Abstract
MicroRNAs (miRNAs), 18–25 nt single-stranded RNAs, act as regulators in fine-tuning gene function. The absence of miRNAs is not life-threatening in early embryonic development of Dicer-knockout zebrafish and mice, which may account for genetic expression of various traits as a result of miRNA complexity in higher animals during natural evolution. The Pol-II-mediated intronic miRNA is a useful tool to validate the function of computer-predicated miRNAs in zebrafish and mice.
Keywords: miRNA, Gene expression, Intronic microRNA, Regulatory gene, Fine-tuning of gene function
MicroRNA (miRNA), a class of 18–25 nt single-stranded RNA, used to be considered as the debris of gene transcripts. In 2000s, the observations that the abundant non-coding RNAs regulate the expression of protein-coding genes became a breakthrough in the understanding of the post-genome gene functions.
The miRNA is a small regulatory single-stranded RNA. The miRNA is transcribed from the DNA segment, which codes for a precursor of miRNA (pri-miRNA), but is not translated into protein. The pri-miRNA includes the miRNA sequence and its reverse-complement base pair to form a small hairpin RNA (shRNA) with a nucleotide loop at one end. The pri-miRNA is cleaved at the base of the shRNA stem region by Drosha, a nuclear enzyme, to form a pre-miR-NA. The pre-miRNA molecule is then actively transported out of the nucleus by Exportin 5. In the cytoplasm, the enzyme Dicer cuts the hairpin loop and one of the stem arm out of the pre-miRNA to form the mature miRNA.
The miRNA functions in gene regulation. It is partially complementary to a part of one or more mRNAs, usually at a site in the 3′-UTR. Binding of the miRNA to the mRNA inhibits protein translation and/or triggers the degradation of the mRNA transcript through a process similar to RNA interference (RNAi).
Recent new evidence suggests that the miRNAs act as regulators in fine-tuning gene functions [1,2]. These observations have pinpointed the important but not life-essential role of miRNAs in regulation of morphogenesis, suggesting a function of miRNAs in fine-tuning genes involved in the brain development. In the study of [2], a family of miR-430 miRNA was identified and the genomic locus composing of multiple miR-430 copies was found to be clustered. This type of conserved clusters was also observed in other animal genomes [4]. Conceivably, more than one miRNAs target on the same genes because the interaction between the miRNA and its target mRNA is based on partial complementarity. Thus, the partial complementarity and multiple miRNA clustering may provide a more delicate means for regulating gene expression. Further, even though important roles for miRNAs during embryonic morphogenesis and differentiation were suggested, none of the signaling pathways known to be affected during embryonic morphogenesis was markedly affected in the Dicer-knockout zebrafish, indicating that the absence of miRNAs is not life-threatening. Similar observations were reported in murine ES cells after Dicer ablation [4].
The fine-tuning of gene functions by miRNAs may account for genetic expression of various traits, consequently, any dysregulation of this miRNA mechanism may lead to genetic diseases. One good example is fragile X syndrome, which represents about 30% of human inherited mental retardation. CGG repeat (rCGG) expansion in the 5′-UTR of FMR1 gene is the causative mutation in 99% of individuals with fragile X syndrome [3]. The FMR1 gene encodes an RNA-binding protein, FMRP, which is associated with polyribosome assembly in an RNP-dependent manner, therefore, is capable of suppressing translation through an RNAi-like pathway. FMRP also contains a nuclear localization signal (NLS) and a nuclear export signal (NES) for shuttling certain miRNAs between the nucleus and cytoplasm. Probably, CpG methylation in the FMR1 rCGG expansion is triggered by a hairpin RNA derived from the 3′-UTR of the FMR1 expanded allele transcript. The hairpin RNA, processed by Dicer, triggers the formation of RNA-induced initiator of transcriptional gene silencing on the homologous rCGG sequences and results in heterochromatin repression of the FMR1 locus [3].
These examples suggest that natural evolution gives rise to more complexity and more variety of miRNAs in higher animals and plants. Again, these miRNAs coordinate the vast gene expression volumes and interactions in higher animals and plants. As a result, any dysregulation of miRNAs may interfere with the fine-tuning mechanism of genes and leads to genetic diseases. These examples also suggest that most miRNAs may not control the life-essential gene expression in a none-or-all fashion, but rather coordinate the fine-tuning of the transcriptional and post-transcriptional gene regulation.
A comprehensive screening of miRNA expression patterns in zebrafish development also revealed that most miRNAs function in tissue fate establishment, differentiation, and maintenance of tissue identity, but not life-threatening [6]. Given that many miRNA genes are clustered in the genome and probably expressed as one primary transcript, it is logic to predict that many of these clustered genes encoding miRNAs showed identical or overlapping expression patterns. Again, these findings suggest that the miRNA functions in specific and diverse, temporal and spatial, fine-tuning for crucial differentiation and development and that the miRNA may be transcribed with the protein-coding gene at the same time by Type II RNA polymerases (Pol- II). Indeed, Wienholds et al. [6] and others have identified numerous miRNAs located in the intron of protein-coding genes (Table 1). However, these intronic miRNAs were predicted based on computational programs and need to be validated for their functional significance.
Table 1.
Up to date, more than one hundred intronic microRNAs have been identified; however, only a few of them were tested
| miRNA | Species | Host gene (intron) [#] | Target gene(s) |
|---|---|---|---|
| Intronic microRNA | |||
| miR-2a, −b2 | Worm | Spi | |
| miR-7b | Mammal | pituitary gland specific factor 1A (2) [NM174947] | paired mesoderm homeobox protein 2b; HLHm5 |
| miR-10b | Mammal | homeobox protein HOX-4 (4) | |
| miR-11 | Drosophila | E2F | |
| miR-13b2 | Drosophila | CG7033 | |
| miR-15b, −16-2 | Mammal | chromosome-associated polypeptide C | |
| miR-25, −93, −106b | Mammal | CDC47 homolog (13) | |
| miR-26a1, −26a2, −26b | Vertebrate | nuclear LIM interactor-interacting factor 1, 2, 3 | |
| miR-28 | Human | LIM domain-containing preferred translocation parterner in lipoma [NM005578] | |
| miR-30c1, −30e | Mammal | nuclear transcription factor Y subunit γ (5) | transcription factor HES-1; PAI-1 mRNA-binding protein |
| miR-33 | Vertebrate | sterol regulatory element binding protein-2 (15) | RNA-dependent helicase p68; NAG14 protein |
| miR-101b | Human | RNA 3′-terminal phospate cyclase-like protein (8) | |
| miR-103, −107 | Human | pantothenate kinase 1, 2, 3 | |
| miR-105-1, −105-2, −224 | Mammal | γ-aminobutyric-acid receptor α-3 subunit precursor, ɛ subunit precursor | |
| miR-126, −126* | Mammal | EGF-like, Notch4-like, NEU1 protein (6) [NM178444] | |
| miR-128b | Mammal | cAMP-regulated phospho-protein 21 (11) | |
| miR-139 | Mammal | cGMP-dependent 3′,5′-cyclic phosphodiesterase (2) | |
| miR-140 | Human | NEDD4-like ubiquitin-protein ligase WWP2 (15) | |
| miR-148b | Mammal | coatomer ζ-1 subunit | |
| miR-151 | Mammal | ||
| miR-152 | Human | coatomer ζ-2 subunit | N-myc proto-oncogene protein; noggin precursor |
| miR-153-1, −153-2 | Human | protein-tyrosine phosphatase N precursors | |
| miR-208 | Mammal | myosin heavy chain, cardiac muscle α isoform (28) | |
| miR-218-1, −218-2 | Human | Slit homolog proteins [NM003062] | |
As listed above, the function and mechanism of some of the most early found intronic miRNAs are currently under investigation.
represents the counterpart of the sense miR-126 sequence, and # denotes the GenBank accession number.
Fig. 1 shows a Pol-II-mediated concurrent synthesis of mRNA and intronic miRNAs. The conventional central dogma of molecular biology is the transcription of DNA to RNA to protein, which forms the backbone of molecular biology and is represented by four major stages. First, the DNA replicates its information in a process that involves many enzymes: replication, (not shown). Second, the DNA codes for the production of messenger RNA (mRNA) during transcription, also known as the RNA synthesis. In chromosomes, DNA acts as a template for the synthesis of RNA. In most mammalian cells, only 1% of the DNA sequence is copied into a functional mRNA. Third, the mRNA is processed and migrates from the nucleus to the cytoplasm in eukaryotes. Fourth, mRNA carries coded information to ribosomes, which ‘‘read’’ this information and use it for protein synthesis. The non-coding sequences are subtracted from the coding sequences of a gene in order to transcribe DNA into mRNA. The non-coding sequences located in introns are removed from the pre-mRNA by RNA splicing. The fragments of the spliced introns are transported out of the nucleus and processed as intronic miRNAs, which bind to the mRNA transcript and suppress the protein-coding gene expression based on partial complementarity.
Fig. 1.
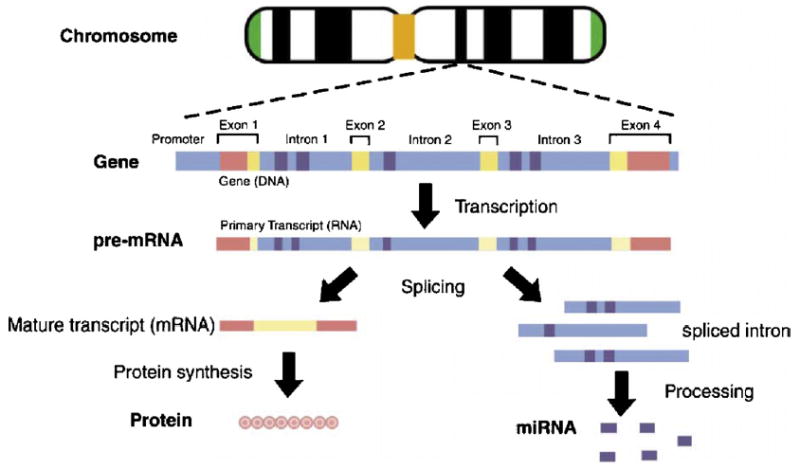
Schematic diagram of protein synthesis and intronic miRNA formation.
The man-made intronic miRNA system has been experimentally demonstrated in zebrafish. Previously, we have discovered that the biogenesis of miRNA-like precursors from the 5′-proximal intron regions of gene transcripts (i.e., pre-mRNAs) is mediated through the mammalian Pol-II. Based on this observation, a unique miRNA-inducing vector construct was developed [1]. Using man-made introns containing hairpin-like miRNA precursors (pre-miRNA), we were able to generate mature miRNA molecules. These miRNA molecules triggered specific gene silencing in several human cancerous cells and rat neuronal stem cells in vitro, as well as in chicken embryo and zebrafish in vivo [5]. The prerequisites for this type of miRNA generation are (a) the coupled interaction of nascent pre-mRNA transcription and intron excision, occurring within certain nuclear regions proximal to genomic perichromatin fibrils, (b) formation of mature miRNAs after Pol-II RNA splicing and excision processing, and (c) the exons of the encoding gene transcript are ligated together to form a mature mRNA for protein synthesis of the encoding gene.
In a nutshell, unique features of the intronic miRNA expression system are the following: First, they are compatible with Pol-II RNA transcription capable of tissue-specific and gene-specific targeted gene silencing. Second, they are compatible with drug-inducible promoters for developing Tet-On or Tet-Off cassettes. Third, the promoters and vectors as well as intronic pre-miRNA inserts are interchangeable. And, lastly, multiple pre-miRNA (cluster) insertion can be developed.
Research into the identification of miRNAs advanced exponentially during 2005, and the view that has emerged is that miRNA may be fine-tuning the minute events of biological development. The challenge now is to validate the function of the computer-searched miRNAs in the diverse biological systems so that the functional significance of miRNAs can be fully established. To this end, man-made miRNA expression systems are readily available tools for the elucidation of the vital function of these non-coding small RNAs in zebrafish.
References
- 1.Lin SL, Chang D, Wu DY, Ying SY. A novel RNA splicing-mediated gene silencing mechanism potential for genome evolution. Biochem Biophys Res Commun. 2003;310:754–760. doi: 10.1016/j.bbrc.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 2.Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP, Schier AF. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 3.Jin P, Alisch RS, Warren ST. RNA and microRNAs in fragile X mental retardation. Nat Cell Biol. 2004;6:1048–1053. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- 4.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SL, Ying SY. Asymmetry of intronic pre-miRNA structures in functional RISC assembly. Gene. 2005 doi: 10.1016/j.gene.2005.04.036. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz RH, Kauppinen S, Plasterk RH. MicroRNA expression in zebrafish embryonic development. Science. 2005 doi: 10.1126/science.1114519. doi:10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]


