Abstract
We have previously demonstrated that PM-Scl-75, a component of the human exosome complex involved in RNA maturation and mRNA decay, can specifically interact with RNAs containing an AU-rich instability element. Through the analysis of a series of deletion mutants, we have now shown that a 266 amino acid fragment representing the RNase PH domain is responsible for the sequence-specific binding to AU-rich elements. Furthermore, we found that the RNase PH domains from two other exosomal components, OIP2 and RRP41, as well as from Escherichia coli polynucleotide phosphorylase, are all capable of specifically interacting with RNAs containing an AU-rich element with similar affinities. Finally, we demonstrate that the interaction of the RNase PH domain of PM-Scl-75 is readily competed by poly(U), but only inefficiently using other homopolymeric RNAs. These data demonstrate that RNase PH domains in general have an affinity for U- and AU-rich sequences, and broaden the potential role in RNA biology of proteins containing these domains.
Keywords: AU-rich, mRNA decay, PM-Scl-75, exoribonuclease
INTRODUCTION
The eukaryotic exosome is a 250–700 kDa complex of nine or 10 3′-to-5′ exonucleases that is present in distinct forms in the nucleus, nucleolus, and cytoplasm (Butler 2002; Raijmakers et al. 2004; Graham et al. 2006). It is involved in the maturation of the 3′ end of numerous RNAs (Perumal and Reddy 2002), as well as in the degradation of both normal and defective mRNAs (LaCava et al. 2005; West et al. 2006). The exosomes of yeast, mammals, and trypanosomes contain six exonucleases related to the RNase PH family of phosphate-specific ribonucleases that are thought to be assembled in a hexameric ring structure at the core of the complex (Zuo and Deutscher 2001; Symmons et al. 2002). The conservation of these six core proteins is generally limited to their RNase PH domains, suggesting an essential role for this region. Why so many 3′-to-5′ exonucleases exist together in an evolutionarily conserved complex is not clear, although this may facilitate the coordinate regulation of their activity.
The exosome plays a significant role in the regulated degradation and quality assurance of mRNAs in yeast and mammalian cells (Butler 2002; Raijmakers et al. 2004). Assays using cytoplasmic extracts have demonstrated an ability of the exosome to participate in the regulated decay of RNA substrates containing an AU-rich element (ARE) (Mukherjee et al. 2002; Gherzi et al. 2004). AREs, which generally consist of one or more copies of an AUUUA element or related nonameric sequence (Chen and Shyu 1995), are found in the 3′ untranslated region (UTR) of many short-lived mRNAs. Significant regulation of mRNA stability occurs through these AREs, and numerous proteins that have been implicated in ARE-regulated mRNA decay, including tristetraprolin (TTP), Hu antigen R (HuR), AU-rich element binding factor (AUF1), and K homology splicing regulatory protein (KSRP), specifically interact with the element (Guhaniyogi and Brewer 2001; Gherzi et al. 2004).
RNase PH domains are ∼250 amino acid regions responsible for the phosphate-dependent 3′-to-5′ exonucleolytic degradation of RNAs (Zuo and Deutscher 2001). The prototype of the family, the bacterial RNase PH protein, essentially contains only the RNase PH domain. The enzyme plays a role in the maturation of the 3′ end of tRNAs that contain an encoded CCA motif—indicating a selectivity of RNA substrates acted upon by RNase PH (Wen et al. 2005). Furthermore, the presence of RNase PH impairs the ability of RNase R to degrade mRNAs in a polynucleotide phosphorylase (PNPase− Bacillus subtilis strain (Oussenko et al. 2005). This suggests that RNase PH may regulate other ribonucleases in bacteria, perhaps in a fashion not unlike the coordinate regulation of exonucleases suggested by the structure of the eukaryotic exosome.
We have previously identified an integral component of the exosome as an ARE-binding protein. PM-Scl-75, a major auto-antigen in patients with polymyositis-scleroderma overlap syndrome (Brouwer et al. 2001), was shown to specifically interact with RNA substrates containing well-characterized AREs from either the TNF-α or GM-CSF mRNAs (Mukherjee et al. 2002). This observation was rather unexpected, as PM-Scl-75 does not contain any domains known to possess sequence-specific RNA binding ability. It was important, therefore, to identify the domain of PM-Scl-75 responsible for the ability of the protein to interact with ARE sequences.
In this study we have used deletion mutagenesis to demonstrate that the RNase PH domain of PM-Scl-75 is both necessary and sufficient for sequence-specific binding to an ARE. Using competition analyses, we determined that the RNase PH domain also has a strong preference for U-rich sequences. Furthermore, we observed that three other RNase PH domains, including one from the Escherichia coli PNPase protein, also can interact in a specific fashion with ARE sequences. Collectively, these data demonstrate that the ability to bind RNA in a specific fashion is a conserved property of RNase PH domains, a property that should be taken into account when assessing the role of proteins that contain this conserved domain in RNA biology.
RESULTS AND DISCUSSION
The RNase PH domain of the PM-Scl-75 protein is responsible for its sequence-specific binding to RNA
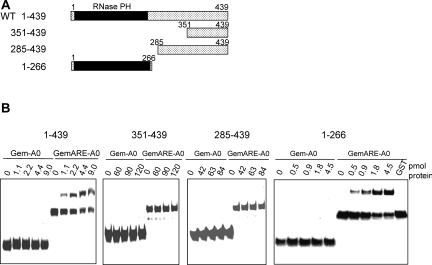
Previously, we have shown that PM-Scl-75 interacts specifically with the AU-rich elements found in both the TNF-α and GM-CSF mRNAs (Mukherjee et al. 2002). This finding was unexpected, as this exosome component does not have an identified RNA binding domain. In order to identify the domain(s) of PM-Scl-75 involved in ARE-specific binding, a series of proteins containing N-terminal and C-terminal deletions were prepared. Key constructs of this series are diagrammed in Figure 1A. Recombinant proteins were purified and incubated with either a polylinker-containing RNA backbone (Gem-A0), or a variant containing a 34 base insertion of the ARE from TNF-α (Gem-ARE-A0). RNA–protein complexes were visualized by gel-shift analyses. As expected, full-length PM-Scl-75 protein specifically formed a shifted complex with the Gem-ARE-A0 substrate (Fig. 1B, panel 1–439). Neither of the two deletion variants that contained only C-terminal fragments of PM-Scl-75 was capable of binding RNA (Fig. 1B, variants 351–439 and 285–439. These data suggest that the presence of the N-terminal region of PM-Scl-75 is required for ARE-specific binding. This region is comprised almost entirely of the RNase PH domain of the protein. In order to assess whether the RNase PH domain was indeed capable of binding to ARE-containing RNAs, we prepared a 266 amino acid fusion protein that represents the intact PM-Scl-75 RNase PH domain plus several flanking amino acids at both termini. As seen in Figure 1B (panel 1–266), this isolated RNase PH domain specifically interacted with RNAs that contain the TNF-α AU-rich element. Collectively from these data and our earlier results, we conclude that the RNase PH domain of PM-Scl-75 can specifically interact with ARE-containing transcripts.
FIGURE 1.
The RNA binding domain of PM-Scl-75 maps to the RNase PH domain. (A) Recombinant wild-type (WT) PM-Scl-75 and three deletion variants were generated as depicted. (B) Increasing amounts of recombinant PM-Scl-75 protein and each of the three variants were incubated with 5 fmol of matched RNA substrates that either contained an AU-rich element (Gem-ARE-A0) or lacked the 34 base insert (Gem-A0). A total of 20 pmol of GST were used as a negative control. RNA–protein complexes were detected by gel-shift analysis on 5% native acrylamide gels.
The RNase PH domain of PM-Scl-75 specifically binds AU-rich sequences
We next investigated the specificity of the interaction with the AU-rich element in more detail. For these experiments, we used a GST-fusion protein bearing just the RNase PH domain, of PM-Scl-75 (amino acids 31–257). We incubated increasing amounts of the fusion protein with either Gem-A0, Gem-ARE-A0, or a variant of Gem-ARE-A0 in which all the AUUUA pentamer motifs in the 34 base AU-rich element had been mutated. The reactions were then separated on a native gel to visualize binding as before. The results of this experiment (Fig. 2A) show that while the PH domain of PM-Scl-75 bound very efficiently to an RNA substrate that contained an AU-rich element (Gem-ARE-A0), the domain displayed negligible binding to RNA substrates that lacked an ARE (Gem-A0) or contained a highly mutated variant (Gem-AREmt-A0). We therefore conclude that the RNase PH domain specifically recognizes AU-rich sequences.
FIGURE 2.
(A) The PM-Scl-75 PH domain is specific for AU-rich sequences. Increasing amounts of recombinant protein were incubated with 5 pmol of Gem-A0, Gem-ARE-A0, or Gem-AREmt-A0 as indicated. RNA–protein complexes were detected by gel-shift analysis on 5% native gels. (B) Poly(U), but not poly(A) or poly(C), is an effective competitor of PM-Scl-75 sequence-specific RNA interaction. The indicated amount of poly(U), poly(C), or poly(A) was added as a competitor to reaction mixtures containing 6 pmol of PM-Scl-75 protein and 5 pmol of Gem-ARE-A0 RNA substrate. RNA–protein complexes were detected by gel-shift analysis on 5% native acrylamide gels.
The RNase PH domain of PM-Scl-75 also shows affinity for poly(U) sequences
We wished to determine whether the RNase PH domain of PM-Scl-75 would show affinity for other RNA sequences. To address this, we assessed the ability of homopolymeric RNAs to serve as competitors for the binding of 6 pmol of the RNase PH domain of PM-Scl-75 to 5 pmol of the Gem-ARE transcript. As seen in Figure 2B, poly(U) competed very effectively for PM-Scl-75 binding. As little as 5 ng of poly(U) was sufficient to compete away PM-Scl-75/Gem-ARE-A0 RNA–protein complexes. Poly(C) and poly(A), on the other hand, were very inefficient competitors with >500 ng required to abrogate binding of PM-Scl-75 to Gem-ARE-A0 RNA. This represents >100× more competitor than is required by poly(U) to compete for binding. Since U-rich tracts in the 3′ UTRs of mRNAs are often associated with AREs and mRNA instability, this preference for poly(U) may reflect an additional way for the exosome to identify and access RNAs destined for 3′-to-5′ exonuclease trimming or degradation.
The conserved RNase PH domains of other exosomal subunits and bacterial PNPase also bind to ARE-containing RNAs
Finally, we wished to determine whether binding to RNA in a sequence-specific fashion was restricted to the RNase PH domain of PM-Scl-75 or could be generalized to other members of the family. As diagrammed in Figure 3A, we prepared a series of GST-fusion proteins containing RNase PH domains from the human exosomal components PM-Scl-75, OIP2, and RRP41, as well as one containing the N-terminal RNase PH domain from E. coli PNPase protein. The purity and integrity of these fusion proteins was demonstrated by the Coomassie-stained SDS-acrylamide gel shown in Figure 3B.
FIGURE 3.
The RNase PH domains of OIP2, RRP41, and PNPase all possess sequence-specific RNA binding ability. A schematic representation of three of the RNase PH domain–containing components of the eukaryotic exosome (OIP2, PM-Scl-75, and RRP41) as well as PNPase from E. coli is shown in A. (B) The RNase PH domain–containing regions (black boxes in A) were prepared as N-terminal GST-fusion proteins, run on a 10% acrylamide gel, and stained with Coomassie blue to assess purity. The molecular weight ladder is indicated at the left of the gel. (C) The indicated pmol amounts of recombinant proteins containing only the RNase PH domain of PM-Scl-75, OIP2, RRP41, or N-terminal RNase PH domain of E. coli PNPase were incubated with 5 fmol of Gem-ARE-A0 RNA or a matched control (Gem-A0) transcript. GST (18 pmol) acted as a negative control. RNA–protein complexes were detected by gel-shift analysis on 5% native acrylamide gels. Kd plots are shown for each protein.
Increasing amounts of these four fusion proteins or GST-alone were incubated with either Gem-ARE-A0 RNA or Gem-A0 RNA, and protein–RNA complexes were analyzed by gel-shift analysis. As seen in Figure 3C, all four RNase PH domain-containing proteins were able to specifically interact in a similar fashion with ARE-containing transcripts. Only extremely low levels of complex were detected using the control Gem-A0 RNA (Fig. 2A; data not shown), and these complexes only occurred at exceptionally high concentrations of recombinant protein in the mixtures. This is likely due to the nonspecific affinity for RNA by RNase PH domains that has been previously inferred by others from structural analysis of archaeal exosome complexes (Buttner et al. 2005; Lorentzen et al. 2005). The affinity for the ARE of each of these RNase PH-containing proteins was also very similar. The dissociation constants (Kd) for the binding of the RNase PH domains to ARE-containing transcript were determined as described in Materials and Methods. The Kds were 5.5 × 10−7 M for PM-Scl-75, 3.0 × 10−7 M for OIP2, 8.4 × 10−7 M for RRP41, and 3.1 × 10−7 M for PNPase. Based on these observations, we conclude that ARE-specific binding is a general property of the RNase PH domain for all of the proteins that we have studied.
The data from this study demonstrate that a variety of RNase PH domains have the ability to interact in a specific fashion with AU- and U-rich RNA sequences. This reveals a novel property for this conserved domain in addition to its known association with exonuclease activity. The Kd of the RNase PH domain for its RNA targets is in the 100 nM range, suggesting a moderate affinity for its substrates, similar to that of the abundant RNA binding protein hnRNP A1 (Abdul-Manan and Williams 1996). Typical ARE-binding factors such as AUF1 and TTP have a Kd in the 10–30 nM range (DeMaria and Brewer 1996; Cao 2004). Many RNase PH domain–containing proteins can be found in multifactor complexes. Coupled with other RNA–protein interactions between the complex and the RNA substrate, the affinity of the RNase PH domain is likely strong enough to make a contribution to binding and substrate selection.
This versatile role for the RNase PH domain, as both an exonucleolytic fold and a sequence-specific RNA binding motif, may explain why the domain is so well conserved throughout evolution. This duality of function of the RNase PH domain may also provide an explanation for why a presumed exonuclease domain is often present in the nonintuitive arrangement of multiple copies in proteins like PNPase or in complexes such as the exosome. To date, we have not identified an RNase PH domain that lacks the ability to interact specifically with AU-rich sequences.
There are numerous biological ramifications of the identification of sequence-specific interactions of the RNase PH domain of human exosomal proteins. First, several exosome components may assist in targeting mRNAs that contain AU-rich or U-rich instability elements for degradation (Wilusz and Wilusz 2004). These types of elements are the most common and best described of the 3′UTR sequences that regulate mRNA decay (Chen and Shyu 1995). Direct interaction of the exosome with elements in the 3′UTR may be sufficient to enhance 3′-5′ decay simply by positioning the complex close to the site of action. Second, RNase PH domain–RNA interactions may help select small RNA precursor transcripts for maturation through 3′ end trimming (Perumal and Reddy 2002). Third, in addition to RNA structures, RNase PH domain–RNA interactions may assist in stalling the exosome at the proper, predetermined sites on the RNA substrate during small RNA maturation. Finally, since many transcripts made by RNA polymerase III contain short U-tracts at their 3′ ends (Perumal and Reddy 2002), it is tempting to speculate that this may serve as a binding site for the exosome through its RNase PH domains. This would allow the exosome to rapidly target and degrade these transcripts when the native secondary and tertiary structures are disturbed. This would facilitate quick removal of misfolded—and potentially deleterious—small RNAs.
Rho-independent terminators of prokaryotic genes usually contain a stem–loop structure followed by a stretch of uridylates (de Hoon et al. 2005). The observation that at least one of the RNase PH domains of PNPase has affinity for U-rich stretches (i.e., the TNF-α ARE) may suggest a rationale for why the polymerase would choose to terminate these transcripts with a U-rich stretch. Perhaps, as for the eukaryotic exosome noted above, PNPase may effectively load onto the terminal uridylates through its RNase PH domain for turnover of these transcripts. Furthermore, the sequence-specific binding property of the RNase PH domain family of 3′-to-5′ exonucleases may also help to explain the observed coevolution of AU-rich 3′ trailer sequences in tRNAs with RNase E cleavage sites (Li et al. 2005). Finally, the propensity for RNase E to cleave at AU-rich sequences may provide an excellent substrate for attack by PNPase due to the ability of the RNase PH domain to bind to these sequences.
The structure of two archaeal exosomal complexes—isoforms of a 240-kDa complex from Archaeoglobus fulgidus and an RNase PH component Rrp41–Rrp42 core complex from Sulfolobus solfataricus—were recently reported (Buttner et al. 2005; Lorentzen et al. 2005). The Rrp41–Rrp 42 core complex was shown to bind RNA using short A5 and U8 oligomers through both ribose-specific and phosphate backbone interactions (Lorentzen et al. 2005). These observations are not inconsistent with the RNA binding results reported here, as longer RNAs may be able to interact in a similar fashion with isolated subunits. For the larger A. fulgidus complex, RNA binding analysis focused on the S1 pore side, which contains the Csl4 and Rrp4-like subunits that possess S1 and KH domains. Binding to the wider PH pore side of the complex could not be ruled out. Furthermore, stoichiometry of exosomal subunits varies in archaeal species (Evguenieva-Hackenberg et al. 2003; Farhoud et al. 2005) and the existence of numerous independent complexes cannot be excluded. In fact, a recent immunolocalization study highlighted the possible existence of numerous subexosomal complexes in Drosophila cells (Graham et al. 2006).
In summary, many targets and substrates for RNA degradation contain AU- or U-rich sequences near their 3′ ends. The observation that the conserved RNase PH domain found in a major family of 3′-to-5′ exoribonucleases can recognize these sequences suggests an excellent way to load the nucleases onto these substrates and promote and/or regulate their degradation. Important future questions include the identification of residues important for sequence-specific recognition in conjunction with modeling of the RNA binding site. The ability to functionally separate RNA binding from exonucleolytic activity would afford us a way to dissect the full function of this novel characteristic in RNA metabolism.
MATERIALS AND METHODS
Plasmids
pGem4 (Promega), with or without the 34-base AU-rich region from TNF-α (5′-ATTATTTATTATTTATTTATTATTTATTTATTTA) was used as a template for the synthesis of RNA. The AU-rich region was ligated into the PstI and HindIII sites of pGem4, forming pGem-ARE-A0. The pGem-AREmt-A0 plasmid was similar except the insert sequence was 5′-GTTAGTATTCATTTGTTTACTATTGATTTCTTTA-3′. pGem4 without the insertion was termed pGem-A0. The pGem plasmids were linearized with HindIII to form templates to use for in vitro transcriptions.
pGEX2TZQ was used for the expression of GST-fusion proteins (Qian and Wilusz 1994). RNase PH-domains of PM-Scl-75 (primers: 5′-CATGGTACCTTGTATAGACACTGAGTCTCTC, 5′-CATGGATTCCTCTCCAATTTGGGCAGTTCC), RRP41 (primers: 5′CGAGGGATCCGAGCTGCGCAAGATCCAGGCG, 5′ACCGAATTCAGCCTGGGCAGCAGCCTCCAAC), and OIP2 (primers: 5′-ACGGTACCTGAATTCAGAACC, 5′-CACTCGAGGTGTCTTGTAACT) were amplified using PCR from plasmids containing the full-length genes or by RT-PCR from total RNA. The RNase PH-domain of PNPase (primers: 5′-CATGGTACCTGGCCAACACACCGTGACTCTG, 5′-CATGAATTCTTGTTCATGACCGAACACTAC) was amplified from genomic DNA of DH5α E. coli. Amplified fragments of DNA were digested with EcoRI and KpnI, or XhoI and KpnI for OIP2, and ligated into pGEX2TZQ.
pTrcHisA (Invitrogen) was used for the expression of His-tagged fusion proteins. The full-length PM-Scl-75 open reading frame was amplified from PM-Scl-75c construct (Raijmakers et al. 2003) using the primers 5′-CCGAGCCTGCAGATGAAGGAAACGCCACTCTCA and 5′-GACCGGTACCTTAATTGGCAGCTCTCCTCTT. Select domains of the protein were amplified from the plasmid as follows: Primers to amplify the C-terminal amino acids 351–439 were 5′-AATAAACTGCAGAGACTCTGAGAAGGAAGATGATGAA and 5′-AATGCTTCGAATTCCCATATGGTAC; primers to amplify the C-terminal amino acids 285–439 were 5′-AATAAACTGCAGAGAGTCTATAGCAAATCAAAGGATC and 5′-AAGCTTCGAATTCCCATATGGTAC. Amplified products were then digested using KpnI and PstI and inserted into the KpnI and PstI sites of pTrcHisA.
In vitro transcriptions
In vitro transcribed RNAs were prepared using SP6 RNA polymerase (Fermentas) and internally labeled with [α32P]UTP. One microgram of linearized template in RNase-free water was incubated with 500 μM cap analog (7MeGpppG), rNTP mix (500 μM ATP, 500 μM CTP, 50 μM GTP, 50 μM UTP), 45 μCi [α32P]UTP (800 Ci/mmol), 20 units of RNase inhibitor (Fisher Biotech), 20 units of SP6 RNA polymerase (Fermentas), and transcription buffer (40 mM Tris-HCl at pH 7.6, 6 mM MgCl2, 2 mM spermidine, 10 mM DTT) for 1 h at 37°C in a total volume of 10 μL. The reaction was stopped by the addition of 90 μL of RNase-free ddH2O and 100 μL of phenol:cholorform:isoamyl alcohol (25:24:1). After ethanol precipitation in 2 M ammonium acetate, the transcripts were centrifuged and the pellets washed with 70% ethanol. The pellets were resuspended in RNA loading buffer (7 M urea, 25 mM Tris at pH 7.6, 1 mM EDTA at pH 8.0, 0.05% bromophenol blue, 0.05% xylene cyanol), denatured at 90°C for 30 sec, and loaded onto a 5% polyacrylamide denaturing gel with 7 M urea.
Following electrophoresis, transcripts were visualized by autoradiography, and the appropriate bands (61-nt for GEM-A0 and 95-nt for GEM-ARE-A0) were excised and incubated in 400 μL of HSCB (0.4 M NaCl, 26 mM Tris at pH 7.6, 0.1% SDS) with 40 μg of proteinase K (Sigma) at 25°C overnight. Eluted RNAs were extracted with an equal volume of phenol:chloroform:isopropyl alcohol (25:24:1), ethanol precipitated, and centrifuged. The pellet was washed with 70% ethanol and resuspended in RNase-free ddH2O. To calculate the yield of the transcripts, 1 μl of sample was counted in a scintillation counter.
Expression and purification of recombinant proteins
GST and GST-tagged PM-Scl-75, RRP41, OIP2, and PNPase RNase PH domains were expressed in BL21(DE3) E. coli cells and purified from cell lysates using glutathione agarose beads (Sigma). One liter of bacterial culture was grown at 30°C to log phase (OD600 = 0.8) and then induced for 3 h with 0.5 mM IPTG (isopropyl β-D-thiogalactopyranoside). Induced cells were harvested by centrifugation at 10,000g for 10 min, then resuspended in 10 mL of lysis buffer (50 mM HEPES at pH 7.9, 150 mM KCl, 1 mM MgCl2, 1% Triton X-100, 0.1 mM PMSF, 2 μg/mL leupeptin). The resuspended cells were lysed after the addition of 8 mg of lysozyme (Sigma) by sonication on ice. The lysate was cleared by centrifugation at 11,000g for 20 min, and the soluble protein fraction was incubated with 200 μL of glutathione agarose beads at 4°C with rocking for 1 h. The beads were washed 10 times with 3 bed volumes of lysis buffer, then the GST-tagged proteins were eluted with 1 bed volume of 50 mM reduced glutathione in lysis buffer. The eluant fractions were dialyzed in lysis buffer for 2 h, brought up to 20% glycerol (v/v), aliquoted, and stored at −80°C.
His-tagged proteins were purified from lysates of E. coli HB101 transformed cells using His-bind resin (Novagen) according to the manufacturer's recommendations. Eluant fractions were collected, dialyzed, adjusted to 20% glycerol (v/v) and stored at −80°C. Protein concentration and purity was verified by separation on a 15% polyacrylamide gel followed by staining with Coomassie blue (Fig. 3B).
RNA gel-shift assay
Gel-shift assays were performed by incubating 5 fmol of 32P-labeled RNA with recombinant proteins in the presence of 20 units of RNase inhibitor, 0.15 mM spermidine, 20 mM HEPES (pH 7.9), 8% glycerol, 100 mM KCl, and 2 mM MgCl2 for 5 min at 30°C in a total volume of 12.5 μL. Low molecular weight heparin (Sigma H3400) was added to a final concentration of 3 μg/μL, and the reactions incubated on ice for an additional 5 min. Five microliters of loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol, 30% glycerol) were added to the reactions, and the samples were electrophoresed on a 5% native polyacrylamide gel at constant voltage at 4°C. Following electrophoresis, the gel was dried and bands were visualized by phosphorimaging.
Dissociation constants (Kd) were calculated by quantifying the amount of bound GEM-ARE-A0 and dividing it by the amount of free GEM-ARE-A0 using Quantity One software (Bio-Rad). This was plotted against the micromolar concentration of the specific protein, and the slope of the subsequent line was determined. The Kd was 1/slope. Only values within the linear range were used. Nonspecific binding was estimated to be 10–20-fold less than specific binding, as judged by binding to Gem-A0 (Fig. 2A; data not shown). We therefore considered this to be negligible and did not take it into account in the calculations.
For gel-shift assays that included RNA competitions, 1 μL of the appropriate concentrated RNA competitor in water was added to the reaction prior to the 5 min incubation at 30°C.
ACKNOWLEDGMENTS
We thank R. Raijmakers and G. Pruijn for supplying the full-length PM-Scl-75 clone. This work was supported by NIH grant GM 072481 to J.W.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.144606.
REFERENCES
- Abdul-Manan, N., Williams, K.R. hnRNP A1 binds promiscuously to oligoribonucleotides: Utilization of random and homo-oligonucleotides to discriminate sequence from base-specific binding. Nucleic Acids Res. 1996;24:4063–4070. doi: 10.1093/nar/24.20.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, R., Pruijn, G.J., van Venrooij, W.J. The human exosome: An autoantigenic complex of exoribonucleases in myositis and scleroderma. Arthritis Res. 2001;3:102–106. doi: 10.1186/ar147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, J.S. The yin and yang of the exosome. Trends Cell Biol. 2002;12:90–96. doi: 10.1016/s0962-8924(01)02225-5. [DOI] [PubMed] [Google Scholar]
- Buttner, K., Wenig, K., Hopfner, K.P. Structural framework for the mechanism of archaeal exosomes in RNA processing. Mol. Cell. 2005;20:461–471. doi: 10.1016/j.molcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Cao, H. Expression, purification, and biochemical characterization of the antiinflammatory tristetraprolin: A zinc-dependent mRNA binding protein affected by posttranslational modifications. Biochemistry. 2004;43:13724–13738. doi: 10.1021/bi049014y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.Y., Shyu, A.B. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- de Hoon, M.J., Makita, Y., Nakai, K., Miyano, S. Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput. Biol. 2005;1 doi: 10.1371/journal.pcbi.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria, C.T., Brewer, G. AUF1 binding affinity to A+U-rich elements correlates with rapid mRNA degradation. J. Biol. Chem. 1996;271:12179–12184. doi: 10.1074/jbc.271.21.12179. [DOI] [PubMed] [Google Scholar]
- Evguenieva-Hackenberg, E., Walter, P., Hochleitner, E., Lottspeich, F., Klug, G. An exosome-like complex in Sulfolobus solfataricus . EMBO Rep. 2003;4:889–893. doi: 10.1038/sj.embor.embor929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhoud, M.H., Wessels, H.J., Steenbakkers, P.J., Mattijssen, S., Wevers, R.A., van Engelen, B.G., Jetten, M.S., Smeitink, J.A., van den Heuvel, L.P., Keltjens, J.T. Protein complexes in the archaeon Methanothermobacter thermautotrophicus analyzed by blue native/SDS-PAGE and mass spectrometry. Mol. Cell. Proteomics. 2005;4:1653–1663. doi: 10.1074/mcp.M500171-MCP200. [DOI] [PubMed] [Google Scholar]
- Gherzi, R., Lee, K.Y., Briata, P., Wegmuller, D., Moroni, C., Karin, M., Chen, C.Y. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol. Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Graham, A.C., Kiss, D.L., Andrulis, E.D. Differential distribution of exosome subunits at the nuclear lamina and in cytoplasmic foci. Mol. Biol. Cell. 2006;17:1399–1409. doi: 10.1091/mbc.E05-08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhaniyogi, J., Brewer, G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- LaCava, J., Houseley, J., Saveanu, C., Petfalski, E., Thompson, E., Jacquier, A., Tollervey, D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–724. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Li, Z., Gong, X., Joshi, V.H., Li, M. Co-evolution of tRNA 3′ trailer sequences with 3′ processing enzymes in bacteria. RNA. 2005;11:567–577. doi: 10.1261/rna.7287505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentzen, E., Walter, P., Fribourg, S., Evguenieva-Hackenberg, E., Klug, G., Conti, E. The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat. Struct. Mol. Biol. 2005;12:575–581. doi: 10.1038/nsmb952. [DOI] [PubMed] [Google Scholar]
- Mukherjee, D., Gao, M., O'Connor, J.P., Raijmakers, R., Pruijn, G., Lutz, C.S., Wilusz, J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussenko, I.A., Abe, T., Ujiie, H., Muto, A., Bechhofer, D.H. Participation of 3′-to-5′ exoribonucleases in the turnover of Bacillus subtilis mRNA. J. Bacteriol. 2005;187:2758–2767. doi: 10.1128/JB.187.8.2758-2767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal, K., Reddy, R. The 3′ end formation in small RNAs. Gene Expr. 2002;10:59–78. [PMC free article] [PubMed] [Google Scholar]
- Qian, Z., Wilusz, J. GRSF-1: A poly(A)+ mRNA binding protein which interacts with a conserved G-rich element. Nucleic Acids Res. 1994;22:2334–2343. doi: 10.1093/nar/22.12.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raijmakers, R., Egberts, W.V., van Venrooij, W.J., Pruijn, G.J. The association of the human PM/Scl-75 autoantigen with the exosome is dependent on a newly identified N terminus. J. Biol. Chem. 2003;278:30698–30704. doi: 10.1074/jbc.M302488200. [DOI] [PubMed] [Google Scholar]
- Raijmakers, R., Schilders, G., Pruijn, G.J. The exosome, a molecular machine for controlled RNA degradation in both nucleus and cytoplasm. Eur. J. Cell Biol. 2004;83:175–183. doi: 10.1078/0171-9335-00385. [DOI] [PubMed] [Google Scholar]
- Symmons, M.F., Williams, M.G., Luisi, B.F., Jones, G.H., Carpousis, A.J. Running rings around RNA: A superfamily of phosphate-dependent RNases. Trends Biochem. Sci. 2002;27:11–18. doi: 10.1016/s0968-0004(01)01999-5. [DOI] [PubMed] [Google Scholar]
- Wen, T., Oussenko, I.A., Pellegrini, O., Bechhofer, D.H., Condon, C. Ribonuclease PH plays a major role in the exonucleolytic maturation of CCA-containing tRNA precursors in Bacillus subtilis . Nucleic Acids Res. 2005;33:3636–3643. doi: 10.1093/nar/gki675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, S., Gromak, N., Norbury, C.J., Proudfoot, N.J. Adenylation and exosome-mediated degradation of cotranscriptionally cleaved pre-messenger RNA in human cells. Mol. Cell. 2006;21:437–443. doi: 10.1016/j.molcel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Wilusz, C.J., Wilusz, J. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 2004;20:491–497. doi: 10.1016/j.tig.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Zuo, Y., Deutscher, M.P. Exoribonuclease superfamilies: Structural analysis and phylogenetic distribution. Nucleic Acids Res. 2001;29:1017–1026. doi: 10.1093/nar/29.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]