Abstract
Cartilage is an avascular and relatively tumor-resistant tissue. Work from a number of laboratories, including our own, has demonstrated that cartilage is an enriched source of endogenous inhibitors of angiogenesis. In the course of a study designed to identify novel cartilage-derived inhibitors of new capillary growth, we have purified an inhibitory protein that was identified by peptide microsequencing and protein database analysis as troponin I (TnI). TnI is a subunit of the troponin complex (troponin-C and troponin-T being the other two), which, along with tropomyosin, is responsible for the calcium-dependent regulation of striated muscle contraction; independently, TnI is capable of inhibiting actomyosin ATPase. Because troponin has never previously been reported to be present in cartilage, we have cloned and expressed the cDNA of human cartilage TnI, purified this protein to apparent homogeneity, and demonstrated that it is a potent and specific inhibitor of angiogenesis in vivo and in vitro, as well as of tumor metastasis in vivo.
Keywords: endothelial cells, neovascularization, metastasis inhibitor
An accumulating body of scientific evidence now shows that the process of new capillary formation, or angiogenesis, is an essential component of a number of serious pathologies including solid tumor growth and metastasis, diabetic retinopathy, rheumatoid arthritis, and many others (1). Given the potential therapeutic benefit that an angiogenesis inhibitor could have in the treatment of these diseases, much of the research attention in this field is now focused on the discovery of suppressors of neovascularization.
A wide variety of experimental strategies have been employed in the course of angiostatic drug discovery. Our approach, and that of other groups, has focused on the study of avascular tissues such as cartilage as enriched sources of inhibitors of angiogenesis (2–8). We have now purified a protein from cartilage that is a potent antiangiogenic molecule and have identified it as being troponin I (TnI).
MATERIALS AND METHODS
Purification of TnI from Cartilage.
Tn I was purified from veal scapulae by using a modification of a protocol previously described (7). Briefly, veal scapulae were vacuum-frozen immediately after slaughter and stored at −20°C until used. Cartilage was scraped first with a periosteal elevator (Arista, New York) and then with a scalpel blade (No. 10, Bard-Parker) until clean of all muscle and connective tissue. Cartilage slices were extracted in 2 M NaCl, precipitated with HCl and ammonium sulfate (25–20%), and fractionated by using a series of chromatography steps: gel filtration on A-1.5 M Sepharose (Bio-Rad) in the presence of 4 M guanidine⋅HCl, ion exchange on a Bio-Rex 70 (Bio-Rad) cation exchange column, gel filtration on a Sephadex G-75 (superfine) (Pharmacia) column, reversed-phase HPLC on a Hi-Pore 304 column (Bio-Rad), and gel filtration on a Progel-TSK G3000SWXL column (30 cm × 7.8 mm) (Supelco). Fractions obtained from each column step were tested for their ability to inhibit capillary endothelial cell (EC) proliferation as described below. Fractions containing inhibitory activity were pooled and concentrated in a Savant SpeedVac concentrator for amino acid and sequence analysis. Unless otherwise stated, all reagents were obtained from Sigma.
Trypsin Digestion, HPLC Separation, and Microsequencing.
Proteins were reduced, S-carboxyamidomethylated, and subjected to digestion with trypsin. The resulting peptide mixtures were fractionated by narrow-bore HPLC by using a Zorbax C18 1.0 mm × 150 mm reverse-phase column on a Hewlett-Packard 1090 HPLC with a 1040 diode array detector. Optimum fractions were chosen based on differential UV absorbance at 205, 277, and 292 nm, peak symmetry, and resolution (9). These fractions were then further screened for length and homogeneity by matrix-assisted laser desorption time-of-flight (MALDI-TOF) MS on a Thermo Bioanalysis Lasermat 2000 (Hemel, England). Tryptic peptide sequences were determined by using electrospray ionization/tandem MS on a Finnigan TSQ7000 (San Jose, CA) triple quadrupole mass spectrometer as described in Nash et al. (10). Alternatively, peptides were submitted to automated Edman degradation on a PE/ABD 477A (PerkinElmer) protein sequencer.
Cloning and Expression of Human TnI.
Human intercostal cartilage tissue was obtained according to the bioethical guidelines of our institutions pertaining to discarded clinical material. The cDNA encoding a fragment of human fast-twitch skeletal muscle TnI was amplified by using standard reverse transcription–PCR (RT-PCR) from the total RNA isolated from a core sample of human cartilage by using primers based on the nucleotide sequence of human fast-twitch skeletal muscle TnI (11): forward primer 5′-GCTCTGCAAACAGCTGCACGCCAAG-3′ and reverse primer 5′-GCCCAGCAGGGCCTTGAGCATGGCA-3′, which was cloned into PCR2.1 (Invitrogen) and sequenced in both directions. The cDNA encoding the full-length ORF of human fast-twitch skeletal muscle TnI was cloned from human skeletal muscle mRNA with Pfu polymerase (Stratagene) under standard PCR conditions, using forward primer (5′-CTCACCATGGGAGATGAGGAGAAGC-3′) and the reverse primer (5′-GCCTCGAGTGGCCTAGGACTCGGAC-3′). The PCR product was cloned into the expression vector Pet 24d (Novagen) by using 5′-NcoI and 3′-XhoI sites and sequenced as above.
Tissue expression of TnI was analyzed by RT-PCR as described above. Total RNA (400 ng per sample) was isolated from rat skeletal muscle, liver (Clontech), xyphoid, and Swarm rat chondrosarcoma. The design of the forward (5′-GAACACTGCCCGCCTCTGCACATC-3′) and reverse (5′-GAGCCCAGCAGCGCCTTCAGCATG-3′) primers was based on the nucleotide sequence of rat fast-twitch skeletal muscle TnI. Recombinant human TnI was expressed according to standard protocols (12).
Purification of Recombinant TnI.
The washed pellet was dissolved in 6 M urea, 0.5 M NaCl, 5 mM Hepes, 2 mM EDTA, and 5 mM DTT (pH 7.5), and nutated in the above buffer for 6–8 hours at 4°C. The sample was then dialyzed against 0.5 M NaCl, 5 mM Hepes, and 5 mM DTT (pH 7.5) and concentrated by using Amicon concentrator (YM-10, molecular weight cutoff 10,000) before application to a Progel-TSK G3000SWXL column (30 cm × 7.8 mm). The sample was eluted by using the above buffer (0.5 M NaCl, 5 mM Hepes, 5 mM DTT, pH 7.5). Some of the inhibitory preparations were further fractionated on a Q-Sepharose HP column (Amersham Pharmacia) and tested as described below with no difference in biological activity. Purified rTnI was dialyzed against PBS containing 0.5 mM DTT prior to testing. Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad).
Western Blot Analysis.
Immunoblotting was conducted on samples of native TnI (purified from cartilage as described above), recombinant TnI (purified as described above), and bovine chondrocyte lysates prepared as described below according to standard protocols. Cultures of primary bovine scapular chondrocytes were established and maintained as described (8). Cells were rinsed with PBS, and to each 10-cm culture dish was added 1 ml of boiling 2× electrophoresis sample buffer (250 mM Tris⋅HCl, pH 6.8, 4% SDS, 10% glycerol, 0.006% bromophenol blue, and 2% 2-mercaptoethanol). Cells were scraped from the dishes by using a disposable cell scraper (Costar), transferred to a microcentrifuge tube, and boiled for an additional 5 min. After several passages through a 26 gauge needle (Becton Dickinson), the sample was clarified by centrifugation (2,000 × g), diluted to 0.1% SDS, and the protein concentration was determined by using a DC protein assay (Bio-Rad). All samples were separated by PAGE on a 4/12% acrylamide mini-gel according to Laemmli (13). Proteins were then transferred to nitrocellulose (Hybond-ECL, Amersham Pharmacia) by using a Transblot apparatus (Bio-Rad), incubated with a monoclonal antibody to rabbit skeletal muscle TnI (Advanced Immunochemical, Long Beach, CA), and developed by using the enhanced chemiluminescence (ECL) Western blotting system according to the manufacturer’s protocol (Amersham Pharmacia).
Cell Culture.
Capillary EC, isolated from bovine adrenal cortex (14), were the generous gift of J. Folkman and K. Butterfield (Children’s Hospital, Boston). Cells were maintained in culture in DMEM (GIBCO) with 10% fetal calf serum (HyClone) (DME/10) supplemented with 3 ng/ml basic fibroblast growth factor (bFGF) in preparation for these assays.
BALB/c mouse 3T3 cells were maintained in DME/10, l-glutamine (292 μg/ml) as described (15). Bovine aortic smooth muscle cells, isolated by explant from the medial layer of bovine aortas, were the kind gift of P. D’Amore and S. Smith (Children’s Hospital). These cells were cultured in DME/10 on uncoated tissue culture plastic as described (16).
Capillary EC Proliferation.
Briefly, capillary EC (2,000 cells per well) were plated on gelatinized 96-well culture plates in DMEM supplemented with 5% (vol/vol) calf serum and incubated for 24 hours. On day 2, cells were treated with bFGF (Scios Nova; 1 ng/ml) and challenged with the test fractions and/or with purified TnI. For experiments in which vascular endothelial growth factor (VEGF) was used as the mitogen, 800 cells per well were plated and allowed to incubate for 3 hours before VEGF (Biomedical Technologies Incorporated; 30 ng/ml) and TnI were added. Control wells contain cells alone and cells stimulated with bFGF or VEGF. On day 5, growth medium was removed from the plates, cells were lysed in buffer containing Triton X-100 and the phosphatase substrate p-nitrophenyl phosphate. After incubation for 2 hours at 37°C, NaOH was added to terminate the reaction. Color development was determined by using a rapid multiwell plate reader (Dynatech MR 5000) (7, 8, 17). EC inhibitory activity was verified by electronic cell counting assays as described (7, 8). [3H]Thymidine incorporation assays were conducted according to the method of Shing (18).
Cell Specificity.
To determine whether the proliferation of bovine aortic SMC and Balb c/3T3 cells was inhibited by TnI, the following assays were conducted. SMC were plated into multiwell dishes (2.1 cm2 per well) at a density of 10,000 cells per well. After allowing the cells to attach overnight, fresh media was applied containing either 3 ng/ml platelet-derived growth factor–BB alone or in combination with increasing concentrations of purified TnI. After incubation for 72 hours at 37°C in 10% CO2, the cells were rinsed in PBS, detached by trypsinization, and counted electronically. The effect of TnI on quiescent BALB/c mouse 3T3 cells was assessed by measuring the incorporation of [3H]thymidine into DNA in 96-well plates as described (18).
Chick Chorioallantoic Membrane (CAM) Assay.
All procedures were carried out in a laminar flow hood under sterile conditions. The eggs were stored in a Favorite Egg Incubator (Leahy, Higginsville, MO) at 37°C and 65% relative humidity. On day 3 of development, fertilized White Leghorn eggs (SPAFAS, Preston, CT) were cracked, and the embryos were removed from their shells and placed in plastic Petri dishes. On day 6, test substances including native rabbit TnI (19) or recombinant human TnI and appropriate buffer controls were mixed in methylcellulose disks and applied to the surfaces of the growing CAMs above the dense subectodermal plexus. Forty-eight hours after implantation of the plastic disc, the eggs were examined for vascular reactions under a dissecting scope (×60) and photographed (7, 8).
Mouse Corneal Pocket Assay.
Inhibition of angiogenesis in vivo was also demonstrated by using the mouse corneal pocket assay (20, 21). Briefly, pellets composed of bFGF (40 ng/ml), sucrose octasulfate, and Hydron were implanted into corneal micropockets of six C57BL/6 mice as previously described. TnI (50 mg/kg) was administered systemically every 12 hours by subcutaneous injection. On the sixth day of treatment, corneal angiogenesis was evaluated by using slit lamp microscopy, and the reaction was photographed.
B16-BL6 Melanoma Model.
Murine melanoma B16-BL6 were cultured in RPMI medium 1640 (GIBCO) supplemented with 10% (vol/vol) fetal calf serum (HyClone), l-glutamine and NaHCO3. Cells were washed with Earl’s balanced salt solution (GIBCO) and trypsinized for 3 to 5 min with 0.25% trypsin/0.2% EDTA to which culture buffer was added for washing. This preparation was then centrifuged for 10 min at ≈100 × g, the cell pellet was resuspended in fresh culture media, cell number was determined by using a Coulter counter, and cell viability was determined with trypan blue (100% viability). The cell suspension was adjusted to 2.5 × 105 cells per ml for implantation. B16-BL6 cells (5 × 105 in 0.2 ml) were injected into the tail veins of C57BL/6 mice (≈6–7 weeks old). One day after tumor cell inoculation, mice were treated with rTnI systemically, twice per week, with a dose of either 1 mg/kg (n = 10) or 20 mg/kg (n = 10) or vehicle (150 mM NaCl, 20 mM citrate, pH 3) over a 28-day period. On day 30, animals were sacrificed, the number of lung surface metastases were counted, and lungs were weighed.
Immunohistochemistry.
Bovine scapular cartilage, obtained within 2 hours of slaughter, was scraped free of muscle and connective tissue as described above. Sagittal slices of 2 mm thickness each were fixed overnight in 10% neutral buffered formalin and embedded in paraffin by using standard protocols. As a positive control, pieces of attached bovine muscle were similarly fixed and embedded. Sections (5 μm thick) were permeabilized with 2 μg/ml proteinase K at 37°C for 15 minutes and washed three times in PBS. Endogenous peroxidase activity was quenched by incubation with 1% H2O2 in MeOH. Sections were incubated for 4 hours at room temperature with mouse anti-rabbit TnI (Advanced ImmunoChemical, Long Beach, CA) diluted 1:10 or 1:50 in 3% goat serum in PBS. After 3 washes in PBS, the sections were incubated with goat anti-mouse antibody conjugated with horseradish peroxidase (diluted 1:50; Dako) for 1 hour at room temperature. Diaminobenzidine was used as a chromagen with methyl green as the counterstain. As a negative control, the primary antibody was omitted and replaced with non-immune serum.
In another series of control experiments designed to verify the specificity of the primary antibody, the TnI antibody was neutralized by preincubation for 2 hours at room temperature with a 100-fold excess of human rTnI or unrelated antigen (in this case, BSA). The immunohistochemical protocol was then followed as described above. As additional controls, sections prepared as above were also subjected to immunohistochemical staining by using antibodies raised against bovine type I and type II collagens as well. Stained sections were photographed by using a Zeiss Axiophot photomicroscope.
RESULTS
Purification and Identification of TnI from Cartilage.
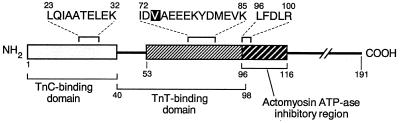
In this study, we utilized an in vitro assay that measures the inhibition of bFGF-stimulated proliferation of capillary ECs to monitor our purification strategy (7, 8, 17). All cartilage-derived fractions obtained from a series of chromatography steps described below were screened for this inhibitory bioactivity. Inhibitory activity eluted at an approximate molecular mass of 25,000 Da from the A-1.5 M size exclusion column, at approximately 0.2 M NaCl from the Biorex 70 cation exchange column, at approximately 23,000 Da from the Sephadex G-75 gel filtration column, at an acetonitrile concentration of approximately 38.5%, and at an approximate Mr of 22,000 Da from the Progel-TSK G3000SWXL column. Inhibitory fractions obtained from the final chromatography step were subjected to tryptic digestion, and the resultant peptides were sequenced by microcapillary liquid chromatography–electrospray ionization tandem MS or automated Edman degradation. The sequences of three peptide fragments were obtained and were identified as fragments of TnI (Fig. 1).
Figure 1.
Schematic representation of amino acid sequences of tryptic peptides purified from cartilage as described in Materials and Methods. Sequence similarity to human TnI is indicated by alignment with the amino acid sequence of the human isoform.
Cloning, Expression and Purification of Human TnI.
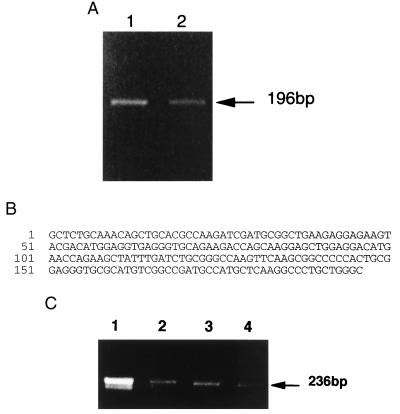
Because there had been no previous reports in the literature that cartilage cells, the chondrocytes, contain TnI, we proceeded to clone the cDNA encoding human cartilage TnI by using a standard PCR strategy (22) (Fig. 2A). Sequencing of the PCR product revealed its identity to human fast skeletal muscle TnI (Fig. 2B). TnI expression levels of rat xiphoid cartilage, Swarm rat chondrosarcoma, and liver also were determined by using RT-PCR and were significantly lower than that of rat skeletal muscle, with the expression level in liver appearing to be slightly lower than that of cartilage or chondrosarcoma (Fig. 2C).
Figure 2.
(A) RT-PCR products amplified from total RNA purified from two separate human intercostal cartilage specimens. Gene-specific primers were designed based on the cDNA sequence of human fast-twitch skeletal muscle TnI. (B) Nucleotide sequence of these PCR products showing identity to the cDNA sequence of human fast-twitch skeletal muscle TnI (nucleotides 189–384). (C) RT-PCR amplification from total RNA (20 ng each lane) purified from rat skeletal muscle (lane 1), xyphoid (lane 2), chondrosarcoma (lane 3), and liver (lane 4). Gene-specific primers were designed based on the cDNA sequence of rat fast-twitch skeletal muscle TnI as described in Materials and Methods.
To obtain sufficient amounts of TnI to investigate its potential as an antiangiogenic factor, a cDNA encoding full-length human fast skeletal muscle TnI was cloned into expression vector pET-24d and transformed into Escherichia coli BL21(DE3) p LysS strain. The expression level of recombinant human skeletal muscle TnI was ≈30–40% of total cellular protein. After purification, recombinant TnI migrated as a single band, at ≈21 kDa, on SDS/PAGE analysis (Fig. 3).
Figure 3.
SDS/PAGE of recombinant human TnI before (lane A) and after (lane B) purification. In both cases, ≈1 μg of total protein was electrophoresed, followed by silver staining as described in Materials and Methods. Recombinant TnI migrates at a molecular mass of ≈21,000 Da.
Inhibition of Capillary EC Growth by TnI.
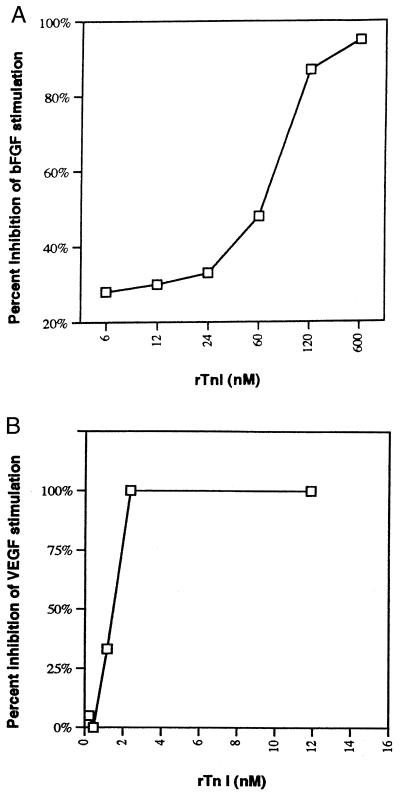
Purified rTnI was tested for its ability to inhibit bFGF and VEGF-stimulated capillary EC and was found to inhibit EC proliferation in a dose-dependent and saturable manner with an IC50 of approximately 65 nM when bFGF was used as the mitogen (Fig. 4A) and ≈1.5 nM when VEGF was used (Fig. 4B). Native TnI inhibited capillary EC proliferation in an equipotent manner. [3H]Thymidine assays demonstrated that recombinant TnI inhibited capillary EC DNA synthesis in a dose-dependent and saturable manner with an IC50 of ≈240 nM. This suppression of proliferation appears to be unique to endothelial cells given the fact that TnI did not suppress the growth of any of the nonendothelial cells tested including bovine aortic smooth muscle cells and BALB/c 3T3 cells even when tested at doses which were >5-fold higher than that required to obtain an IC50 value for capillary EC.
Figure 4.
Inhibition of capillary EC proliferation by rTnI. Percent inhibition was determined by comparing wells exposed to the angiogenic stimulus bFGF (A) and VEGF (B) with those exposed to stimulus and inhibitor. Each point represents the mean of duplicate control and inhibitor wells. This is a representative experiment of four different EC proliferation assays, each testing different TnI preparations.
Inhibition of Angiogenesis in Vivo by TnI.
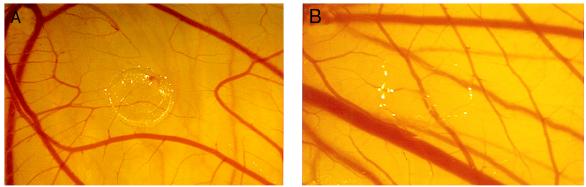
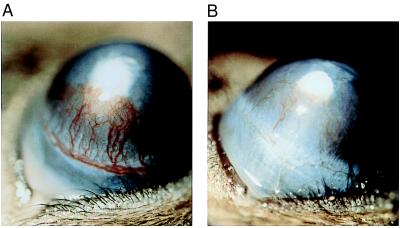
To determine whether rTnI was an inhibitor of angiogenesis in vivo, two different model systems were used: (i) the chicken CAM and (ii) the mouse corneal pocket assay. Fig. 5 A and B demonstrates the significant inhibition of embryonic neovascularization as evidenced by the large avascular zone caused by 130 pmol of rTnI. This effect was observed in 66% of the eggs tested at this dose and 100% of the eggs tested at a dose of ≈380 pmol. This observation was reproduced in three separate sets of CAM assays by using three different TnI preparations. Over 125 CAMs were tested in this series of experiments. In the second in vivo assay, the mouse corneal pocket assay, we found that systemic administration of rTnI significantly inhibited bFGF-induced angiogenesis (Fig. 6B) when compared with corneas of control mice that received vehicle alone (Fig. 6A). Taken together, these in vivo studies show rTnI to be a potent inhibitor of neovascularization when compared with other inhibitors tested in these same assays (23).
Figure 5.
Inhibition of embryonic angiogenesis in vivo by rTnI. After a 48-hour exposure to rTnI as described in Materials and Methods, avascular zones, free of capillaries and small vessels, were observed by using a binocular dissecting microscope at ×7–10 magnification. This zone was produced by ≈380 pmol of TnI (A). A normal CAM implanted with a methylcellulose disk containing buffer alone is shown in (B).
Figure 6.
Inhibition of bFGF-induced angiogenesis by systemic administration of TnI. Human rTnI (50 mg/kg) was administered systemically every 12 hours to mice whose corneas had been implanted with pellets containing bFGF (40 ng/ml) on day 1. After 6 days of treatment, significant inhibition of bFGF-induced neovascularization was observed in TnI-treated corneas (B) as compared with control corneas (A).
Inhibition of Metastasis by TnI.
In a third in vivo assay, rTnI was tested for its ability to inhibit lung metastasis caused by a very aggressive variant of the B16 melanoma cell line, B16-BL6 (24). Recombinant TnI, administered systemically, inhibited lung metastases by 52% (P < 0.04; one-tailed t test) at a dose of 1 mg/kg when given only twice weekly (n = 10) and by 64% (P < 0.02; one-tailed t test) at a dose of 20 mg/kg twice weekly (n = 10), (lung metastases: control, 68.6 ± 7.5 SEM, n = 10; 1 mg/kg, 32.8 ± 4.8 SEM; 20 mg/kg, 25.0 ± 7.5 SEM) with no observed toxicity (i.e., no weight or appetite loss, etc.). Lung weights were comparable in control and treated groups. When compared with other angiogenesis inhibitors tested in this same model, TnI is among the most inhibitory (25, 26, 27).
Immunohistolocalization of TnI in Cartilage.
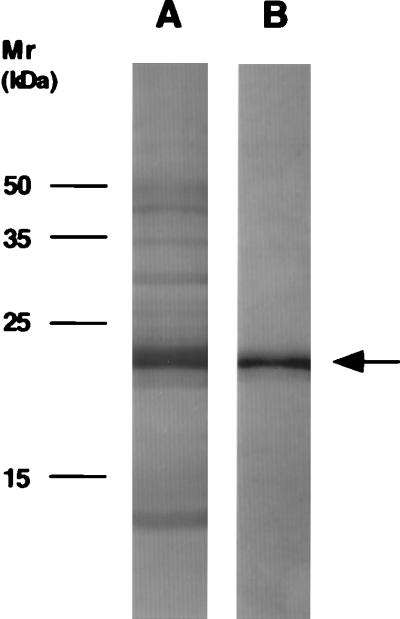
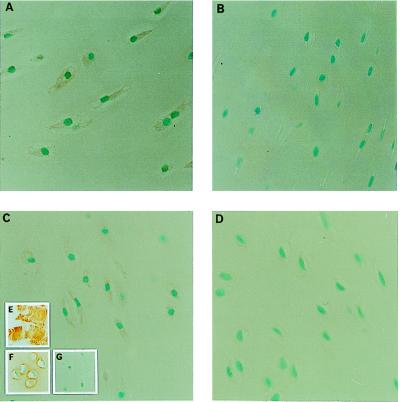
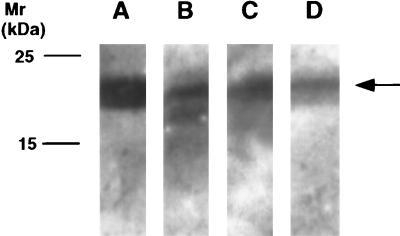
Immunohistolocalization studies to determine where TnI was located in cartilage were conducted and demonstrated the intracellular, extranuclear presence of TnI in individual chondrocytes (Fig. 7A and B). The specificity of the immunoreactive signal was confirmed by competition studies in which the addition of a 100-fold excess of TnI successfully blocked the immunoreactive signal, in contrast to the same study conducted using a 100-fold excess of BSA as control (Fig. 7 A, C, and D). Both recombinant and native TnI were recognized by a monospecific TnI antibody in a Western blot analysis as were TnI obtained from the RP-HPLC purification step and TnI-containing chondrocyte lysate preparation (Fig. 8).
Figure 7.
Immunohistolocalization of TnI in bovine chondrocytes. Sections of bovine scapular cartilage were prepared as described in Materials and Methods and incubated overnight with a monoclonal antibody (mAb1691) against bovine cardiac muscle TnI (Chemicon) according to standard protocols. Positive staining (×40) was observed intracellulary (A) in contrast to a control section (B) incubated in the absence of the primary antibody. Competition studies were performed by using either BSA (C) or rTnI (D). Positive staining was blocked by incubation with a 100-fold excess by weight of rTnI (D). Other controls include immunohistochemistry of bovine skeletal muscle stained with MAB1691 (E) and of bovine scapular cartilage sections stained with antibodies against Type II collagen (F) and type I collagen (Southern Biotechnology Associates) (G).
Figure 8.
Immunoblot analysis of recombinant human TnI (lane A), bovine cartilage-derived TnI (lane B), bovine chondrocyte lysate (lane C), and human skeletal muscle TnI (Advanced ImmunoChemical) (lane D). Approximately 3 μg of each protein was immunoblotted against a monoclonal antibody to rabbit skeletal muscle TnI (Advanced ImmunoChemical) by using standard ECL protocols.
DISCUSSION
The discovery that a contractile protein such as TnI is present in nonmuscle tissues, although novel with respect to cartilage, is not unprecedented with respect to other nonmuscle tissues. A number of contractile proteins, including troponin C, actin, tropomyosin, myosin heavy chain (28–30), and dystrophin (31) have been shown to be present in nonmuscle tissues. These studies demonstrating the presence of a variety of contractile proteins in nonmuscle tissues suggest that these proteins can function in processes other than mediating and regulating contraction of striated muscle.
A variety of contractile elements and cytoskeletal components have been identified and shown to play a role in cellular compartments other than their conventional location including α-actin and β-tubulin (32, 33). Of particular interest are reports that actin can be localized to the external face of different cell surfaces, including endothelial cells (34, 35). In one report, EC-associated actin functioned as a binding protein for angiogenin, a potent angiogenic molecule (35). Of interest, a reduction in the synthesis of various actin-binding proteins has been demonstrated in some tumor and transformed cells, consistent with the fact that transformation has often been associated with changes in cell shape and cytoskeletal architecture via changes in cell adhesion proteins and in microfilaments (36). Furthermore, certain tropomyosin isoforms have been demonstrated to have tumor suppressor activity in transformed cells (36, 37).
The mechanism(s) by which TnI inhibits capillary EC proliferation in vitro and angiogenesis in vivo is currently unknown. It is widely accepted that the modulation of EC cell shape via manipulation of the cytoskeleton and its regulatory elements, including actin-associated proteins, can exert a profound effect on EC cell growth and capillary morphogenesis (38). In the current study, capillary EC treated with TnI appeared as flat, elongated, and refractile-appearing cells, morphological features previously associated with growth inhibition and increased adhesivity (39). Of interest is the report that transfection of the human calponin gene into smooth muscle cells resulted in the inhibition of smooth muscle cell proliferation and migration. The inhibitory effect caused by the overexpression of calponin, a troponin homolog thought to be involved in thin filament-based regulation of smooth muscle contraction, was associated with enhanced matrix adhesion (40). The existence of an endothelial TnI receptor may be a means by which TnI could induce changes in EC shape that, in turn, would suppress growth of these cells. The possibility also exists that, under physiological conditions, TnI (pI 8.88), with its relatively rich lysine content, could, through its affinity for heparin, bind to and compete with bFGF and perhaps VEGF for heparin sulfate proteoglycan on the EC surface. The fact that TnI inhibited only mitogen-stimulated, but not basal, levels of EC proliferation (data not shown) may support this hypothesis.
TnI is liberated from cells into the immediate tissue microenvironment and its blood supply during cardiovascular injury (41). Based on this observation, results from a recent multicenter trial demonstrated that TnI is a sensitive serum marker whose presence is tightly correlated to the degree of myocardial damage (41). Monitoring of troponin levels is now used to provide prognostic information and to permit early identification of patients with acute disease who are at a higher risk of death and who may require immediate medical intervention. In light of TnI’s ability to inhibit neovascularization, it may be possible that the difficulty encountered in revascularizing the ischemic myocardium after cardiovascular injury (42) may be due, at least in part, to high local levels of this angiogenesis inhibitor.
Taken together, the results presented above suggest that TnI may have the potential to be a useful inhibitor of the large number of serious diseases characterized by deregulated angiogenesis.
Acknowledgments
The authors thank Dr. Judah Folkman and Geraldine Jackson for help with the chick CAM assays, R. Robinson, J. Neveu, V. Bailey, T. Addona, and E. Spooner of the Harvard Microchemistry Facility for expertise in mass spectrometry and peptide sequencing, and Southern Research Institute Labs for their help with the metastasis model. We thank Drs. Michael O’Reilly, Michael R. Freeman, and Frank McGowan for helpful discussions and Atlantic Veal and Lamb Co., New York, for their generous gifts of veal cartilage. This study was supported by a sponsored research grant from Boston Life Sciences (B.L.S.), Boston, Massachusetts. R.L. and Children’s Hospital hold equity in B.L.S.; T.T. is supported by Grant AR21673 from the National Institutes of Health.
ABBREVIATIONS
- TnI
troponin I
- EC
endothelial cell
- bFGF
basic Fibroblast Growth Factor
- CAM
chorioallantoic membrane
- RT
reverse transcription
- VEGF
vascular endothelial growth factor
References
- 1.Folkman J. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 2.Brem H, Folkman J. J Exp Med. 1975;141:427–439. doi: 10.1084/jem.141.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenstein R, Kuettner K E, Neopolitan C, Soble L W, Sorgente N. Am J Pathol. 1975;81:337–347. [PMC free article] [PubMed] [Google Scholar]
- 4.Sorgente N, Kuettner K E, Soble L, Eisenstein R. Lab Invest. 1975;32:217–222. [PubMed] [Google Scholar]
- 5.Langer R, Brem, Falterman H, Klein K, Folkman J. Science. 1976;193:70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- 6.Langer R, Conn H, Vacanti J, Haudenschild C, Folkman J. Proc Natl Acad Sci USA. 1980;77:4331–4335. doi: 10.1073/pnas.77.7.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moses M A, Sudhalter J, Langer R. Science. 1990;2488:1408–1410. doi: 10.1126/science.1694043. [DOI] [PubMed] [Google Scholar]
- 8.Moses M A, Sudhalter J, Langer R. J Cell Biol. 1992;119:474–481. doi: 10.1083/jcb.119.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane W S, Galat A, Harding M W, Schreiber S L. J Protein Chem. 1991;10:151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- 10.Nash H M, Bruner SD, Scharer O D, Kawate T, Addona T A, Spooner E, Lane W S, Verdine G L. Curr Biol. 1996;6:968–980. doi: 10.1016/s0960-9822(02)00641-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Perez-Alvarado G, Wade R. Biochim Biophys Acta. 1994;1217:338–340. doi: 10.1016/0167-4781(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. [Google Scholar]
- 13.Laemmli K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J, Haudenschild C, Zetter B R. Proc Natl Acad Sci USA. 1979;76:5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klagsbrun M, Langer R, Levenson R, Smith S, Lillehei C. Exp Cell Res. 1977;105:99–108. doi: 10.1016/0014-4827(77)90155-0. [DOI] [PubMed] [Google Scholar]
- 16.D’Amore P A, Smith S R. Growth Factors. 1993;8:61–75. doi: 10.3109/08977199309029135. [DOI] [PubMed] [Google Scholar]
- 17.Connolly D J, Knight M B, Harakas N K, Wittwer A J, Feder J. Anal Biochem. 1986;152:136–140. doi: 10.1016/0003-2697(86)90131-4. [DOI] [PubMed] [Google Scholar]
- 18.Shing Y. In: Methods in Enzymology. Barnes D, Mather J P, Sato G H, editors. New York: Academic; 1990. pp. 91–95. [Google Scholar]
- 19.Greaser M L, Gergely J. J Biol Chem. 1971;246:4226–4233. [PubMed] [Google Scholar]
- 20.Chen C, Parangi S, Tolentino M J, Folkman J. Cancer Res. 1995;55:4230–4233. [PubMed] [Google Scholar]
- 21.O’ Reilly M S, Holmgren L, Chen C, Folkman J. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 22.Wu I, Moses M A. Gene. 1996;18:243–246. doi: 10.1016/0378-1119(95)00783-0. [DOI] [PubMed] [Google Scholar]
- 23.Moses M A, Klagsbrun M, Shing Y. Int Rev Cytol. 1995;161:1–48. doi: 10.1016/s0074-7696(08)62495-x. [DOI] [PubMed] [Google Scholar]
- 24.Saiki I, Iida J, Murata J, Ogawa R, Nishi N, Sugimura K, Tokura S, Azuma I. Cancer Res. 1989;49:3815–3822. [PubMed] [Google Scholar]
- 25.Yamaoka M, Yamamoto T, Masaki T, Ikeyama S, Sudo K, Fujita T. Cancer Res. 1993;53:4262–4267. [PubMed] [Google Scholar]
- 26.Chivri R G S, Garfalo A, Crimmin M J, Bawden L J, Stoppacciaro A, Brown P, Giavazzi R. Int J Cancer. 1994;58:460–464. doi: 10.1002/ijc.2910580326. [DOI] [PubMed] [Google Scholar]
- 27.Oku T, Ata N, Yonezawa K, Tokai H, Fujii H, Shinagawa A, Ohuchi E, Saiki I. Biol Pharm Bull. 1997;20:843–849. doi: 10.1248/bpb.20.843. [DOI] [PubMed] [Google Scholar]
- 28.Perry S V, Margreth A, Adelstein R S, editors. Contractile Systems in Nonmuscle Tissues. New York: North–Holland; 1976. pp. 93–99. [Google Scholar]
- 29.Pollard T D. J Cell Biol. 1981;91:156–165. doi: 10.1083/jcb.91.3.156s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard T D, Weihing R R. CRC Crit Rev Biochem. 1974;2:1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- 31.Nudel U, Zuk D, Einat P. Nature (London) 1989;337:76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- 32.Pittinger M F, Kazzaz J A, Helfman D M. Curr Opin Cell Biol. 1994;6:96–104. doi: 10.1016/0955-0674(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 33.Smalheiser N R. Mol Biol Cell. 1996;7:1003–1014. doi: 10.1091/mbc.7.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardridge W, Nowlin D, Choi T, Yang J, Calacay J, Shively J. J Cereb Blood Flow Metab. 1989;9:675–680. doi: 10.1038/jcbfm.1989.95. [DOI] [PubMed] [Google Scholar]
- 35.Moroianu J, Fett J, Riordan J, Vallee B. Proc Natl Acad Sci USA. 1993;90:3815–3819. doi: 10.1073/pnas.90.9.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastinejad F, Conboy M J, Rando T A, Blau H M. Cell. 1993;75:1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- 37.Prasad G L, Fuldner R A, Cooper H L. Proc Natl Acad Sci USA. 1993;90:7039–7043. doi: 10.1073/pnas.90.15.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingber D E. Proc Natl Acad Sci USA. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braunhut S J, Palomares M. Microvasc Res. 1991;41:47–62. doi: 10.1016/0026-2862(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi, K., Takagi, M., Ohgami, K., Nakai, M., Kojuma, A., Nadal-Ginard, B. & Shibata, N. (1993) J. Am. Heart Assoc. (Abstr.) 95, no. 010379.
- 41.Antman E M, Tanasijevic M J, Thompson B, Schactman M, McCabe C H, Cannon C P, Fischer G A, Fung A Y, Thompson C, Wybenga D, Braunwald E. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 42.Ware J A, Simons M. Nat Med. 1997;3:158–164. doi: 10.1038/nm0297-158. [DOI] [PubMed] [Google Scholar]