Abstract
Cocaine- and amphetamine-regulated transcript (CART) is a recently discovered hypothalamic peptide regulated by leptin and with a potent appetite-suppressing activity. In the rat, the CART gene encodes a peptide of 116 amino acid residues (or a splice variant 13 residues longer). The predicted signal sequence is 27 amino acid residues, resulting in a prohormone of 89 residues. The CART prohormone contains several potential posttranslational processing sites in the form of mono- and dibasic sequences. In the present study we have purified CART peptides from extracts of adrenal gland, hypothalamus, nucleus accumbens, and pituitary gland (anterior and neurointermediate lobe) of the rat and determined the peptide structures by using microsequencing and mass spectrometry. In none of the tissues examined the long splice variant was found. From the adrenal gland, the CART(1–89) and CART(10–89) peptides were isolated, in contrast to the hypothalamus and nucleus accumbens, from which the shorter form peptides CART(42–89) and CART(49–89) were purified. From the anterior lobe of the pituitary gland, CART(42–89) was isolated, in contrast to the neurointermediate lobe, which contains only CART(49–89). This tissue-specific processing indicates that CART peptides may have different biological functions in the periphery and in the central nervous system.
Keywords: brain, adrenal gland, appetite regulation
Over the last several years, a number of neuropeptides have been identified as central signaling molecules with an overall effect on feeding behavior and have thus attracted interest in relation to the development of antiobesity drugs. These molecules include neuropeptide Y, bombesin, cholecystokinin, galanin, and glucagon-like peptide 1 (GLP-1), as well as the newly discovered agouti-related peptide and orexin/hypocretin (for review, see ref. 1). Recently, the peptide CART was added to this list (2). CART stands for cocaine- and amphetamine-regulated transcript and refers to the way this peptide was originally discovered. Douglass and coworkers (3) identified the CART mRNA by differential-display techniques that allowed identification of genes specifically expressed after acute administration of cocaine and amphetamine to rats. In both rats and mice, CART exerts a very potent anorectic effect (2, 4) and is able to completely block the feeding response induced by neuropeptide Y (2). CART is the third most abundant mRNA in rat hypothalamus after subtraction of hippocampal and cerebellar mRNAs (5). Furthermore, CART expression in the arcuate nucleus is regulated by leptin (2), indicating that CART is integrated in the circuit that controls the overall regulation of food intake. The rat CART gene encodes a peptide of either 129 or 116 amino acid residues in length (3). The predicted leader sequence is 27 amino acid residues, thus resulting in a mature CART peptide consisting of either 102 (long form) or 89 (short form) amino acid residues (3). In contrast to the rat, only the short form exists in humans (6). The mature peptide contains several potential cleavage sites (mono- and dibasic sequences), and CART may be posttranslationally processed in vivo into several biologically active fragments. Posttranslational processing at a Lys–Arg sequence in the middle of the short form of CART would generate an N-terminal CART(1–39) and a C-terminal CART(42–89) fragment. The latter of these fragments has actually been isolated from ovine hypothalamic extracts (7), thus indicating that CART is processed at the Lys40–Arg41 sequence at least in the ovine brain. So far, no systematic studies have been carried out to determine the naturally occurring forms of CART. Because immunoreactive CART peptides have been reported to be present in adrenals glands (8), hypothalamus (8, 9), and nucleus accumbens (10), we have chosen these three tissues, together with the pituitary gland, as starting material for the isolation and structure determination of naturally occurring forms of CART peptides. By using microsequencing and mass spectrometry, we here demonstrate a tissue-specific processing of CART, which may point to different biological functions of CART peptides in the periphery and in the central nervous system.
MATERIALS AND METHODS
Animal Tissue.
Wistar female rats (M & B, Ry, Denmark) (weight ≈250 g) were allowed to acclimatize for 1 week before the experiment. Animals were decapitated and tissue was removed and immediately frozen on dry ice.
In Situ Hybridization.
In situ hybridization analysis was performed as described (11) on saggital cryostat sections from rat brain by using antisense RNA probes directed against the rat CART cDNA (base pairs 226–411 (GenBank accession no. U10071). Post-hybridization washes were performed at 62°C and 67°C in 50% formamide. After hybridization, sections were exposed on BetaMax film (Amersham Pharmacia) for 1 week.
Preparation of Monoclonal CART Antibodies (MCAs).
Purified recombinant CART(41–89) (4) were conjugated to the carrier ovalbumin by using carbodiimide. Mice of the RBF strain were injected s.c. with the antigen in Freund’s complete adjuvant and boosted twice every other week with the antigen in Freund’s incomplete adjuvant. Spleen cells from an i.v.-boosted mouse were fused by the polyethylene glycol method to FOX myeloma cells (12). Supernatants from the resulting hybridoma lines were screened in a direct ELISA by using CART(41–89) as antigen. Positive hybridoma lines were cloned. The MCA (mAb from clone Ca6-1F4D4) was purified by Protein A (Amersham Pharmacia) affinity chromatography.
Tissue Extraction.
Tissue from 18 animals was used with a total weight of 2.59 g (adrenal glands), 2.68 g (hypothalamus), 2.65 g (nucleus accumbens), 0.31 g (pituitary gland, anterior lobe), and 0.11 g [pituitary gland, neurointermediate (intermediate and posterior) lobe]. The tissue was frozen immediately after dissection and subsequently extracted at 4°C with 25 ml of 3:1 (vol/vol) ethanol/HCl (0.7 M) by using an Ultra-Turrax type TP18/10 homogenizer equipped with a 10N head. After 3 min of homogenization, the extraction mixture was stirred at 4°C for 18 h. After centrifugation at 38,000 × g for 30 min, the supernatant was concentrated to 20% by vacuum centrifugation and lyophilized. The lyophilized material was extracted with 20 ml of 2M acetic acid, and after centrifugation at 38,000 × g for 30 min, the supernatant was lyophilized. The lyophilized powder was redissolved in 20 ml of PBS buffer (50 mM sodium phosphate, 100 mM NaCl, pH 7.2) containing a mixture of protease inhibitors as described by the manufacturer (Complete, Boehringer Mannheim, catalog no. 1697498).
Affinity Column.
A solution of 10 ml of MCA containing 2.17 mg/ml in PBS buffer was added to 10 ml of 0.6 M sodium hydrogen carbonate (pH 8.6) and solid polyethylene glycol (Mr = 20,000) to a final concentration of 15% (wt/vol). Five milliliters of Mini-Leak gel (Kem-En-Tek, Copenhagen, catalog no. 1011F) was added. The coupling was carried out for 5 days at 4°C. The gel was washed with 0.15 M NaCl and added to 10 ml of 0.2 M ethanolamine/HCl, pH 9.0. After 3 h at room temperature, the gel was washed three times with 0.1 M disodium hydrogen phosphate buffer (pH 11.0), three times with 0.1 M glycine/HCl buffer (pH 3.0), and stored in 0.1 M NaCl containing 15 mM sodium azide. Before use in the CART peptide purification from tissue extracts, an aliquot of the affinity gel was tested for its ability to bind recombinant CART(42–89).
Purification of CART Peptides.
Small affinity columns (3 × 10 mm) were equilibrated in PBS (pH 7.2), and the neutral tissue extracts were passed over the columns with a flow rate of 1 ml/min. The columns were washed with 30 ml of PBS, and the bound peptides were eluted with 2.5 ml of 50 mM glycine/HCl buffer (pH 2.5). To avoid cross contamination, new column material was used for each tissue extract. The eluates were concentrated to 0.25 ml by vacuum centrifugation and injected onto a Vydac 214TP54 reversed-phase C4 HPLC column (4.6 × 250 mm) equilibrated at 30°C at a flow rate of 1.0 ml/min with 0.1% (vol/vol) trifluoroacetic acid in 5% (vol/vol) acetonitrile. The concentration of acetonitrile in the eluting solvent was raised to 65% (vol/vol) over 30 min, and the absorbance was measured at 214 nm. Peptide material corresponding to UV peaks were collected and further analyzed.
Characterization of CART Peptides.
N-terminal amino acid sequences were determined by automated Edman degradations by using an Applied Biosystem Model 494 Protein Sequencer essentially as described by the manufacturer. By using an optimized system, it was possible to determine the partial sequence of as little as 300–500 fmol of peptide.
Mass spectrometry analysis was performed on a Voyager RP matrix-assisted laser desorption-ionization time of flight instrument (Perseptive Biosystems, Framingham, MA) equipped with a nitrogen laser (337 nm). The instrument was operated in linear mode with delayed extraction, and the accelerating voltage in the ion source was 25 kV.
Sample preparation was done as follows: 1 μl of sample solution was mixed with 1 μl of matrix solution [α-cyano-4-hydroxycinnamic acid dissolved in a 5:4:1 (vol/vol/vol) mixture of acetonitrile/water/3% (vol/vol) trifluoroacetic acid], and 1 μl was deposited on the sample plate and allowed to dry. Calibration was performed by using external standards, and the accuracy of the mass determinations was within 0.1%.
RESULTS
Characterization of MCA to CART.
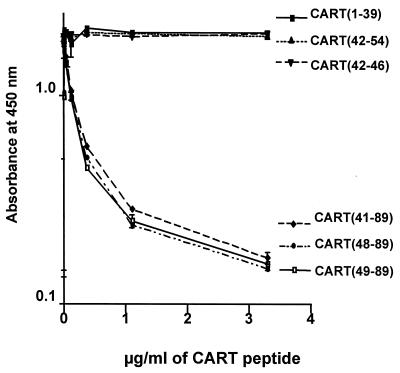
The MCA reacted equally well with the CART(41–89), CART(48–89), and CART(49–89) peptides, all of which were produced in yeast (4) (Fig. 1). To further clarify the epitope to which the MCA binds, we displaced binding of the antibody to CART(41–89) with the synthetic peptides CART(1–39), CART(42–54), and CART(42–46). As expected, the CART MCA did not react with the synthetic peptides from the N-terminal end of the CART(1–89) molecule (Fig. 1), indicating that the C-terminal part of the molecule is necessary for binding to the MCA. Furthermore, reduced CART(42–89) did not displace the binding of the MCA to active CART(42–89), indicating that the three-dimensional structure, including the three disulfide bonds, is essential for binding.
Figure 1.
Displacement of MCA by CART peptides.
CART Peptides in Hypothalamus and Nucleus Accumbens.
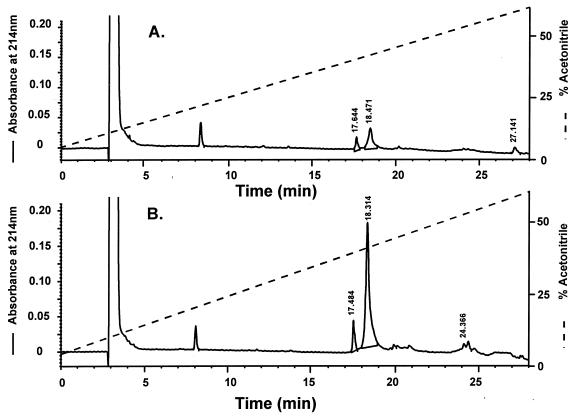
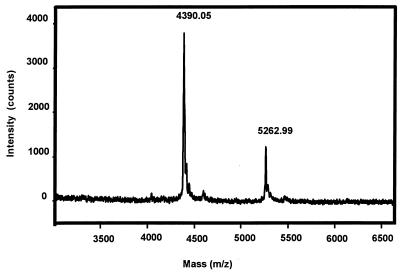
Fig. 2 shows the HPLC chromatograms of the CART peptides isolated from extracts of nucleus accumbens and hypothalamus, respectively. As can be seen from this figure, the total amount of CART peptides isolated from the hypothalamus is ≈7-fold higher than from nucleus accumbens (approximately the same amount of tissue was used). This is in good agreement with the relative intensities observed when comparing mRNA amounts based on the findings using in situ hybridization (Fig. 3). By sequence and mass spectrometry analysis, the two peptides eluting at 17.5–17.6 min and 18.3–18.5 min were found to correspond to CART peptides. The sequence analysis of the peptide eluting at 18.3 min from the hypothalamic extract is shown in Table 1, and the corresponding mass spectrum of this sample is shown in Fig. 4. From these sequence data, it can be seen that the peptide material eluting in this fraction is a mixture of CART(42–89) and CART(49–89). The molecular mass of rat CART(42–89) and CART(49–89), as calculated from the published amino acid sequence (1), is 5,259.3 and 4,387.2 Da, respectively. The molecular mass of the two hypothalamic CART peptides were found to be 5,263 ± 5 and 4,390 ± 4 Da, respectively (Fig. 4), in good agreement with the theoretical values. Sequencing and mass analysis of the peptides from nucleus accumbens also identified the CART(42–89) and CART(49–89) (results not shown).
Figure 2.
Reversed-phase HPLC on a Vydac 214TP54 column of partially purified CART peptides from nucleus accumbens (A) and hypothalamus (B).
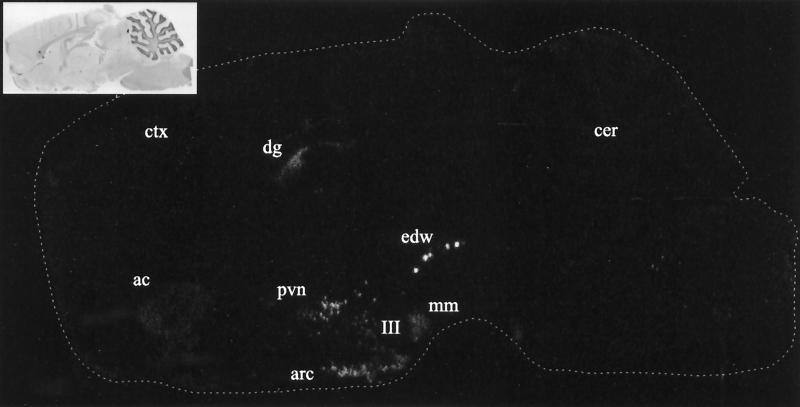
Figure 3.
Film image of saggital section from rat brain demonstrating the overall distribution of CART mRNA by in situ hybridization using 35S-labeled antisense RNA. Inset shows hematoxylin and eosin staining of the same section. ac, accumbens nucleus; arc, arcuate nucleus; cer, cerebellum; ctx, cerebral cortex; dg, dentate gyrus; edw, edinger-westphal nucleus; mm, medial mammary nucleus; III, third ventricle. Sections were exposed to film for 5 days.
Table 1.
Automated Edman degration of CART peptides from hypothalamus
| Cycle | CART (42–89) PTH-AA | Yield, pmol | CART (49–89) PTH-AA | Yield, pmol |
|---|---|---|---|---|
| 1 | Ile | 6 | Tyr | 11 |
| 2 | Pro | 4 | Gly | 10 |
| 3 | Ile | 4 | Gln | 11 |
| 4 | Tyr | 4 | Val | 10 |
| 5 | Glu | 3 | Pro | 8 |
| 6 | Lys | 4 | Met | 8 |
| 7 | Lys | 5 | (Cys) | — |
| 8 | Tyr | 4 | Asp | 6 |
| 9 | Gly | 8 | Ala | 8 |
| 10 | Gln | 6 | Gly | 11 |
| 11 | Val | 4 | Glu | 4 |
| 12 | Pro | 6 | Gln | 8 |
| 13 | Met | 3 | (Cys) | — |
| 14 | (Cys) | — | Ala | 7 |
| 15 | Asp | 5 | Val | 5 |
| 16 | Ala | 5 | Arg | 10 |
| 17 | Gly | 8 | Lys | 7 |
| 18 | Glu | 2 | Gly | 10 |
| 19 | Gln | 5 | Ala | 8 |
| 20 | (Cys) | — | Arg | 12 |
| 21 | Ala | 6 | Ile | 5 |
| 22 | Val | 4 | Gly | 11 |
| 23 | Arg | 12 | Lys | 7 |
| 24 | Lys | 7 | Leu | 7 |
| 25 | Gly | 9 | (Cys) | — |
| 26 | Ala | 5 | Asp | 7 |
| 27 | Arg | 12 | (Cys) | — |
| 28 | Ile | 3 | Pro | 7 |
| 29 | Gly | 9 | Arg | 13 |
| 30 | Lys | 5 | Gly | 10 |
| 31 | Leu | 5 | Thr | 4 |
| 32 | (Cys) | — | Ser | 5 |
| 33 | Asp | 6 | (Cys) | — |
| 34 | (Cys) | — | Asn | 2 |
| 35 | Pro | 6 | Ser | 5 |
| 36 | Arg | 12 | Phe | 4 |
| 37 | Gly | 8 | Leu | 4 |
| 38 | Thr | 3 | Leu | 4 |
| 39 | Ser | 4 | Lys | 4 |
| 40 | (Cys) | — | (Cys) | — |
| 41 | Asn | 2 | Leu | 4 |
| 42 | Ser | 4 | ||
| 43 | Phe | 3 | ||
| 44 | Leu | 4 | ||
| 45 | Leu | 4 | ||
| 46 | Lys | 4 | ||
| 47 | (Cys) | — | ||
| 48 | Leu | 3 |
PTH, phenylthiohydration. Cysteine residues (Cys) were not identified.
Figure 4.
Mass spectrum of purified CART peptides from hypothalamic tissue. The two peaks corresponds to CART(49–89) (theoretical mass = 4,387.2 Da) and CART(42–89) (theoretical mass = 5,259.2 Da), respectively.
CART Peptides in the Pituitary Gland.
Although the amount of pituitary tissue extracted (0.31 g anterior lobe and 0.11 g intermediate and posterior lobe) was considerably less than the corresponding nucleus accumbens and hypothalamic tissue (both ≈2.7 g), the total amount of CART peptides purified from each tissue was approximately the same. In contrast to the brain sections, only one CART peptide was isolated from each lobe of the pituitary gland; from the anterior lobe, CART(42–89) was identified, and from the neurointermediate lobe, CART(49–89) was isolated (results not shown).
CART Peptides in Adrenal Glands.
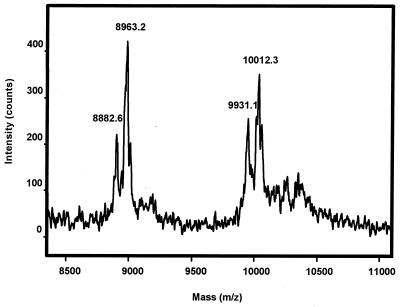
CART peptides from the adrenal gland extract also were subjected to affinity purification followed by HPLC using the same system as described for the brain extracts. The HPLC chromatogram (not shown) revealed a single broad peak eluting at a retention time of 20.8 min, thus significantly different from the brain and pituitary extracts. A partial amino acid sequence analysis on peptide material corresponding to this peak identified the sequence of: Ala-Leu-Asp-Ile-Tyr-Ser-Ala-Val-Asp-Asp-, corresponding to CART(10–89). The corresponding mass spectrum obtained on this fraction is shown in Fig. 5. The calculated mass of the identified CART(10–89) is 8,882.4 Da. In the mass spectrum, two peaks corresponding to 8,882.6 and 8,963.2 Da are found (Fig. 5). The first of these peaks corresponds to CART(10–89), whereas the latter peak seems to correspond to CART(10–89) with an unknown posttranslational modification of 80.6 mass units. In the high molecular end of the spectrum, the mass 9,931.1 corresponds to CART(1–89) with an N-terminal pyrGlu. The blocked N terminus also explains why this form does not show up on the sequence analysis. Also, the CART(1–89) exists with an additional unknown posttranslational modification of 81.2 mass units. Thus, in the adrenal gland, two forms of CART have been found, CART(1–89) and CART(10–89). Each of these forms exists with or without an additional posttranslational modification of ≈80 mass units. Because of the small amount of peptide material purified, it has not been possible to further characterize this modification; however, both phosphorylation (on a Ser, Thr or Tyr residue) or sulfation (on a Tyr residue) would fit with the observed data.
Figure 5.
Mass spectrum of purified CART peptides from adrenal glands. The two peaks correspond to CART(10–89) (theoretical mass = 8,882.4 Da) and CART(1–89) with an N-terminal pyrGlu (theoretical mass = 9,931.6 Da). Both peaks are accompanied by a larger peak representing an additional unknown modification of ≈80 mass units.
CART Processing.
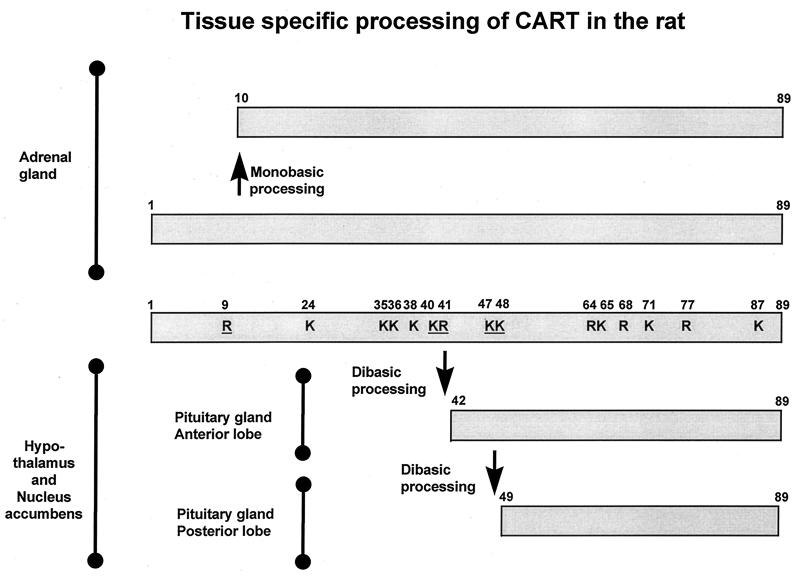
In Fig. 6, the different CART peptides isolated and characterized in the present study have been summarized. In the adrenal gland, CART(1–89) and CART(10–89) were found. The latter of these forms might be generated by a proline-directed arginyl cleavage at the Pro8–Arg9 sequence of CART(1–89), a well known cleavage site in many other peptide precursors (13). In both hypothalamus and nucleus accumbens, two forms of CART were found: CART(42–89) and CART(49–89). Both of these forms are generated as a result of processing at dibasic sequences, in this case at the Lys40–Arg41 and Lys47–Lys48 of the CART(1–89) precursor. Dibasic processing sites are also found in proinsulin (14, 15), proglucagon (16), and a long series of other peptide hormone precursors. The same two forms of CART were found in the pituitary gland, but interestingly, only the CART(42–89) was found in the anterior lobe, and only the CART(49–89) form was found in the posterior lobe.
Figure 6.
Schematic representation of the tissue-specific processing of CART in the rat. The scheme is based on the amino acid sequence deduced from rat cDNA (3) and the CART peptides identified in the present study. The lysine and arginine residues are indicated by K and R, and the underlined residues represent the identified mono- and dibasic processing sites.
DISCUSSION
By using in situ hybridization and immunohistochemistry techniques, CART mRNA and immunoreactivity have been localized in a series of different tissues from several species (3, 5, 6, 8–10, 17). In the present study, we have purified the CART peptides present in these different tissues and organs from the rat and determined the actual structure of the peptides and thus elucidated some of the posttranslational processing pathways of the CART precursor. The power of the affinity purification technique in combination with high-sensitivity mass spectrometry and sequencing is illustrated by the fact that it has been possible to isolate and characterize the CART peptides by using tissue from as little as 18 rats. The only previous study in which a naturally occurring CART peptide was isolated goes back to 1981, where Spiess and coworkers (7) used hypothalamic tissue from 490,000 sheep. The results obtained, however, are limited to the techniques used, and because the crucial step in the isolation is an affinity purification, only CART peptides recognized by the MCA will be isolated. Because previous studies (2, 4) have shown that the C-terminal part of the CART precursor, containing the three disulfide bridges, is biologically active, the MCA was prepared by immunization with the CART(41–89) fragment. As expected, this antibody recognizes CART peptides with intact C-terminal but not N-terminal fragments of the precursor e.g., CART(1–39) (Fig. 1).
The proteolytic processing of the CART precursor was studied both in central and in peripheral tissues from the rat. The CART precursor was found to be processed differently in central and peripheral sites, but differences in processing patterns also were observed between the two peripheral tissues studied, the adrenal gland and the anterior lobe of the pituitary gland.
In the adrenal gland, the full-length unprocessed CART precursor and CART(10–89) are present in a 7:1 ratio. Cleavage of unprocessed CART to CART(10–89) takes place on the C-terminal side of a single arginine in position nine. N-terminal to this basic residue, a proline residue is present, thus adding this processing site to the group of proline-directed arginine-processing sites described by Schwartz (13). Both CART(1–89) and CART(10–89) were found to exist in two forms with and without an unknown posttranslational modification of ≈80 mass units. As this heterogeneity was absent in CART(42–89) and CART(49–89), this modification is most likely associated with one of the residues in position 10–42. These 80 mass units could correspond to a sulfation or a phosphorylation. Examining the sequence from positions 10 to 42 identifies Ser39 as the most likely site for a phosphorylation, because this residue is surrounded by five basic residues (18). However, applying the rules of Tyr sulfation (28) suggests that sulfation of Tyr14 may also be a possibility.
None of the potential dibasic cleavage sites are processed in the adrenal gland CART. This is in contrast to the findings from the anterior pituitary lobe, where the precursor is efficiently cleaved after Lys40–Arg41, resulting in CART(42–89). This is the only processing product that can be identified in the anterior lobe, demonstrating that the anterior lobe completely spares the Lys47–Lys48 sequence from processing. This lack of processing at the second Lys–Lys in the anterior lobe is in agreement with the processing pattern of other hormone precursors in the anterior lobe, e.g., proopiomelanocortin processing (19). Of the specific dibasic prohormone convertases (PCs), only PC1 (also called PC3) is easily detectable in the anterior lobe (20, 21) demonstrating that also in the CART precursor, the Lys–Lys sequence is a poor substrate for PC1. A similar differentiation in processing between Lys–Arg and Lys–Lys cleavage sites in the proopiomelanocortin precursor has also been observed in an anterior lobe-derived cell line, AtT-20 (22, 23). Like the cells of the anterior lobe, these cells mainly express PC1 and very low (or nondetectable) levels of PC2 (24, 25, 26).
In the hypothalamus and nucleus accumbens, two forms of CART is identified, CART(42–89) and CART(49–89), showing that cleavage occurs both after Lys40–Arg41 and Lys47–Lys48 in these tissues. From the present data, it is not possible to determine whether there are two subpopulations of CART-expressing neurons, one containing CART solely cleaved after Lys40–Arg41 and one capable of additional cleavage after Lys47–Lys48, or whether the CART-expressing neurons express both forms of CART. An immunohistochemical study using antibodies specific for the two different CART forms is needed to answer this question.
Only CART(49–89) is found in the extracts from the intermediate and posterior lobe of pituitary. Examination of both mRNA and protein expression (8, 17) has shown that CART is not expressed in the intermediate lobe of the pituitary gland. This observation indicates that the CART-expressing hypothalamic neurons that project to the posterior lobe all cleave efficiently at Lys47–Lys48. The identity of the processing enzyme responsible for the processing at Lys47–Lys48 is unknown. Both PC1 and PC2 are expressed in a number of nuclei in the hypothalamus (21, 27). Furthermore, a high degree of coexpression between PC2 and the posterior lobe hormones vasopressin and oxytocin has been demonstrated (27), and PC2 could be a possible candidate for cleavage at this site. However, other processing enzymes, e.g., PC5, could also be involved, and colocalization studies with CART and the processing enzymes is needed in order resolve this question.
Within the CART precursor, other dibasic sequences are found, and because of the limitations of the techniques used, it is not possible to determined whether further processing of the N-terminal part of CART [CART(1–39)] takes place in the neuroendocrine cells studied (pituitary, hypothalamus, and accumbens). An additional dibasic site in found in the C-terminal part of CART, Arg64–Lys65. This site, however, is positioned in the cysteine-rich region with disulfide bridges, and is probably not available as a cleavage site.
The CART field is relatively new, and only few reports about its biological activity have been published. Presently, no data are available to support a biological significance of the differential processing reported in the present study. Future biological studies with a panel of different CART fragments will hopefully answer this question.
ABBREVIATIONS
- CART
cocaine and amphetamine regulated transcript
- MCA
monoclonal antibody from clone Ca6-1F4D4
- PC
prohormone convertase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Campfield L A, Smith F J, Burn P. Science. 1998;280:1383–1387. doi: 10.1126/science.280.5368.1383. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen P, Judge M E, Thim L, Ribel U, Christjansen K N, Wulff B S, Clausen J T, Jensen P B, Madsen O D, Vrang N, et al. Nature (London) 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 3.Douglass J, McKinzie A A, Couceyro P. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thim L, Nielsen P F, Judge M E, Andersen A S, Diers I, Egel-Mitani M, Hastrup S. FEBS Lett. 1998;428:263–268. doi: 10.1016/s0014-5793(98)00543-2. [DOI] [PubMed] [Google Scholar]
- 5.Gautvik K M, deLecca L, Gautvik V K, Danielson P E, Tranque P, Dopazo A, Bloom F E, Sutcliffe J G. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglass J, Daoud S. Gene. 1996;169:241–245. doi: 10.1016/0378-1119(96)88651-3. [DOI] [PubMed] [Google Scholar]
- 7.Spiess J, Villareal J, Vale W. Biochemistry. 1982;1981:1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- 8.Koylu E O, Couceyro P R, Lambert P D, Ling N C, DeSouza E B, Kuhar M J. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- 9.Koylu E O, Couceyro P R, Lambert P D, Kuhar M C. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- 10.Smith Y, Koylu E O, Couceyro P, Kuhar M J. Synapse. 1997;27:90–94. doi: 10.1002/(SICI)1098-2396(199709)27:1<90::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen P, Eriksen J, Danø K. Scand J Clin Lab Invest. 1990;50:229–245. [Google Scholar]
- 12.Taggart R T, Samloff I M. Science. 1983;219:1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz T W. FEBS Lett. 1986;200:1–10. doi: 10.1016/0014-5793(86)80500-2. [DOI] [PubMed] [Google Scholar]
- 14.Steiner D F, Cunningham D D, Spigelman L, Aten B. Science. 1967;157:697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- 15.Chance R E, Ellis R M, Bromer W W. Science. 1968;161:165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- 16.Tager H S, Steiner D F. Proc Natl Acad Sci USA. 1973;70:2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couceyro P R, Koylu E O, Kuhar M J. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- 18.Aitken A. In: Identification of Protein Consensus Sequences. Aitken A, editor. New York: Ellis Horwood; 1990. pp. 35–53. [Google Scholar]
- 19.Eipper B A, Mains R E. Endocr Rev. 1980;1:1–29. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Seidah N G, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei M G, Lazure C, Mbikay M, Chretién C. Mol Endocrinol. 1991;5:111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- 21.Steiner D F. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 22.Mains R E, Eipper B A. J Biol Chem. 1976;251:4115–4120. [PubMed] [Google Scholar]
- 23.Akil H M, Watson S J, Young E, Lewis M E, Kachaturian H, Walker J M. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- 24.Seidah N G, Gaspar L, Mion P, Marcinkiewicz M, Mbikay M, Chrétien M. DNA Cell Biol. 1990;9:415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- 25.Smeekens S P, Avruch A S, LaMendola J, Chan S J, Steiner D S. Proc Natl Acad Sci USA. 1991;88:340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wulff B S, Johansen T E, Dalbøge H, O’Hare M M T, Schwartz T W. J Biol Chem. 1993;268:13327–13335. [PubMed] [Google Scholar]
- 27.Dong W, Seidel B, Marcinkiewicz M, Chrétien M, Seidah N G, Day R. J Neurosci. 1997;15:563–575. doi: 10.1523/JNEUROSCI.17-02-00563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hortin G, Folz R, Gordon J I, Strauss A W. Biochem Biophys Res Commun. 1986;141:326–333. doi: 10.1016/s0006-291x(86)80372-2. [DOI] [PubMed] [Google Scholar]