Abstract
Nuclear hormone receptors have been shown to repress transcription in the absence of ligand. This repression is mediated by a corepressor complex that contains the Sin3A protein and histone deacetylases (HDAC1 and 2). Studies by several groups demonstrate that this complex is recruited to nuclear receptors through the highly related corepressors SMRT (silencing mediator of retinoid acid and thyroid hormone receptor) and N-CoR (nuclear receptor corepressor). We describe here the cloning, characterization, and chromosomal mapping of forms of human and mouse SMRT that includes a 1,000-aa extension, which reveals striking homology to the amino terminus of N-CoR. Structure and function studies of wild-type and natural splicing variants suggest the presence of 3–4 amino terminal domains that repress in a cooperative as well as mechanistically distinct fashion.
Steroid nuclear hormone receptors contribute to physiologic homeostasis by functioning as both repressors and activators of transcription (reviewed in ref. 1). In the absence of hormone, a DNA-bound receptor mediates repression by recruiting putative corepressor proteins (2, 3). When isolated, the nuclear receptor corepressors SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) and N-CoR (nuclear receptor corepressor) were found to be large “platform proteins” with approximately 40% identity (4, 6). Both proteins directly bind to the nonliganded ligand binding domain of the thyroid hormone receptor (TR), retinoic acid receptor (RAR), and other nuclear receptors via two discrete and independent receptor interaction domains (4–9). Several lines of evidence demonstrate both the mechanism and importance of repression in hormone action and disease (reviewed in ref. 10). Mutations in the TR that result in constitutive repression give rise to erythroleukemia in chickens (11–13). The oncogenic activity of this TR mutant, v-erbA, was shown to depend on its ability to bind DNA and repress transcription (14). A single amino acid substitution in the ligand binding domain, Pro-160–Arg, abolishes the ability of v-erbA to transform cells, repress basal transcription, and to interact with SMRT (15). Similarily, mutations in the human RAR that increase repression give rise to acute promyelocytic leukemia (16–18). Additionally, microinjection of neutralizing antibodies targeting N-CoR and SMRT (19, 20) or coexpression of dominant negative forms of these corepressors result in the loss of transcriptional repression by unliganded receptors (5).
Several studies have provided clues to the mechanism by which these factors may affect transcription. Both SMRT and N-CoR have been shown to form a large protein complex (19, 21–24) that includes histone deacetylase I (HDAC1) (25, 26) and Sin3A (27, 28). It is hypothesized that the ability of this complex to deacetylate histones results in an altered chromatin state that is inhibitory to transcription (29, 30). This mechanism of transcriptional repression also is used by the Myc/Mad family of basic helix–loop–helix proteins (27, 28), and work in yeast has shown it to be evolutionarily conserved (25, 26, 31). Recently it was demonstrated that the recruitment of this complex to the protein product of the promyelocytic leukemia zinc finger (PLZF)-retinoic acid receptor (RAR) translocation results from the direct binding of SMRT to PLZF, as well as RAR (16). This finding may provide an explanation to the poor response of these patients to retinoic acid therapy (32). Additionally, the SMRT/N-CoR proteins have been shown to regulate the Notch signaling pathway through interaction with the transcription factor CBF/RBP-Jk (33). These findings predict that the SMRT/N-CoR class of corepressors will be found to play a role in regulating a variety of transcriptional systems.
Although SMRT functions as an intrinsic corepressor, the size discrepancy with N-CoR suggested the existence of a longer isoform (4). A systematic screen resulted in the isolation of mouse and human homologues of 2,473 and 2,517 aa, respectively. We designate the longer form as SMRT, and the earlier shorter version as s-SMRT. Functional analysis reveals multiple repressor domains in the amino terminal extension, as well as blocks of striking homology and divergence to N-CoR. Surprisingly, a small (208 aa) and naturally occurring splice variant displays dramatically altered repressor function, suggesting the potential for intramolecular cooperativity and cross-talk.
MATERIALS AND METHODS
cDNA Cloning.
An examination of the s-SMRT sequence reveals that the first eight amino acids and upstream sequences are derived from a portion of ribonucleoprotein K sequence. Accordingly, a mouse spleen cDNA lamba ZAP II library (Stratagene) was screened at low stringency with a probe corresponding to approximately the 5′ 1,000 bp of the previously identified human s-SMRT. A 3.5-kb cDNA fragment was obtained that contained unique sequence in addition to existing SMRT sequence. The 5′ end of this, and subsequent clones, was used in successive rounds of screening of the spleen and mouse brain cDNA libraries (Stratagene) to obtain the full-length cDNA of SMRTα and SMRTβ isoforms. The mouse SMRT (m-SMRT) 5′ sequence was used at low stringency to screen a human pituitary cDNA library (Stratagene) to obtain the full-length human SMRT (h-SMRT) cDNA. All cDNA clones were sequenced on both strands by using standard methods.
Plasmids, Cell Culture, and Transfections.
The plasmids pCMX-GAL4 DBD (21) and pMH100-TK-luc (21) have been described. Standard PCR amplifications were used to generate the GAL4 fusion constructs. All constructs were verified by double-stranded sequencing to confirm identity and reading frame. Detailed information is available on request. Monkey CV-1 cells were grown in DMEM supplemented with 10% resin-charcoal stripped FBS, 50 units/ml of penicillin G, and 50 μg/ml of streptomycin sulfate at 37°C in 7% CO2. CV-1 cells (60–70% confluence, 48-well plate) were cotransfected with 16 ng of pCMX-GAL4, 100 ng of pMH100-TK-luc, and 100 ng of pCMX-β-galactosidase in 200 μl of DMEM containing 10% super stripped FCS by the N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP)-mediated procedure (21). The amount of DNA in each transfection was kept constant by addition of pCMX. After 24 hr, the medium was replaced and cells were harvested and assayed for luciferase activity 36–48 hr after transfection. The luciferase activity was normalized by the level of β-galactosidase activity. Each transfection was performed in triplicate and repeated at least three times.
Northern Blot Analysis.
Total RNA was prepared from adult CB6F1 mouse tissues by using TRIZOL reagent (GIBCO/BRL). Poly(A) RNA was purified from total RNA by using an Oligotex mRNA Kit (Qiagen, Valencia, CA). RNA was separated on 1.25% agarose-6% formaldehyde gel and transferred to a NYTRAN membrane (Scheicher & Schuell). The probes used were a 720-bp m-SMRT/PstI fragment. The filters subsequently were stripped and rehybridized with a murine glyceraldehyde-3-phosphate dehydrogenase cDNA probe for RNA loading normalization.
Chromosomal Localization.
To determine the chromosomal localization of SMRT, the 5.3-kb cDNA clone was used for fluorescence in situ hybridization. The probe was labeled by nick-translation with biotin-11-dUTP and hybridized to normal male human metaphase chromosomes. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Chromosome identification was carried out by computer inversion of the gray scale DAPI image on a PSI Imaging System (Perceptive Scientific Instruments, League City, TX). Chromosome 12 confirmation was carried out by using a chromosome 12-specific alpha satellite probe (Vysis, Downers Grove, IL).
RESULTS
Sequence Analysis of SMRT.
Longer forms of SMRT were isolated by systematically screening a variety of mouse and human cDNA libraries with successive 5′ proximal probes. By sequentially shifting between mouse spleen and brain cDNA libraries, several clones containing a potential starting methionine and 5′ untranslated region sequences were obtained. These clones then were used to pull out the long form of h-SMRT from a pituitary cDNA library. The complete protein sequences and alignments of m-SMRT and h-SMRT are presented in Fig. 1. In the mouse sequence, amino acids 36–254 are underlined to highlight the region that is deleted from a splice variant isolated from the brain cDNA library. These two forms of SMRT are designated SMRTα and SMRTβ and are discussed further below. An arrow marks the first amino acid residue from s-SMRT. As illustrated by the sequence comparison in Fig. 1, there is a high degree of sequence conservation (84% identity) between m-SMRT and h-SMRT.
Figure 1.
Alignment of h-SMRT and m-SMRT sequences. Proteins were aligned by using the clustal alignment program. The underlined region of m-SMRT corresponds to the region deleted from the β isoform (see text). The arrow indicates the start point of the human s-SMRT identified previously. The nucleotide sequences are available on request and have been submitted to GenBank (accession nos. h-SMRT: AF113003; m-SMRTα: AF113001; m-SMRTβ: AF113002).
SMRT and N-CoR Homology Domains.
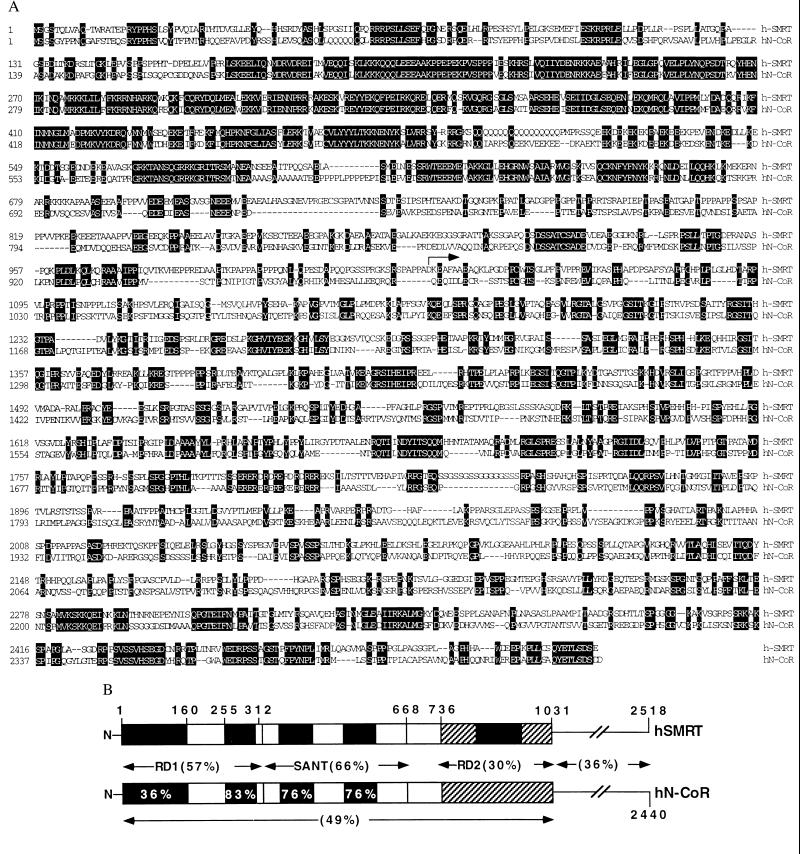
A direct sequence comparison between h-SMRT and N-CoR reveals the N-terminal extension (1–1031) harbors striking regions of homology that identify potentially new functional domains. These domains and their alignments are shown in Fig. 2 A and B. The first 160 aa of N-CoR are not well conserved in SMRT, with a similarity in this region of 36%. As this area has been shown to interact with Siah2 in N-CoR (34), it will be interesting to determine whether Siah2 also interacts with SMRT. If this interaction is maintained, there are two small regions of identity that would be candidates for the interaction site. A 52-aa segment from N-CoR (255–312 amino acids) has been demonstrated to mediate an interaction with Sin3A (19) and was presumed to represent the core of the larger repression domain (RD) 1 region (6). This small interaction domain is highly conserved (83%), with an overall identity between SMRT and RD1 of 57%. An adjacent stretch of amino acids from 312 to 668 is also well conserved (66%) with two internal sequence blocks of even higher similarity. These blocks are both homologous to each other and to part of the SANT domain that was identified in the yeast chromatin remodeling factor SWI3, the yeast adapter protein ADA2, the basal transcription factor TFIIIB, and other proteins (reviewed in ref. 35). The high percent sequence identity of the SANT domains between SMRT and N-CoR (76%) suggests a common, albeit undetermined, function for this region. Finally, the amino acids described in N-CoR as RD2 are the least conserved (30%) (6). The sequence similarity between the N-CoR and SMRT N-terminal region is approximately 41%, and the overall percent identity between the full-length proteins is 36%.
Figure 2.
Alignment of h-SMRT and human N-CoR (hN-Cor). (A) The protein sequences were aligned by using the clustal alignment program. Identical residues are white with a black background. The arrow indicates the start of s-SMRT. (B) The diagram highlights the regions of homology between the SMRT N-terminal 1,031 aa and the corresponding N-CoR sequence (GenBank accession no. AF044209). The amino acids 1–160 are colored black to indicate this region as the Siah2 interacting domain. Amino acids 255–312 represent the Sin3A interaction domain mapped in N-CoR. Within the SANT region (amino acids 312–668), the actual SANT repeats are highlighted in black. The black-shaded region in the RD2 homology region represents the further defined repression core between amino acids 845 and 986. Percent similarities were calculated for each region by using the blastp program with a bosum62 matrix.
Tissue Distribution and Chromosomal Location of SMRT.
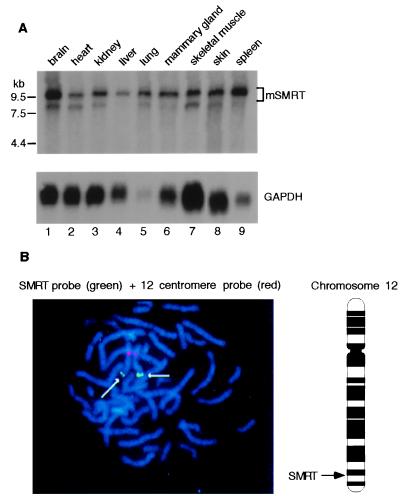
The expression pattern of s-SMRT has been examined and suggested to be ubiquitous. To verify this pattern by using full-length m-SMRT, we repeated the Northern analysis by using a probe consisting of bp 2760–3620 from m-SMRT. The results in Fig. 3A indicate that the expression pattern is indeed ubiquitous, although higher levels are detected in lung, spleen, and brain. We have confirmed these levels of expression for h-SMRT by using a multiple tissue blot from CLONTECH (not shown). It is noteworthy that two isoforms of SMRT are present in the majority of the mouse tissues and may correspond to the α and β isoforms.
Figure 3.
Tissue distribution and chromosomal localization of SMRT. (A) Poly(A) RNA derived from 20 μg of total RNA from each indicated tissue was subjected to Northern blot analysis. The filter was hybridized with 32P-labeled m-SMRT/PstI fragment (720 bp) (Upper). The relative position of RNA marker (kb) is labeled at the left. The filter was stripped and subsequently reprobed with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA for the purpose of loading control (Lower). A 10-kb major transcript was detected in all tissues examined, with relatively high expression in brain, lung, and spleen (lanes 1, 5, and 9). A minor transcript of 8.5 kb in size was present in most tissues. (B) Fluorescence in situ hybridization analysis of h-SMRT is shown with a SMRT-specific probe (green) and a chromosome 12-specific alpha satellite probe that hybridizes to the pericentromeric region (red). The arrows indicate the localization of the SMRT clone at band 12q24. The schematic diagram of chromosome 12 highlights the relative position of SMRT on the chromosome as being close to the 12q24 band. A more defined location is available from genemap 98 (see text).
As a step to establishing genetic evidence for the SMRT/N-CoR function, we have mapped the chromosomal location of h-SMRT and N-CoR. The SMRT clone hybridized to the q arm of one of the C group chromosomes. Computer-mediated banding of the 4′,6-diamidino-2-phenylindole-stained chromosomes identified the labeled chromosome as chromosome 12, band q24. The chromosome 12 localization was confirmed by cohybridization of SMRT and a chromosome 12 alpha satellite probe (D12Z3) (Vysis), which labels the pericentromeric region of chromosome 12 (Fig. 3B). The location for human N-CoR was determined through a mapped human bacterial artificial chromosome clone, hCIT529I10, that is 158 kb of genomic N-CoR and resides on chromosome 11p11.2. Both SMRT and N-CoR chromosomal locations can be accessed through genemap98 from the Human Genome Project (http://www.ncbi.nlm.nih.gov/genemap).
SMRT N Terminus Contains Multiple Repression Domains.
The high degree of identity between the SMRT N terminus and the corresponding N-CoR region suggests functional conservation in repression. To investigate this possibility, a nested series of mammalian expression vectors was generated with each testing segment fused to the GAL4 DNA binding domain (GAL-DBD) (see Fig. 4A). These constructs were cotransfected with a GAL4-TK-luciferase reporter to determine the regulatory properties of the GAL4-SMRT fusions. Repression was determined relative to the basal activity of the reporter in the presence of the GAL-DBD alone. The greatest repression (38-fold) was achieved with the entire region [GAL4-SMRT(1–1031)], which virtually extinguishes reporter activity. In terms of isolated domains, GAL4-SMRT(1–303), equivalent to RD1, demonstrated 6-fold repression (Fig. 4 A and B). GAL4-SMRT(736–1031), the region equivalent to RD2, retains modest repressor activity (2.6-fold) despite the rather low homology between SMRT and N-CoR in this region (Fig. 4 A and B). Surprisingly, the well-conserved SANT domain shows significant repression (3.3-fold).
Figure 4.
Characterization of SMRT amino terminus. (A) The schematic illustrates the various GAL4-SMRT fusions constructed, their corresponding amino acid numbers, and repressing ability. The patterns of colored and striped regions are described in the Fig. 2B legend. The GAL4-DBD is depicted as a gray oval. The fold repression represents the ability of the GAL-4-SMRT constructs to repress the activity of a GAL4-TK-luc reporter in cotransfection experiments and is relative to GAL-DBD alone. (B) Representative data comparing the repressing abilities of various regions of the m-SMRT N terminus. The constructs are shown in A. Amino acids 1–301 correspond to RD1, 427–663 to the SANT region, 736-1031 to RD2, and 1–1031 to the full m-SMRT N terminus. (C) Representative data narrowing down the RD2 region to a 150-aa core from 845 to 986. (D) Representative data demonstrating the differing repression abilities of the two m-SMRT isoforms. GAL4-SMRTβ (1–85) corresponds to RD1 with the deletion shown in Figs. 1 and 4A. GAL4-SMRTβ (1–813) corresponds to the full N terminus of m-SMRT with the β isoform deletion.
Within the RD2 homology region, a smaller segment between amino acids 845 and 986 shows increased conservation. Several constructs were generated to determine whether this minimal region was sufficient for the repression activity. Deletion of flanking amino acids 736–845 or downstream amino acids 987-1055 did not affect repression (Fig. 4 A and C), suggesting that the repressor function is contained within a 141-aa core of RD2.
Two SMRT Isoforms Identified in Mouse.
Two variant cDNA clones were identified in the screening for the N-terminal region of the m-SMRT, which potentially represent products of an alternative splicing event. These large clones span from the 5′ untranslated regions, to regions present in s-SMRT. The longer clone, designated SMRTα, refers to full-length m-SMRT. The shorter clone is designated SMRTβ and harbors a deletion of amino acids 36–254 (see underlined sequence in Fig. 1). Based on sequence similarity to N-CoR, this deletion removes the majority of RD1, including a portion of the Sin3A binding region. The effect of this deletion on SMRT function was assessed by cotransfection experiments comparing repression by SMRTα to SMRTβ. As expected, SMRTβ shows a severely impaired repressor activity (see Fig. 4 A and D), suggesting that alternative splicing may add further diversity to expand the function of SMRT gene products.
DISCUSSION
These studies present the isolation of forms of h-SMRT and m-SMRT that contain an approximately 1,000-aa extension to the previously characterized s-SMRT product. The results, although not formally ruling out the presence of isoforms of SMRT equivalent to s-SMRT, indicate that proteins of approximately 2,500 aa likely represent the major forms of SMRT found in vivo. It also should be noted that another form of SMRT, the TRAC-1 clone (8), is also a fusion with DNA from the human multiple endocrine neoplasia type 1 locus. This work provides direct sequence comparison with N-CoR in this region and reveals striking regions of conservation and divergence. The most well-conserved region is the 57-aa segment comprising the Sin3A interaction domain, which would represent the third such characterized region in SMRT (21). Interestingly these regions share little homology to each other, suggesting they most likely bind to different surfaces of the Sin3A protein and thus may be subject to different regulatory influences. The second best conserved region spans the SANT repeats, and surprisingly, harbors repressor function. As SANT domains participate in a variety of regulatory roles, it is not yet possible to predict how this repressor activity is mediated. However, their structural distinctness suggests it may contribute to repression in a novel way. Further we show that, like h-SMRT, m-SMRT exhibits a ubiquitous tissue distribution, suggesting it may contribute to a universally exploited repression pathway.
The region of SMRT corresponding to N-CoR RD1 has the most potent repressor activity, which is presumably caused by the presence of the Sin3A interaction domain. However, an analysis of sequence identity suggests the extreme amino terminus also is conserved and therefore may possess an independent function. Recently it has been demonstrated that SAP-30, a Sin3A-associated protein, and Siah2 also bind within RD1 (34, 36). Despite the plentitude of interactions, the isolated RD1 region is only a modest repressor relative to the larger NH2-terminal extension. This finding suggests repression is a multivarient phenomena emerging out of a collective of independent associations. The cloning of the full-length SMRT allows for testing to explore the potential shared activity of these domains and their associated factors. It is possible that the strong repression activity of the combined domains depends on their linear order in the protein or from the grouping of the domains in the N terminus. The presence of the multiple interaction sites for Sin3A, and their correlation with several of the mapped repression domains, suggests that a strict linear order may not be necessary for potent repression. It may, however, be important for each domain to be placed in the correct context within the protein to provide for the correct structural framework enabling multiple and simultaneous protein–protein interactions. The naturally occurring alternative splicing isoform (SMRTβ) loses 90% of the repressor activity of the NH2 terminus, providing a dramatic example of cooperativity as well as providing a means to control the action of SMRT gene products. It will be important to assess the consequences of this deletion on the repression activity of the intact full-length protein.
Among the recently identified functional regions of SMRT, the RD2 domain bears the lowest homology to the equivalent region in N-CoR. Also, how RD2 contributes to repression is not known but may be mechanistically different than RD1 or the other repressor regions in SMRT and N-CoR because it lacks an apparent Sin3A interaction domain. In any case, its deletion results in marked reduction of repression, re-enforcing the importance of cooperativity in this process. Interestingly, it recently has been reported that components of the basal transcription machinery (e.g., TFIIB, TAF 70/32) may directly contact corepressors, adding another layer of complexity to the problem of repression (37).
To conclude, we have reported here the identification of mouse and human forms of SMRT that contain the full-length sequence for this protein. Perhaps the most challenging and important part of future studies will be to understand the molecular diversity of pathways by which SMRT and N-CoR repress target genes, why so many pathways are needed, and the mechanisms by which each of these functions are integrated to achieve transcriptional silencing.
Acknowledgments
We thank Richard Lin and David Egan for comments on the manuscript and Elaine Stevens for administrative assistance. We acknowledge Henry Juguilon for help in tissue culture and Julie Malchiodi for technical services. P.O. is a fellow of the Hewitt Foundation for Medical Research. M.D. is a C. J. Martin Research fellow from the National Health and Medical Research Council of Australia. R.M.E. is the March of Dimes Chair of Molecular and Developmental Biology at the Salk Institute for Biological Sciences and an Investigator of the Howard Hughes Medical Institute. This work was supported by the Howard Hughes Medical Institute (R.M.E.) and National Institutes of Health grants (R.M.E. and N.B.S.).
ABBREVIATIONS
- SMRT
silencing mediator of retinoid acid and thyroid hormone receptor
- s-SMRT
short version of SMRT
- h-SMRT
human SMRT
- m-SMRT
mouse SMRT
- N-CoR
nuclear receptor corepressor
- TR
thyroid hormone receptor
- RD
repression domain
Footnotes
References
- 1.Mangelsdorf D J, Evans R M. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 2.Casanova J, Helmer E, Selmi-Ruby S, Qi J S, Au-Fliegner M, Desai-Yanjik V, Koudinova J, Yarm F, Raaka B M, Samuels H H. Mol Cell Biol. 1994;14:5756–5765. doi: 10.1128/mcb.14.9.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baniahmad A, Leng X, Burris T P, Tsai S Y, Tsai M J, O’Malley B W. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J D, Evans R M. Nature (London) 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen D J, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1996;93:7567–7571. doi: 10.1073/pnas.93.15.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamai Y, Soderstrom M, Glass C K, Rosenfeld M G. Nature (London) 1995;377:397–403. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 7.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld M G, Glass C K. Nature (London) 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 8.Sande S, Privalsky M. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- 9.Lee J W, Choi H S, Gyuris J, Brent R, Moore D D. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 10.Torchia J, Glass C K, Rosenfeld M G. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 11.Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. Nature (London) 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger C, Thompson C C, Ong E S, Lebo R, Gruol D J, Evans R M. Nature (London) 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 13.Graf T, Beug H. Cell. 1983;34:7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- 14.Damm K, Beug H, Graf T, Vennstrom B. EMBO J. 1987;6:375–382. doi: 10.1002/j.1460-2075.1987.tb04765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damm K, Evans R M. Proc Natl Acad Sci USA. 1993;90:10668–10672. doi: 10.1073/pnas.90.22.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin R J, Nagy L, Inoue S, Shao W, Miller W H, Jr, Evans R M. Nature (London) 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 17.Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthhardt M, Ferrara F F, Zamir I, et al. Nature (London) 1998;391:815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 18.Hong S H, David G, Wong C W, Dejean A, Privalsky M L. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C, Torchia J, Yang W M, Brard G, Ngo S, Davie J, et al. Nature (London) 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 20.Lavinsky R M, Jepsen K, Heinzel T, Torchia J, Mullen T M, Schiff R, Del-Rio A L, Ricote M, Ngo S, Gemsch J, et al. Proc Natl Acad Sci USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy L, Kao H Y, Chakravarti D, Lin R, Hassig C, Ayer D, Schreiber S, Evans R M. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 22.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Nature (London) 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 23.Laherty C D, Yang W M, Sun J M, Davie J R, Seto E, Eisenman R N. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 25.Taunton J, Hassig C, Schreiber S. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 26.Yang W M, Inouye C, Zeng Y, Bearss D, Seto E. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayer D E, Lawrence Q A, Eisenman R N. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber-Agus N, Chin L, Chen K, Torres R, Rao G, Guida P, Skoultchi A I, DePinho R A. Cell. 1995;80:777–786. doi: 10.1016/0092-8674(95)90356-9. [DOI] [PubMed] [Google Scholar]
- 29.Grunstein M. Nature (London) 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 30.Wolffe A P, Wong J, Pruss D. Genes Dev. 1997;2:291–302. doi: 10.1046/j.1365-2443.1997.1260323.x. [DOI] [PubMed] [Google Scholar]
- 31.Sternberg P W, Stern M J, Clark I, Herskowitz I. Cell. 1987;48:567–577. doi: 10.1016/0092-8674(87)90235-2. [DOI] [PubMed] [Google Scholar]
- 32.Miller W H, Jr, Jakubowski A, Tong W P, Miller V A, Rigas J R, Benedetti F, Gill G M, Truglia J A, Ulm E, Shirley M, et al. Blood. 1995;85:3021–3027. [PubMed] [Google Scholar]
- 33.Kao H Y, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner C R, Evans R M, Kadesch T. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Guenther M G, Carthew R W, Lazar M A. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aasland R, Stewart A F, Gibson T. Trends Biochem Sci. 1996;21:87–88. [PubMed] [Google Scholar]
- 36.Laherty C D, Billin A N, Lavinsky R M, Yochum G S, Bush A C, Sun J M, Mullen T M, Davie J R, Rose D W, Glass C K, et al. Mol Cell. 1998;2:33–42. doi: 10.1016/s1097-2765(00)80111-2. [DOI] [PubMed] [Google Scholar]
- 37.Muscat G E O, Burke L J, Downes M. Nucleic Acids Res. 1998;26:2899–2907. doi: 10.1093/nar/26.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]