In addition to reduced thymic and circulating lymphocytes, a 4-fold increase in the number of circulating monocytes is present in ob/ob mice. Therefore, it is likely that the absence of leptin will lead to reduced sensitivity to T cell-activating stimuli and enhanced responses to monocyte activators. The interactions between leptin and TNF-α appear to be of particular interest. TNF-α induces leptin (20), whereas leptin can modulate both the production and the response to TNF-α. In fact, leptin is clearly a protective factor when TNF-α is either administered exogenously or induced by LPS (9–11). However, we have shown that the absence of leptin is responsible for reduced production of TNF-α from T cells. The role of leptin in the protection from TNF-α toxicity is probably direct, since short-term treatment with exogenous leptin can protect mice (9, 10). In contrast, a longer treatment schedule is necessary to restore the response of ob/ob mice to Con A, suggesting a more indirect activity of leptin, likely mediated by the reconstitution of lymphoid cellularity.
Howard et al. (19) demonstrated that administration of exogenous leptin reconstitutes lymphocyte cellularity in ob/ob mice, which have dramatically reduced (>90%) numbers of lymphocytes in the thymus and altered CD4+CD8+/CD4−CD8− ratios. Although we also demonstrated reduced cellularity in the thymus of ob/ob mice and reconstitution after leptin administration, only a 2-fold difference between +/? and ob/ob mice was present in our experiments. Furthermore, significant changes in the percentage of CD4+ or CD8+ cells between ob/ob and +/? mice were not observed. Differences in the age (4-wk-old vs. 10-wk-old mice) and gender (females vs. males) of the animals may account for these discrepancies. Importantly, in the present report, we demonstrate that leptin replacement leads to the appearance of functional lymphocytes, as administration of exogenous leptin restores the ability of ob/ob mice to respond to Con A.
Leptin, initially discovered as a regulator of food intake and energy expenditure, is emerging as a pleiotropic molecule involved in a variety of physiological and pathological functions (43). Alterations in leptin levels and/or in the responsiveness to leptin have been reported not only during starvation and obesity, but also in patients affected by diabetes, renal failure, hypothyroidism, and AIDS (43). T lymphocytes and cytokines are critical mediators of hepatic inflammation in patients affected by viral, allergic, autoimmune, and possibly alcoholic liver disease (44–47). Because of the close interactions between leptin, cytokines, and lymphocytes, our observations suggest that the study of the role of leptin in disease may help to better understand the regulation of the immune and inflammatory response.
Acknowledgments
We thank Dr. C.K. Edwards III for the kind gift of TNFsRp55. These studies were supported by National Institutes of Health Grants AI-15614 (to C.A.D.) and DK-49448 (to C.G.) and by the Research Service of the Department of Veterans (to C.G. and K.R.F.).
Abbreviations
- TNF-α
tumor necrosis factor α
- PEA
Pseudomonas aeruginosa exotoxin A
- TNFsRp55
TNF-α soluble receptor p55
- LPS
lipopolysaccharide
- ALT
alanine aminotransferase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040561297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040561297
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 3.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 4.Truett G E, Bahary N, Friedman J M, Leibel R L. Proc Natl Acad Sci USA. 1991;88:7806–7809. doi: 10.1073/pnas.88.17.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, et al. Nature (London) 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 6.Clement K, Vaiss C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmellen M, Dina C, Chambaz J, Lacorte J-M, et al. Nature (London) 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 7.Gainsford T, Willson T A, Metcalf D, Handman E, McFarlane C, Ng A, Nicola N A, Alexander W S, Hilton D J. Proc Natl Acad Sci USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierra-Honigmann M R, Nath A K, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa W C, Madge L A, Schechner J S, Scwabb M B, Polverini P J, Flores-Riveros J R. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 9.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello C A, Feingold K R, Grunfeld C. Am J Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi N, Waelput W, Guisez Y. J Exp Med. 1999;189:207–212. [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S Q, Lin H Z, Lane M D, Clemens M, Diehl A M. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuboi R, Rifkin D B. J Exp Med. 1990;172:245–251. doi: 10.1084/jem.172.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffreda S, Yang S Q, Lin H Z, Karp C L, Brengaman M L, Wang D J, Klein A S, Bulkley G B, Bao C, Noble P W, et al. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 14.Mandel M A, Mahmoud A A F. J Immunol. 1978;120:1375–1377. [PubMed] [Google Scholar]
- 15.Meade C J, Sheena J, Mertin J. Int Arch Allergy Appl Immunol. 1979;58:121–127. doi: 10.1159/000232183. [DOI] [PubMed] [Google Scholar]
- 16.Barbul A, Sisto D A, Wasserkrug H L, Levenson S M, Efron G, Seifter E. J Parenter Enteral Nutri. 1981;5:492–495. doi: 10.1177/0148607181005006492. [DOI] [PubMed] [Google Scholar]
- 17.Chandra R K, Au B. Int Arch Allergy Appl Immunol. 1980;62:94–98. doi: 10.1159/000232498. [DOI] [PubMed] [Google Scholar]
- 18.Boissonneault G A, Harrison D E. J Nutr. 1994;124:1639–1646. doi: 10.1093/jn/124.9.1639. [DOI] [PubMed] [Google Scholar]
- 19.Howard J K, Lord G M, Matarese G, Vendetti S, Ghatei M A, Ritter M A, Lechler R I, Bloom S R. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunfeld C, Zhao C, Fuller J, Pollock A, Moser A, Friedman J, Feingold K R. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faggioni R, Fantuzzi G, Fuller J, Dinarello C A, Feingold K R, Grunfeld C. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 22.Lord G M, Matarese G, Howard J K, Baker R J, Bloom S R, Lechler R I. Nature (London) 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 23.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. Immunol Rev. 1999;169:175–194. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 24.Bradham C A, Plumpe J, Manns M P, Brenner D A, Trautwein C. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 25.Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. J Exp Med. 1994;179:1529–1537. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ksontini R, Colagiovanni D B, Josephs M D, Edwards C K I, Tannahill C L, Solorzano C C, Norman J, Denham W, Clare-Salzler M, MacKay S L, Moldawer L L. J Immunol. 1998;160:4082–4089. [PubMed] [Google Scholar]
- 27.Schumann J, Angermuller S, Bang R, Lohoff M, Tiegs G. J Immunol. 1998;161:5745–5754. [PubMed] [Google Scholar]
- 28.Dinarello C A, Novick D, Puren A J, Fantuzzi G, Shapiro L, Muhl H, Yoon D-Y, Reznikov L L, Kim S-H, Rubinstein M. J Leukocyte Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 29.Tsutsui H, Nakanishi K, Matsui K, Higasino K, Okamura H, Miyazawa Y, Kaneda K. J Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- 30.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, et al. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 31.Tsutsui H, Matsui K, Kawada Y, Hyodo N, Hyashi H, Okamura H, Higashino K, Nakanishi K. J Immunol. 1997;159:3961–3967. [PubMed] [Google Scholar]
- 32.Tiegs G, Hentschel J, Wendel A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gantner F, Leist M, Lohse A W, Germann P G, Tiegs G. Hepatology. 1995;21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 34.Tiegs G. Acta Gastroenterol Belg. 1997;60:176–179. [PubMed] [Google Scholar]
- 35.Edwards C K I. Ann Rheum Dis. 1999;58:I73–I81. doi: 10.1136/ard.58.2008.i73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantuzzi G, Puren A J, Harding M W, Livingston D J, Dinarello C A. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 37.Fantuzzi G, Reed D A, Dinarello C A. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsutsui H, Kayagaki N, Kuida K, Nakano H, Hayashi N, Takeda K, Matsui K, Kashiwamura S-I, Hada T, Akira S, et al. Immunity. 1999;11:359–367. doi: 10.1016/s1074-7613(00)80111-9. [DOI] [PubMed] [Google Scholar]
- 39.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 40.Kusters S, Gantner F, Kunstle G, Tiegs G. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 41.Shirin H, Dotan I, Papa M, Maaravi Y, Aeed H, Zaidel L, Matas Z, Bruck R, Moss S F, Halpern Z, Oren R. Liver. 1999;19:206–211. doi: 10.1111/j.1478-3231.1999.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 42.Gardella S, Andrei C, Costigliolo S, Poggi A, Zocchi M R, Rubartelli A. J Leukocyte Biol. 1999;66:237–241. [PubMed] [Google Scholar]
- 43.Mantzoros C S, Moschos S J. Clin Endocrinol (Oxford) 1999;49:551–567. doi: 10.1046/j.1365-2265.1998.00571.x. [DOI] [PubMed] [Google Scholar]
- 44.Chang K M, Rehermann B, Chisari F V. Springer Semin Immunopathol. 1997;19:57–68. doi: 10.1007/BF00945025. [DOI] [PubMed] [Google Scholar]
- 45.Castell J V. Clin Exp Allergy. 1998;28, Suppl. 4:13–19. [PubMed] [Google Scholar]
- 46.Mieli-Vergani G, Vergani D. Semin Liver Dis. 1998;18:271–279. doi: 10.1055/s-2007-1007163. [DOI] [PubMed] [Google Scholar]
- 47.Batey R G, Clancy R L, Pang G T, Cao Q. Alcohol Clin Exp Res. 1999;23:1207–1209. [PubMed] [Google Scholar]