Abstract
We report the results of x-ray reflectivity measurements of thin films formed by different water-soluble proteins at the air–aqueous solution interface. It is demonstrated that glucose oxidase, alcohol dehydrogenase, and urease molecules denaturate at the air–aqueous solution interface to form 8- to 14-Å-thick peptide sheets. X-ray reflectivity data indicate that the spreading of a lipid monolayer at the aqueous solution surface before protein injection does not prevent proteins from unfolding. On the other hand, crosslinking of proteins results in intact enzyme layers at the subphase surface. A model that involves interaction of glucose oxidase molecules with a phospholipid monolayer is proposed. In this model, an observed decrease of the lipid electron density in the protein presence is explained in terms of “holes” in the monolayer film caused by protein molecule adsorption.
Further development of the engineering of two-dimensional protein arrays is extremely important for their potential applications in micro-optics, microelectronics, and biotechnology, and in particular as biosensors. Among the most successful applications of biosensors are receptor surfaces for electro-optical devices (1). For example, bacteriorhodopsin arrays can be used for photosensors (2) and photomemories (3).
The design of biosensors whose main component is a thin protein film is one of the most challenging problems of modern biophysics and biochemistry (4–7). One of the most effective ways of immobilizing proteins in a two-dimensional matrix is the Langmuir–Blodgett (LB) technique (8–9). However, the LB technique requires the transfer of monolayers from the air–water interface onto a solid support, and the quality of the resulting LB film is in large part dependent on the quality of the precursor monolayer.
It is essential that enzymes used for biosensors be immobilized in a matrix to prevent their denaturation. The problem to be avoided is “surface denaturation” of water-soluble proteins, discovered more than 50 years ago (10–12). It has been inferred (10) that in most cases water-soluble proteins are so constructed that the hydrocarbon groups are buried in the interior, leaving the surface covered by polar groups. When a molecule with this structure reaches the surface, there is a strong tendency for the hydrocarbon parts of the protein molecule to cover the surface, and this is accomplished by unfolding the protein molecule to form a flat sheet. To prevent surface denaturation, a number of immobilization methods have been developed, for example, noncovalent adsorption onto the physical transducer (13), covalent linking (14–15), and polymer matrix immobilization (16–18).
In this paper we use a specular x-ray reflectivity to study the structural integrity of water-soluble enzymes of glucose oxidase (GOx), alcohol dehydrogenase, and urease monolayers at the air–aqueous interface.
Experimental Details
Glucose oxidase (type X-S, Sigma), urease (type VII from jack beans, Sigma), and alcohol dehydrogenase (from baker’s yeast, Sigma) were used without further purification. All other chemical reagents [dibehenoyl phosphatidylcholine (C22:0), glucose, Mes, glutaraldehyde, NaCl, and CdSO4 (all from Sigma)] were of the highest obtainable quality. In all experiments, Millipore purified water was used.
Glucose oxidase crosslinked with glutaraldehyde was prepared according to a protocol used by Sun et al. (19) for preparation of active Langmuir–Blodgett GOx films. This procedure involved solution of 40 mg of GOx in 2 ml of water and addition of 0.5 ml of 50% glutaraldehyde (10,000:1 mol ratio of glutaraldehyde to GOx), and then 1.5 ml of H2O. This solution was allowed to stand for about 5 min and then was diluted with methanol/H2O mixture to 10 ml, giving a solution of 4 mg/ml of GOx, 2.5% glutaraldehyde, and 20% methanol. After dilution, the solution was allowed to react for 24 h at room temperature. The solution of glucose oxidase, crosslinked with glutaraldehyde, was deposited on a glass rod contacting the liquid surface (20) in a fashion such that it became evenly distributed into a thin layer before contacting the meniscus of the liquid surface/rod interface.
The aqueous subphase developed by Yoshimura et al. for growing two-dimensional crystals of the ferritin (21) and apoferritin (22) proteins at the air–water interface was used as the subphase for spreading the urease films. This subphase, which consists of 2% glucose (wt/vol), 10 mM Mes, 150 mM NaCl, and 10 mM CdSO4 (pH 5.8) has a higher density and surface tension than does the protein solution. Three milliliters of aqueous urease solution (4.2 mg/ml) was deposited on a glass rod contacting the liquid surface (20). Immediately after deposition of the urease, the surface pressure rose to 25 mN/m, indicating adsorption of the protein at the interface. To prevent unfolding of the protein at the interface, glutaraldehyde [3 ml, 50% (wt/vol)] was injected underneath the surface shortly after the protein film was spread.
X-ray reflectivity (XR) experiments were carried out at beamline X19C of the National Synchrotron Light Source, Brookhaven National Laboratory (Upton, NY) by using a custom-built liquid surface diffractometer (23) with incident wavelength of λ = 1.54 Å. A sealed and thermostated Langmuir trough equipped with a Wilhelmy balance was supported on a vibration isolation stage on the diffractometer. Compression of the monolayer was achieved by moving a motorized Teflon ribbon, which confines the monolayer to one side of the trough. Both the trough and the ribbon assembly were enclosed in a temperature-controlled aluminum housing with a Kapton window.
The monochromatic x-ray beam was deflected toward the water surface by means of Bragg reflection from a Si (111) crystal. The x-rays were collimated with a set of four-jaw slits. These slits were always set to optimize the resolution in the scattering plane while increasing the signal by lowering the resolution out of the scattering plane. A beam monitor before the sample provided data for the normalization of the incident beam intensity. The scattered beam intensity was measured with a NaI scintillation detector. Calibrated attenuators between the sample and the detector were inserted and removed as necessary during the course of the XR scan to use only the linear regime of the detector. All x-ray scattering measurements were performed at 5°C.
X-Ray Scattering Measurements and Discussion
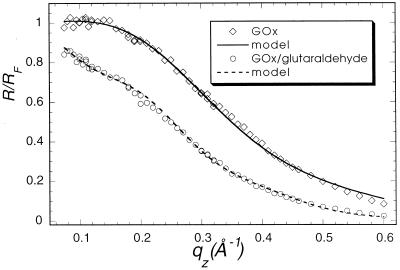
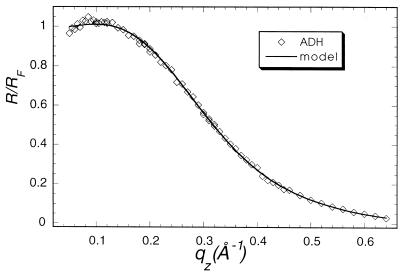
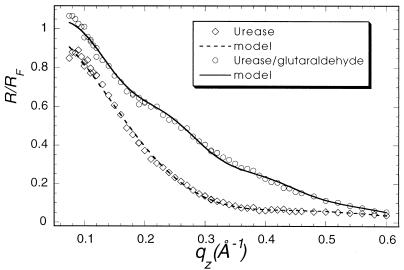
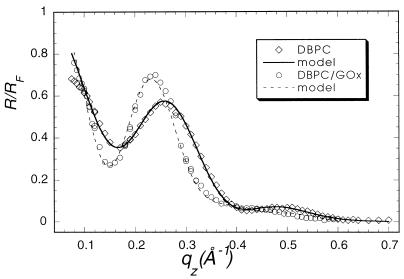
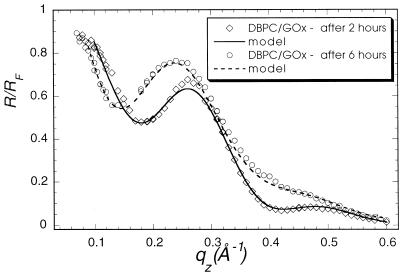
Specular x-ray reflection measurements record the intensity of those x-rays scattered from the air–water interface with momentum transfer strictly perpendicular to that interface. The structure factor associated with the XR profile is determined by the Fourier transform of the gradient of the electron density perpendicular to that interface. The data cannot, in general, be uniquely inverted to yield the electron density profile. As have many others (24), we have fit our XR data to a model of the interface consisting of a stack of uniform slabs, each with a different electron density ρi and thickness Li.. The effect of capillary waves on the density distribution in the interface is modeled by a single Gaussian roughness, σ, for all of the interfaces. The data accumulated at the highest x-ray count rates at angles close to the critical angle had a recorded intensity about 45% of the actual value because of incipient nonlinearity in the beamline electronics. Only the data for qz > 0.07 Å−1 were used in analysis. The absolute intensity scaling factor was one of the least square fitting parameters. X-ray reflectivity data from films of glucose oxidase (Fig. 1, squares), alcohol dehydrogenase (Fig. 2), and urease (Fig. 3, squares) spread on an aqueous solution surface (Fig. 1) were best fitted with a one-slab representation. All three protein monolayers appear to be 8- to 14-Å thick (Table 1), indicating the presence of unfolded peptide chains at the air–aqueous solution interface. One way of preventing proteins from unfolding at the air–water interface is to create artificially a hydrophilic boundary at that interface. Spreading of a Langmuir monolayer with polar headgroups onto the aqueous surface is one of the simplest ways to achieve this goal. Indeed, the uniform layer of the polar headgroups penetrating the water surface should prevent the hydrocarbon parts of the protein molecule from reaching the surface and then unfolding to a flat sheet. To examine this hypothesis, the following experiment was designed. A monolayer of dibehenoylphosphatidyl choline (DBPC) phospholipid was deposited on the PBS buffered (pH = 7.4) aqueous surface. After compression of the monolayer to Π = 35 mN/m, x-ray reflectivity measurements were carried out (Fig. 4, circles). Then an aqueous solution of glucose oxidase was injected underneath the monolayer, such that the concentration of GOx in the subphase reached 1 mg/ml. The surface tension dropped almost 25% immediately after the protein injection, indicating the adsorption of protein molecules at the interface. The monolayer was then recompressed and left for 2 hr to let the system reach equilibrium. X-ray reflectivity measurements of the DBPC/GOx system were then carried out under the same conditions as for the pure DBPC monolayer (Fig. 5, circles). Both sets of reflectivity data were best fitted with a two-slab model. The phosphatidyl choline headgroup of the pure DBPC monolayer was modeled (Fig. 4) with a 9.0-Å-thick slab of electron density ρ1/ρs = 1.07, whereas the hydrocarbon tails were represented by a 23-Å-thick slab with electron density ρ2/ρs = 0.87, indicating a 30° tilt from the normal to the aqueous surface. Surprisingly, the presence of glucose oxidase in the subphase did not alter much the structure of the interface layer. The best fit (Fig. 5) to the measurements yielded a 7.7-Å-thick slab with electron density ρ1/ρs = 1.09, which corresponds to the headgroup region of the monolayer, and a 23.1-Å-thick slab with electron density ρ2/ρs = 0.93, corresponding to the hydrocarbon chains. The results of this XR measurement did not suggest the presence of any glucose oxidase at the interface. The reflectivity measurements were repeated again 6 hr after the protein injection (Fig. 5, squares). The best fit was again represented by a two-slab model with the first slab of thickness 6.3 Å and electron density ρ1/ρs = 0.89, and the second slab of thickness 18.8 Å and electron density ρ1/ρs = 0.81. This result indicates that over the 4-hr period, the electron density of the interface layer decreased almost by 15%.
Figure 1.
Measured (points) and calculated (solid line) x-ray specular reflectivities from glucose oxidase protein. (squares) pure glucose oxidase; (circles) covalently crosslinked glucose oxidase.
Figure 2.
Measured (points) and calculated (solid line) x-ray specular reflectivities from pure alcohol dehydrogenase protein.
Figure 3.
Measured (points) and calculated (solid line) x-ray specular reflectivities from urease layer at the air–aqueous interface. (squares) Pure urease; (circles) in situ covalently crosslinked urease.
Table 1.
Fitted parameters for slab model of electron density corresponding to x-ray reflectivity curves shown in Figs. 1–5
| Number of slabs | ρ1/ρs | L1, Å | ρ2/ρs | L2, Å | σ | |
|---|---|---|---|---|---|---|
| Glucose oxidase | 1 | 1.05 | 13.6 | — | — | 2.5 |
| Glucose oxidase crosslinked | 1 | 0.97 | 27.0 | — | — | 3.3 |
| Urease | 1 | 0.75 | 8.8 | — | — | 2.9 |
| Urease crosslinked | 2 | 1.02 | 26.0 | 0.96 | 9.6 | 2.9 |
| Alcohol dehydrogenase | 1 | 1.06 | 13.3 | — | — | 2.9 |
| DBPC/buffer | 2 | 1.07 | 9.0 | 0.87 | 23.1 | 3.3 |
| DBPC/GOx (injected) after 2 hr | 2 | 1.09 | 7.7 | 0.93 | 23.1 | 3.4 |
| DBPC/GOx (injected) after 6 hr | 2 | 0.89 | 6.3 | 0.81 | 18.8 | 3.1 |
| DBPC spread over GOx | 2 | 1.1 | 15.0 | 0.88 | 24.4 | 3.6 |
ρs, electron density of the subphase; ρ1, ρ2 and L1, L2, electron densities and thicknesses of the first and second slabs, respectively; σ, surface roughness parameter.
Figure 4.
X-ray specular reflectivities from DBPC monolayer over pure phosphate buffer (circles) and over glucose oxidase film (squares).
Figure 5.
X-ray specular reflectivities from DBPC monolayer with glucose oxidase injected 2 hr before measurement (circles) and 6 hr before measurement (squares).
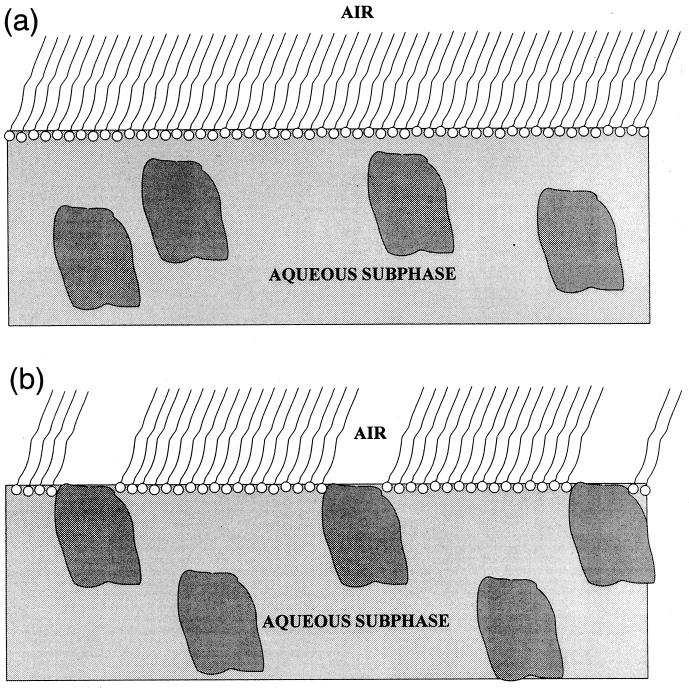
We propose the following model to explain our observation. The molecules of glucose oxidase do not bind to the phosphatidyl choline headgroups when they reach the interface region, but rather push them aside creating a network of “holes” in a DBPC layer (Fig. 6). In that case, the overall density of the illuminated DBPC region would decrease, and the tilt of the hydrocarbon chains would increase, as is observed. The electron density of water-soluble protein molecules is, in general, very close to that of water itself, so if the occupation of the surface sites by protein molecules is sporadic they would be practically “invisible” to the x-rays.
Figure 6.
The proposed arrangement of DBPC monolayer and glucose oxidase molecules at the air–aqueous interface (a) 2 hr after GOx injection and (b) 6 hr after GOx.
XR measurements (Fig. 4, squares) of a DBPC film spread on top of a glucose oxidase layer (as opposed to injection of GOx underneath the monolayer) were analyzed with a two-slab model with a 15-Å-thick “headgroup slab” (ρ1/ρs = 1.1) and a 24.5-Å-thick “tails slab.” The increase of the “headgroup slab” thickness from 9 Å to 15 Å (comparable with the DBPC monolayer over the pure buffer) is consistent with the presence of an unfolded peptide sheet at the interface region, as in the case of pure protein layers.
Another way to prevent the surface denaturation of water-soluble proteins is to crosslink them covalently to make their unfolding much more difficult. The crosslinked layers of glucose oxidase and urease at the air–aqueous interface were examined with x-ray reflectivity. Crosslinking of glucose oxidase by glutaraldehyde was carried out before the measurements, as described above. Crosslinked glucose oxidase was deposited onto the glass rod contacting the water surface (20). The reflectivity measurements (Fig. 1, circles) were analyzed with a one-slab model to yield a uniform protein layer of 27 ± 5 Å thickness (ρ/ρs = 0.97). To minimize the damage to protein molecules, the urease aqueous solution was also deposited with the glass rod contacting the subphase. For in situ crosslinking of the urease layer, 6 ml of 4 mg/ml was injected directly under the subphase surface. The system was left for 1.5 hr and then XR data were collected. The urease data were best fitted with a two-slab model (Fig. 3, circles), which yielded a 26 ± 5 Å-thick layer for the first slab (ρ1/ρs = 1.02) and a 9.6 ± 2 Å layer for the second slab (ρ1/ρs = 0.96), giving about 35 to 36 Å total thickness.
According to three-dimensional crystal structure data, the monomeric molecule of glucose oxidase is approximately 60 Å × 52 Å × 37 Å (25) (it forms a dimer in three-dimensional crystals). As has recently been determined by small-angle x-ray scattering in aqueous solution (26), the radius of gyration of urease is about 48 Å. These experimental data were successfully modeled (26) assuming that urease hexamers are composed of six cylindrical subunits of 28.7 Å radius and 44.4 Å height. Evidently, the thicknesses of both the glucose oxidase and the urease layers are close to the real protein dimensions in three-dimensional crystals. In any event, the XR data show that covalent crosslinking prevents (or partially prevents) the unfolding the protein molecules at the air–aqueous interface.
In conclusion, the structure of water-soluble protein layers at the air–water interface has been examined with x-ray reflectivity. It was demonstrated that glucose oxidase, alcohol dehydrogenase, and urease unfold at the aqueous solution surface to form flat peptide sheets. Our measurements showed no indication that the presence of the polar layer of the headgroups of a DBPC monolayer prevents glucose oxidase from unfolding. It was, however, observed that there is a time delay effect in adsorption of GOx molecules on the DBPC monolayer. Although there were no notable changes in the DBPC electron density profile 2 hr after GOx injection into the subphase, after 6 hr a significant decrease in the DBPC electron density was observed. The proposed model suggests that glucose oxidase molecules, when they adsorb onto a DBPC monolayer, push aside the phospholipid molecules to create “holes” in the monolayer. It has been shown that when crosslinked, glucose oxidase and urease molecules most likely remain intact at the interface.
Acknowledgments
We thank Paul A. Heiney for critically reviewing this paper. We are grateful to Robert S. Eisenberg for several fruitful discussions and for his insightful suggestions concerning protein crosslinking. This work has been supported by a grant from the National Science Foundation.
ABBREVIATIONS
- GOx
glucose oxidase
- XR
x-ray reflectivity
- DBPC
dibehenoylphosphatidyl choline
References
- 1.Stelzle M, Weissmuller G, Sackmann E. J Phys Chem. 1993;97:2974–2981. [Google Scholar]
- 2.Miyasaka T, Koyama K, Itoh I. Science. 1992;255:342–344. doi: 10.1126/science.255.5042.342. [DOI] [PubMed] [Google Scholar]
- 3.Oesterhelt D, Brauchle C, Hampp N. Q Rev Biophys. 1991;24:425–478. doi: 10.1017/s0033583500003863. [DOI] [PubMed] [Google Scholar]
- 4.Sackmann E. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 5.Nagayama K. Advances in Biophysics. Vol. 34. 1996. pp. 3–23. [DOI] [PubMed] [Google Scholar]
- 6.Nicolini C. Thin Solid Films. 1996;284–285:1–5. [Google Scholar]
- 7.Diederich A, Làsche M. In: Protein Array: An Alternate Biomolecular System. Nagayama K, editor. Vol. 34. Tokyo: Limerick: Japan Scientific Societies Press/Elsevier; 1997. pp. 205–230. [Google Scholar]
- 8.Langmuir I. Trans Faraday Soc. 1920;15:62–74. [Google Scholar]
- 9.Blodgett K B. J Am Chem Soc. 1935;57:1007–1022. [Google Scholar]
- 10.Adam N K. The Physics and Chemistry of Surfaces. London: Oxford Univ. Press; 1941. [Google Scholar]
- 11.Rothen A. Adv Protein Chem. 1947;3:123–128. doi: 10.1016/s0065-3233(08)60078-9. [DOI] [PubMed] [Google Scholar]
- 12.Cheesman D F, Davies J T. Adv Protein Chem. 1954;9:439. doi: 10.1016/s0065-3233(08)60211-9. [DOI] [PubMed] [Google Scholar]
- 13.Clarck L C, Lions C. Ann Acad Sci Fenn Ser A. 1962;102:29. [Google Scholar]
- 14.Guilbault G G, Montavlo J G. J Am Chem Soc. 1969;91:2164–2169. doi: 10.1021/ja01036a083. [DOI] [PubMed] [Google Scholar]
- 15.Tran-Minh C, Broun G. Anal Chem. 1975;45:1359–1364. doi: 10.1021/ac60358a075. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara H, Aizawa M, Shirakawa H. J Chem Soc Jpn. 1986;3:465. [Google Scholar]
- 17.Eremenko A, Kurochkin I, Chernov S, Barmin A, Yaroslavov A, Moskvitina T. Thin Solid Films. 1995;260:212. [Google Scholar]
- 18.Nicolae C A, Cantin-Riviere S, El Abed A, Peretti P. Langmuir. 1997;13:5507–5510. [Google Scholar]
- 19.Sun S, Ho-Si P-H, Harrison D J. Langmuir. 1991;7:727–737. [Google Scholar]
- 20.Trurnit H J. J Colloid Interface Sci. 1960;15:1. [Google Scholar]
- 21.Yoshimura H, Scheybani T, Baumeister W, Nagayama K. Langmuir. 1994;10:3290–3295. [Google Scholar]
- 22.Scheybani T, Yoshimura H, Baumeister W, Nagayama K. Langmuir. 1996;12:431–435. [Google Scholar]
- 23.Schlossman M L, Synal D, Guan Y M, Meron M, SheaMcCarthy G, Huang Z Q, Acero A, Williams S M, Rice S A, Viccaro P J. Rev Sci Instrum. 1997;68:4372–4384. [Google Scholar]
- 24.Kjaer K, Als-Nielsen J, Helm C A, Tippmankrayer P, Mohwald H. J Phys Chem. 1989;93:3200–3206. [Google Scholar]
- 25.Hecht H J, Kalisz H M, Hendle J, Schmid R D, Schomburg D. J Mol Biol. 1993;229:153–172. doi: 10.1006/jmbi.1993.1015. [DOI] [PubMed] [Google Scholar]
- 26.Hirai M, Kawai-Hirai R, Hirai T, Ueki T. Eur J Biochem. 1993;215:55–61. doi: 10.1111/j.1432-1033.1993.tb18006.x. [DOI] [PubMed] [Google Scholar]