Abstract
Human chorionic gonadotropin (hCG) preparations contain activity against HIV type 1 (HIV-1). However, there has been controversy about whether some biological activities of hCG β-subunit (hCGβ) preparations are caused by the β-subunit itself or other proteins present in the preparations. We report here the purification, characterization, and identification of three enzymes with anti-HIV activity present in the β-core fraction of hCGβ prepared from the urine of pregnant women. The N-terminal amino acid sequence of one protein is identical to human urinary lysozyme C, and those of the other two are identical to human RNase A and urinary RNase U. We thus refer to these proteins as AVL (antiviral lysozyme) and AVR (antiviral RNases). In addition to HIV-1 inhibition, AVL is capable of lysing Micrococcus lysodeikticus. AVR digests a variety of RNA substrates, including RNA from HIV-1-infected cells. We also find that lysozyme from chicken egg white, human milk, and human neutrophils and RNase A from bovine pancreas possess activity against HIV-1. These findings may offer additional strategies for the treatment of HIV-1 infection.
Keywords: AIDS, muramidase, urinary proteins, antiviral agents
The transmission of HIV type 1 (HIV-1) from mother to fetus is rare during the first trimester of pregnancy when the secretion of human chorionic gonadotropin (hCG) is high in the placenta (1, 2). We and others found that the β-subunit of hCG (hCGβ), but not the α-subunit, is active against HIV-1 virus (3) and AIDS-related Kaposi’s sarcoma (4) in AIDS patients (5) and HIV-1 transgenic mice (6). These studies were conducted by using heterogeneous commercial preparations with different potencies reported for different source materials. There recently has been controversy as to whether the activity against Kaposi’s sarcoma found in hCGβ preparations is caused by hCGβ itself or other proteins (7–10).
To determine whether proteins other than hCGβ itself might contribute to the anti-HIV activity of hCGβ preparations, we fractionated commercial preparations derived from the urine of pregnant women. We found that a significant portion of the antiviral activity is associated with the β-core fraction. The β-core is a dimer of two peptide fragments of hCGβ linked by disulfide bridges. Here we report the purification, characterization, and identification of three anti-HIV proteins associated with β-core preparations from the urine of pregnant women. The N-terminal 15-aa sequence of one protein is identical to human urinary lysozyme. The N-terminal amino acid sequences of the other two proteins are identical to human RNase A and urinary RNase U. We also report here that lysozymes and RNases from other sources inhibit HIV-1. These findings may offer additional strategies for the treatment of HIV-1 infection.
METHODS
Purification of Antiviral Lysozyme (AVL) and Antiviral RNases (AVR).
A crude preparation of β-core was isolated from a commercial hCG preparation from the urine of pregnant women (Diosynth, Oss, The Netherlands). Intact hCG, intact hCGβ, and hCGα were removed by Sephadex G-100 gel chromatography (11). Crude β-core in 0.01 M trifluoroacetic acid (TFA) was fractionated by reverse-phase chromatography on a C18 Vydac (218TP510) column. The column was eluted with a linear gradient of acetonitrile in 0.01 M TFA. Seven protein fractions were obtained (P1–P7). Each fraction was lyophilized, characterized by SDS/PAGE, and further purified by gel filtration on Sephadex G25 superfine in 10 mM Tris⋅HCl, 50 mM NaCl, pH 7.8. Pure β-core was obtained from P1 and P2, RNase U from P5 and P6, and lysozyme C from P6.
Electrophoresis, Electroblotting, and N-Terminal Amino Acid Sequencing.
SDS/PAGE was carried out by a discontinuous buffer system (12) using 15% polyacrylamide separating gels containing 0.1% SDS (wt/vol) in 0.375 M Tris, pH 8.8 with 4% stacking gels containing 0.1% SDS (wt/vol) in 0.125 M Tris, pH 6.8. Electrophoresis was performed in Tris-glycine buffer (25 mM Tris, pH 8.3/192 mM glycine) containing 0.1% SDS, at 100 V for 2.5 hr. For visual analysis, the gels were stained with 0.1% Coomassie blue R250.
For N-terminal amino acid sequencing, the gels were transferred to Immobilon-P membranes [poly(vinylidene difluoride), Millipore] by electroblotting in 10 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid), pH 11 in 10% methanol. The blot was rinsed with deionized water, then saturated with methanol, stained lightly with 0.1% Coomassie blue R250, destained in 50% MeOH, and blot-dried with filter paper. Individual bands were cut out from the membrane and subjected to N-terminal amino acid sequencing, by using Perkin–Elmer/Applied Biosystem Procise Sequencer model 494.
Anti-HIV Assay.
Anti-HIV activity was assayed by HIV-1 core protein p24 expression in chronically infected ACH-2 lymphocytes and U1 monocytes. ACH-2 and U1 cells, obtained through the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, originally were contributed by T.M. Folks (13). The cell lines were cultured in RPMI medium 1640 containing 100 units/ml of penicillin, 100 μg/ml of streptomycin, 2 mM l-glutamine, and 10% heat-inactivated FCS. The expression of viral core protein p24 was assayed as described (3).
Lysozyme Assay.
Lysozyme activity was determined by the turbidimetric assay (14). Briefly, 0.015% (wt/vol) cell suspension of Micrococcus lysodeikticus (ATCC 4698) was used as the substrate. One unit of enzyme is defined as the amount of protein that produces a decrease in absorbance of 0.001/min at 450 nm, pH 6.24 at 25°C.
RNase Assay.
Total RNA was prepared from HIV-1-infected ACH2 cells by using the Biotecx Ultraspec RNA Isolation System (Houston) according to the manufacturer’s protocol. Four micrograms of total cellular RNA was incubated with 1 μg of sample in diethyl pyrocarbonate water at 37°C for 1 hr. RNase activity was measured by the degradation of intact total cellular RNA by formaldehyde agarose gel (1.2%). The RNA samples were electrophoresed at 50 V for 90 min, stained with ethidium bromide, and visualized by UV illumination.
RESULTS
Purification, Identification, and Characterization of Lysozyme and RNases in the β-Core Preparations from Urine of Pregnant Women.
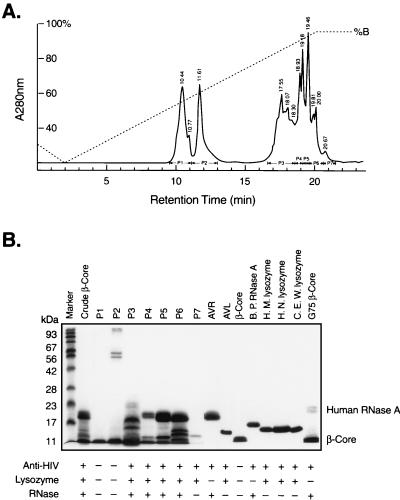
To investigate the active components responsible for anti-HIV activity in the β-core fraction from urine of pregnant women, we subjected a preparation of β-core fragment to reverse-phase HPLC. Fig. 1A represents a typical elution profile, showing seven pooled fractions (P1–P7). We characterized these fractions by SDS/PAGE and anti-HIV assays (Fig. 1B) as well as N-terminal amino acid sequencing (Table 1).
Figure 1.
(A) HPLC separation of hCG β-core fragment, human urinary AVL, and AVR from a crude β-core preparations. The elution profile of a representative reverse-phase liquid chromatogram of 2.5 mg run is shown. Details are described in Methods. (B) Characterization of AVL and AVR. SDS/PAGE was carried out on crude β-core (Sephadex G100 gel chromatography fractionated), fractions P1-P7 of C18 reverse-phase HPLC, pure β-core, AVL, AVR, lysozyme, and RNase. Each lane contains about 5–10 μg of sample. The presence (+) or absence (−) of anti-HIV, lysozyme, and RNase activities are shown under the SDS/PAGE pattern. Proteins smaller than 11 kDa were not separated in this SDS/PAGE system.
Table 1.
N-terminal amino acid sequence of hCGβ-core fractions
| Fractions | SDS/PAGE position | Amino acid sequence* | Analogous proteins |
|---|---|---|---|
| P1 & P2 | <11 kDa | RPR(C)RPI(N)ATLAVEK | β-core |
| VV(C)NYRDVRFESIRL | |||
| P5 & P6 | 18.5 kDa | KPPQFTWAQWFETQHI(N) | Human RNase U |
| P5 & P6 | 18 kDa | PQFTWAQWFETQHI(N) | Human RNase U |
| P5 & P6 | 14 kDa | VFER(C)ELARTLKRLG | Human lysozyme C |
| β-core (G75) | 23 kDa | KESRAKKFQRQHMDS | Human RNase A |
(C) and (N) were not identified during sequencing by the Edman degradation. The absence of (N) at the indicated position is consistent with glycosylation.
The N-terminal amino acid sequences of P1 and P2 are identical to that of urinary β-core, and these fractions exhibit little, if any, anti-HIV activity. Peaks 5 and 6 contain the bulk of anti-HIV activity. The sizes of the major protein bands are 18 and 18.5 kDa in P5 and 14, 18, and 18.5 kDa in P6. The N-terminal amino acid sequence of the 14-kDa protein is identical to that of human lysozyme C, also known as milk or urinary lysozyme. The N-terminal amino acid sequence of the 18- and 18.5-kDa protein is identical to that of RNase U, also known as eosinophil-derived neurotoxin. Further purification of P5 and P6 by Sephadex G25 superfine yielded homogeneous human urinary lysozyme C and RNase U, both of which showed potent anti-HIV activity. Thus these proteins were designated AVL and AVR, respectively.
We identified another RNase with anti-HIV activity in a β-core preparation. This RNase remains with the β-core even after Con A-Sepharose, DEAE-Sephacryl, and Sephadex G75 Superfine fractionation (11). This preparation is designated as G75 β-core. N-terminal sequence analysis of this preparation revealed two major sequences identical to those of β-core and a minor sequence (approximately 10% of the major sequences). The minor sequence is identical to human RNase A. SDS/PAGE of G75 β-core yields a major protein band migrating at the front (β-core) and a minor doublet migrating at 23 kDa (Fig. 1B). The N-terminal 15-residue sequence of the 23-kDa doublet gave only the sequence of human RNase A (Table 1). Because of the limited supply of this 23-kDa protein, further purification was not attempted. Nevertheless, the presence of RNase A in this highly purified β-core preparation is intriguing. The series of purification processes did not remove, but rather concentrated RNase A.
AVL, AVR, Lysozymes, and RNases Are Effective Against HIV-1.
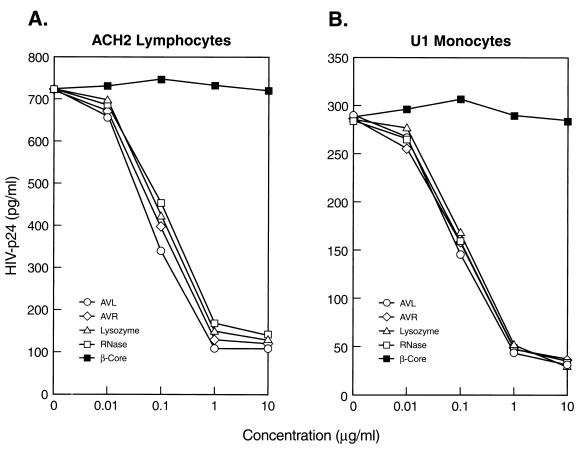
To ensure that the anti-HIV activities of AVL and AVR are protein specific, we tested crude β-core, fractions P1-P7, β-core purified from P1 and P2 (hereafter referred as pure β-core), AVL, and AVR for their ability to inhibit viral production in chronically infected ACH2 T lymphocytes and U1 monocytes (13). To relate the anti-HIV activity to authentic lysozyme and RNase, we also tested the antiviral activity of homogeneous human milk lysozyme, human neutrophil lysozyme, chicken egg white lysozyme, and bovine pancreatic RNase A in HIV-1 p24 release by ELISA as described (3). Fig. 2 A and B shows the dose–response curves of pure β-core, AVL, AVR, lysozyme, and RNase on viral production from ACH2 lymphocytes and U1 monocytes, respectively. AVR, lysozyme, and RNase exhibit profound effects on viral production with EC50 in the range of 50 to 80 nM, whereas pure β-core shows negligible effect.
Figure 2.
AVR and AVL are active in HIV-1 inhibition. Dose–response anti-HIV activities of AVL, AVR, pure β-core, chicken egg white lysozyme, and bovine pancreatic RNase were assayed by viral production (p24 ELISA) in (A) ACH2 lymphocytes and (B) U1 monocytes. Each data point is the mean of triplicate. Identical dose–response anti-HIV activity was obtained for human milk lysozyme and human neutrophil lysozyme (data not shown).
AVL, AVR, Lysozymes, and RNases Are Not Cytotoxic.
To ensure that inhibition of viral production by AVL, AVR, lysozymes, and RNases is not the result of nonspecific inhibition of cell proliferation, we examined their cytotoxicity by measuring [3H] thymidine incorporation into cellular DNA in HIV-infected ACH2 lymphocytes and U1 monocytes. No cytotoxic effect was detected for these samples over the entire concentration range of the anti-HIV assay. These results indicate that inhibition of viral production is not caused by inhibition of cell growth. Cell viability, as determined by trypan blue dye exclusion and CellTiter 96 AQueousNon-Radioactive Cell Proliferation Assay (Promega) as described (15), also was unaffected by AVL, AVR, lysozymes, or RNases.
AVL and AVR Exhibit Authentic Lysozyme and RNase Activity, Respectively.
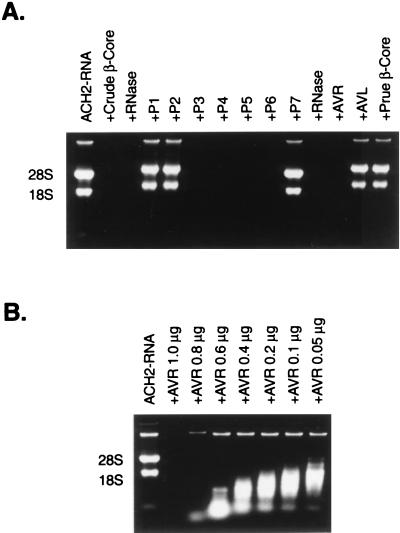
Our initial identification of the anti-HIV proteins present in the β-core preparations was based on N-terminal amino acid sequencing. We thus tested AVL and AVR for lysozyme and RNase activity. AVL demonstrated bona fide lysozyme activity as measured by the turbidimetric assay with Micrococcus lysodeikticus cell suspension at 450 nm (14). The specific activity of AVL (108,000 units/mg) is comparable to that of authentic lysozyme. AVR exhibits RNase activity, including digestion of RNA isolated from HIV-1 infected cells. Fig. 3A shows the RNase activity of AVR by using RNA isolated from ACH2 cells infected with HIV-1. Based on degradation of RNA as analyzed by electrophoresis in 1.2% formaldehyde agarose gels, AVR exhibits potent RNase activity, whereas no RNase activity is detected in P1, P2, or pure β-core. Fig. 3B shows the dose-dependent RNase activity of AVR. Complete digestion of both substrates is obvious at 0.8 μg of AVR, and cleavages are apparent at 0.05 μg.
Figure 3.
RNase activity of AVR. (A) RNase activity of AVR was assayed by degradation of total RNA isolated from HIV-infected ACH2 lymphocytes. AVR demonstrated potent RNase activity comparable to that of bovine pancreatic RNase A, whereas pure β-core and AVL showed no RNase activity. (B) Dose-dependent RNase activity of AVR.
DISCUSSION
In the present study, we identify lysozyme and RNases as enzymes that contribute to anti-HIV activity of crude preparations of β-core. We purified these enzymes from a crude β-core preparation and refer to these enzymes as AVL and AVR. We also show that lysozymes from chicken egg white, human milk, and human neutrophils, as well as RNase from bovine pancreas have similar activity against HIV-1. Previously, an amphibian RNase, also know as onconase, but not human RNase, has been shown to exhibit inhibition of HIV-1 production and selective degradation of viral RNA (16).
The β-core is a fragment from hCGβ made up of two peptide chains, one corresponding to amino acids 6–40 and the other to amino acids 55–92 with interchain disulfide bridges. The β-core is hormonally inactive and found in the urine of pregnant women and the urine of many patients with ovarian, breast, and colon cancer (11). Its levels correlate with tumor grade and disease progression. We found that as β-core preparations are fractionated, the antiviral activity becomes separated from β-core, and purified β-core itself contains little or no antiviral activity.
Lysozyme activity is found in the urine of the pregnant women (17). Human placental lysozyme reduces the adsorption of ectromelia virus and may play important protective roles during pregnancy (18). However, under normal and nonpregnant conditions, lysozyme activity is almost absent from urine, bile, and spinal fluid. Serum and urine levels of lysozyme are significantly elevated in patients with monocytic and mono-myelocytic leukemia (19). Macrophages are an important source of lysozyme, which mediates some of their antitumor activities (20). In contrast to lysozyme, an 18-kDa urinary RNase was found to copurify with β-core (7, 21). Although exact mechanisms of their action have not been explored, it is reasonable to assume that the antiviral effect of lysozymes and RNases may be caused by degradation of viral polysaccharides and RNA transcripts or viral genomic RNA, respectively. A recent report (10) indicating that an unidentified hCG associate factor with in vitro and in vivo anti-HIV activities eluted as two peaks corresponding to 15–30 kDa (major) and 2–4 kDa (minor) in gel chromatography is consistent with our present findings.
What are the molecular mechanisms by which lysozymes and RNases block HIV-1 replication in infected cells? One possibility is that the antiviral effect is caused by the hydrolytic activities of these enzymes. If so, it will be important to determine whether AVL and AVR enter cells as in the case of onconase (16) or whether they act on extracellular substrates.
Because AVL and AVR are proteins that occur and circulate naturally during pregnancy, they are likely to be well-tolerated physiologically and free of toxicity. We do not know whether AVL and AVR function as natural antiviral proteins during pregnancy or whether they have additional biological activities. In any case, present findings of AVL and AVR as anti-HIV proteins in crude β-core preparations hopefully will lead to additional strategies for the treatment of HIV and other viral infections in patients.
Acknowledgments
We thank Dr. Carl Dieffenbach for his advice and support. This work was supported in part by National Institutes of Health Grant Al-31343 to S.L.-H.
ABBREVIATIONS
- hCG
human chorionic gonadotropin
- HIV-1
HIV type 1
- AVL
antiviral lysozyme
- AVR
antiviral RNases
References
- 1.De Rossi A, Ometto L, Mammano F, Zanotto C, Giaquinto C, Chieco-Bianchi L. AIDS. 1992;6:1117–1120. [PubMed] [Google Scholar]
- 2.Krivine A, Firtion G, Cao L, Francoual C, Henrion R, Lebon P. Lancet. 1992;339:1187–1189. doi: 10.1016/0140-6736(92)91131-q. [DOI] [PubMed] [Google Scholar]
- 3.Bourinbaiar A S, Lee-Huang S. Immunol Lett. 1995;44:13–18. doi: 10.1016/0165-2478(94)00191-s. [DOI] [PubMed] [Google Scholar]
- 4.Lunardi-Iskandar Y, Bryant J L, Zeman R A, Lam V H, Samaniego F, Besnier J M, Hermans P, Thierry A R, Gill P, Gallo R C. Nature (London) 1995;375:64–68. doi: 10.1038/375064a0. [DOI] [PubMed] [Google Scholar]
- 5.Gill P S, Lunardi-Ishkandar Y, Louie S, Tulpule A, Zheng T, Espina B M, Besnier J M, Hermans P, Levine A M, Bryant J L, Gallo R C. N Engl J Med. 1996;335:1261–1269. doi: 10.1056/NEJM199610243351702. [DOI] [PubMed] [Google Scholar]
- 6.De S K, Wohlenberg C R, Marinos N J, Doodnauth D, Bryant J L, Notkins A L. J Clin Invest. 1997;99:1484–1491. doi: 10.1172/JCI119310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths S J, Adams D J, Talbot S J. Nature (London) 1997;390:568. doi: 10.1038/37510. [DOI] [PubMed] [Google Scholar]
- 8.Albini A, Paglieri I, Orengo G, Carlone S, Aluigi M G, DeMarchi R, Matteucci C, Mantovani A, Carozzi F, Donini S, Benelli R. AIDS. 1997;11:713–721. doi: 10.1097/00002030-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hopp T. Nat Biotech. 1997;12:834–835. [Google Scholar]
- 10.Lunardi-Isakandar Y, Bryant J L, Blattner W A, Hung C L, Flamand L, Gill P, Hermans P, Birken S, Gallo R C. Nat Med. 1998;4:428–434. doi: 10.1038/nm0498-428. [DOI] [PubMed] [Google Scholar]
- 11.Blithe D L, Akar A H, Wehmann R E, Nisula B C. Endocrinology. 1988;122:173–180. doi: 10.1210/endo-122-1-173. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Folks T, Benn S, Rabson A, Theodore T, Hoggan M D, Martin M, Lightfoote M, Sell M. Proc Natl Acad Sci USA. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shugar D. Biochim Biophys Acta. 1952;8:302. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- 15.Cory A H, Owen T C, Barltrop J A, Cory J G. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 16.Saxena S K, Gravell M, Wu Y N, Milkulski S M, Shogen K, Ardelt W, Youle R J. J Biol Chem. 1996;271:20783–20788. doi: 10.1074/jbc.271.34.20783. [DOI] [PubMed] [Google Scholar]
- 17.Hankiewicz J, Swierczek E. Clin Chim Acta. 1974;57:205–209. doi: 10.1016/0009-8981(74)90398-2. [DOI] [PubMed] [Google Scholar]
- 18.Arimura H. Acta Virol. 1973;17:130–137. [PubMed] [Google Scholar]
- 19.Osserman E F, Klockars M, Halper J, Fischel R E. Nature (London) 1973;243:331–335. doi: 10.1038/243331a0. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S, Todd J, Cohn Z A. J Exp Med. 1974;139:1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths S J, Bramley T A, Menzies G S, Adams D J. Mol Cell Endocrinol. 1997;134:69–76. doi: 10.1016/s0303-7207(97)00174-3. [DOI] [PubMed] [Google Scholar]