Abstract
Binding enhancement by tertiary interactions is a strategy that takes advantage of the higher order folding of functionally important RNAs to bind short nucleic acid-based compounds tightly and more specifically than possible by simple base pairing. For example, tertiary interactions enhance binding of specific hexamers to a group I intron ribozyme from the opportunistic pathogen Pneumocystis carinii by 1,000- to 100,000-fold relative to binding by only base pairing. One such hexamer, d(AnTnGnAnCn)rU, contains an N3′ → P5′ phosphoramidate deoxysugar–phosphate backbone (n) that is resistant to chemical and enzymatic decay. Here, it is shown that this hexamer is also a suicide inhibitor of the intron’s self-splicing reaction in vitro. The hexamer is ligated in trans to the 3′ exon of the precursor, producing dead-end products. At 4 mM Mg2+, the fraction of trans-spliced product is greater than normally spliced product at hexamer concentrations as low as 200 nM. This provides an additional level of specificity for compounds that can exploit the catalytic potential of complexes with RNA targets.
Most human therapeutics have been discovered by screening natural products. Synthetic organic chemistry has made it possible to synthesize such natural products and derivatives thereof in large quantities, thus broadening the range of compounds that can be used clinically (1–3). Synthetic methodology coupled with the outpouring of protein structural information has also allowed rational design of completely new therapeutic compounds (4, 5). Similarly, the recent explosion in nucleic acid sequence information is providing a knowledge base for structure-based targeting of RNA. The first generation of such therapeutics consists of antisense nucleic acids that bind mRNA through Watson-Crick base pairing and thereby regulate translation (6, 7). Because of the long sequences employed, typically 15–20 nucleotides, potential disadvantages include high cost of synthesis (8) and lack of specificity (9). Cost of synthesis can be reduced and specificity increased by designing short antisense agents whose binding to RNA targets is enhanced by tertiary interactions (10, 11). Here we show that one such hexanucleotide can also be a suicide inhibitor (12, 13) of RNA function. This provides an additional design principle for increasing specificity of compounds that can exploit the catalytic potential of complexes with RNA targets.
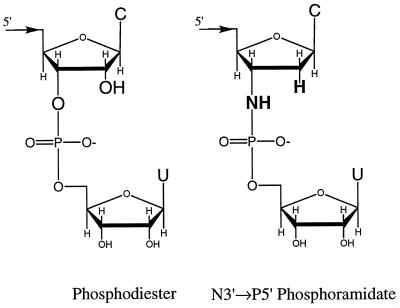
Our model system is a large-subunit ribosomal RNA (rRNA) precursor from the opportunistic pathogen Pneumocystis carinii, which is a common cause of death in immunocompromised patients (14, 15). The rRNA precursor contains a group I self-splicing intron (10, 16) that provides a potential therapeutic target (16, 17) because self-splicing is required for assembly of active ribosomes (18). Hexamers that mimic this intron’s 5′ exon can bind to the catalytic core of a ribozyme derived from the intron as much as 100,000-fold more tightly than expected if the hexamers bound by simple base pairing (10). Much of this binding enhancement by tertiary interactions (BETI) can be retained when these hexamers are modified to be resistant to nuclease degradation (11). In particular, d(AnTnGnAnCn)rU with N3′ → P5′ phosphoramidate (19) linkages (Fig. 1) binds 2,000-fold more tightly than expected for base pairing.
Figure 1.
The 3′ end of the 5′ exon (phosphodiester) after the first step of splicing (see B1 in Fig. 2), and of the phosphoramidate hexamer, d(AnTnGnAnCn)rU. The atomic substitutions are shown in bold. C, cytosine; U, uracil.
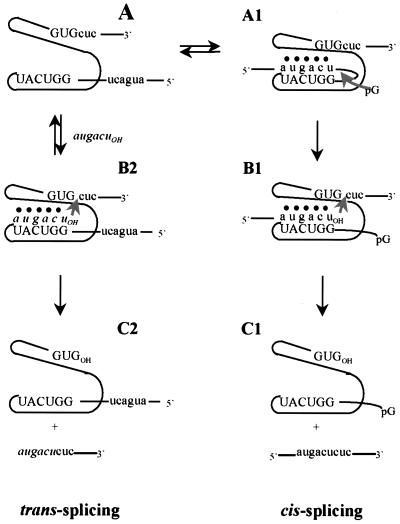
In this report, we show that d(AnTnGnAnCn)rU is ligated to the 3′ exon of a truncated ribosomal RNA precursor in a reaction that mimics the second step of splicing (Fig. 2). At Mg2+ concentrations lower than that required for optimal self-splicing in vitro, this trans-splicing can compete with the natural cis-splicing. Such trans-splicing produces dead-end products that would not lead to functional RNA. Thus, an oligonucleotide that is resistant to chemical and nuclease degradation can act as a suicide inhibitor of an RNA-catalyzed reaction in vitro. This suggests a strategy for enhancing specificity in targeting some RNAs.
Figure 2.
Schematic representation of the self-splicing (cis-splicing) and trans-splicing reactions. The self-splicing reaction follows the pathway A → A1 → B1 → C1. The trans-splicing reaction follows the pathway A + augacuOH → B2 → C2. Steps B → C are shown as irreversible because of the low concentration of the spliced products. The upper case letters and intervening line represent the group I intron; the lower case letters and terminal lines represent the 5′ and 3′ exons; the italicized lower case letters represent the exogenous N3′ → P5′ phosphoramidate hexanucleotide; ● represents tertiary interactions with the intron’s catalytic core. The internal guide sequence is 5′-GGUCAU-3′. The reaction product monitored depends on the position of the radiolabel; 5′ labeled hexamer monitors the trans-spliced products, internal labeled precursor monitors products containing intron, and 3′ end-labeled precursor monitors products containing 3′ exon.
MATERIALS AND METHODS
DNA and RNA Synthesis and Purification.
The truncated P. carinii rRNA precursor (P-h), the derived ribozyme (P-8/4x), and hexanucleotides were synthesized and purified essentially as described (10, 20). Hexamers were 5′ end radiolabeled, and the P-h rRNA precursor was internally radiolabeled as described (10).
The P-h rRNA precursor was 3′-end radiolabeled by incubating 1 μM [5′-32P]pCp, 440 nM P-h RNA transcript, 10 mM MgCl2, 5 μM ATP, 3 mM DTT, 250 ng of BSA, 50 mM Hepes (pH 8.3), and 30 units of T4 RNA ligase in a total volume of 25 μl for 5 h at 22°C. The reaction mixture was passed through a Chromaspin G100 size-exclusion spin column (CLONTECH) to remove unincorporated [5′-32P]pCp, and then added to 12.5 μl of 2× stop buffer (10 M urea, 3.1 mM EDTA, 10 mM Tris, and 9 mM boric acid at pH 8.4) and 2 μl glycerol. The labeled precursor was purified on a 5% polyacrylamide, 8M urea denaturing gel. The precursor band was excised from the gel and eluted by pulverizing at room temperature overnight in 1 ml sterile water with a sterile stir bar (the spin-soak procedure). The resultant solution was spin-filtered (Isolab) to remove gel particulate and ethanol precipitated twice to remove residual salts and urea.
Inhibition of Self-Splicing.
Reactions were conducted in HxMg buffer consisting of 50 mM Hepes (25 mM Na+), 135 mM KCl, and x mM MgCl2 at pH 7.5, where x refers to the amount of MgCl2 in mM in the buffer (listed in the figures). For splicing reactions conducted with internally radiolabeled precursor RNA, about 180 nM RNA was annealed by heating at 55°C for 5 min in the appropriate buffer in a volume of 3 μl and then slow-cooling to 37°C. A 3-μl solution of buffer at 37°C containing either 2 mM pG and/or 60 μM d(AnTnGnAnCn)rU (or neither) was added and allowed to react for 1 h at 37°C. An equal volume of 2× stop buffer was added, and the products and reactants were separated on a 5% acrylamide, 8 M urea gel. To check sequence specificity, the self-splicing reaction was conducted with the control oligonucleotide d(CnAnGnTnAn)rU as above by using H2Mg buffer and 1 mM pG, conditions that maximize production of the 5′ exon–intron band with d(AnTnGnAnCn)rU. Gels were dried under vacuum, and the bands were quantified on a Molecular Dynamics PhosphorImager. The intensity of each band was corrected for the number of adenines in each sequence. A final concentration of 1 mM pG was used in these assays because 3 mM pG, although resulting in marginally more spliced product (11), also doubles the amount of 5′ exon–intron hydrolysis product (data not shown).
The fate of the hexamers, d(AnTnGnAnCn)rU and d(CnAnGnTnAn)rU, also was analyzed by using radiolabeled hexamer and unlabeled precursor in the presence and absence of pG cofactor. Approximately 300 nM unlabeled P-h precursor was annealed by heating at 55°C in 3 μl of the appropriate buffer for 5 min and slow-cooling to 37°C. A 3-μl solution of 8 nM 5′-end radiolabeled hexamer in the same buffer at 37°C was added, and the reaction was allowed to proceed for 1 h. The reaction was quenched by the addition of 6 μl of 2× stop buffer, and the reactants and products were separated on a 10% polyacrylamide, 8 M urea gel. The gel was dried under vacuum, and the bands were quantified with a Molecular Dynamics PhosphorImager.
To directly monitor the cis- and trans-spliced products, the self-splicing reaction was analyzed as a function of d(AnTnGnAnCn)rU concentration by using 3′-end radiolabeled P-h precursor. The reaction was conducted essentially as described by using internally radiolabeled precursor RNA, except that the concentration of d(AnTnGnAnCn)rU ranged from 10 nM to 30 μM, the concentration of precursor was approximately 5 nM, and H4Mg buffer was used. Bands were identified by their migration relative to the precursor, the 3′ exon hydrolysis product, and the properly spliced product bands (the latter two determined in the absence of added hexamer).
P-8/4x Ribozyme Binding Assays.
The dissociation constant for d(AnTnGnAnCn)rU binding to the P-8/4x ribozyme was determined by direct band-shift PAGE assays by using H15Mg, H5Mg, H4Mg, and H3Mg as the binding and electrophoresis buffers (10). In these assays, 6.56 μl of serially diluted P-8/4x ribozyme at concentrations ranging from 0.005 to 1.5 μM in the appropriate buffer with 3.4% (vol/vol) glycerol were incubated at 55°C for 5 min and then slowly cooled to 37°C. Approximately 8 nM 32P-radiolabeled 5′ exon mimic in 0.94 μl of the appropriate buffer at 37°C was added, and the solution was allowed to equilibrate for 90 min. The fraction of mimic bound was partitioned from unbound on a 37°C, 10% native polyacrylamide gel, which was made with the same buffer as the binding buffer. The gel was then dried under vacuum, and the bands were quantified with a Molecular Dynamics PhosphorImager. Dissociation constants were calculated as described (10).
RESULTS
Reactivity of Internally Radiolabeled Precursor as a Function of Mg2+ Concentration [Mg2+].
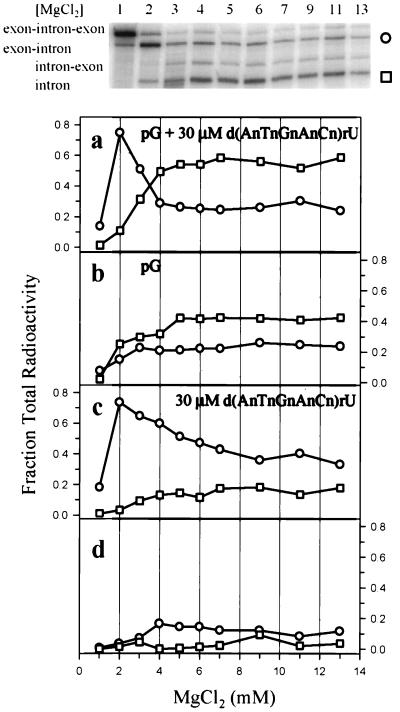
The effects of [Mg2+] on the formation of various products derived from internally radiolabeled precursor RNA in the presence and absence of 30 μM d(AnTnGnAnCn)rU and 1 mM pG are shown in Fig. 3. Fig. 3a shows that in the presence of d(AnTnGnAnCn)rU and pG, the 5′ exon–intron product reaches a maximum at 2 mM Mg2+, where it is 7.5-fold more prevalent than the completely excised intron product. Mg2+ concentrations higher than 3 mM, however, result in a predominance of completely excised intron. The 5′ exon–intron product could arise from either trans-splicing of d(AnTnGnAnCn)rU (Fig. 2) or hydrolysis of the precursor at the intron-3′ exon junction.
Figure 3.
Magnesium dependence of products from internally radiolabeled precursor. Reactions were run for 1 h in HxMg buffer consisting of 50 mM Hepes (25 mM Na+) at pH 7.5, 135 mM KCl, and x mM MgCl2, where x is listed below the plots and above the corresponding lanes of the gel. The gel shows a typical reaction with 1 mM pG and 30 μM d(AnTnGnAnCn)rU. Each plot is the average of two independently run assays, and the error of each point is typically ±6% of the average value. Circles represent the 5′ exon–intron product generated by either trans-splicing or intron–3′ exon junction hydrolysis. Squares represent the intron products formed either by splicing or by hydrolysis at both the 5′ exon–intron and intron–3′ exon junctions. Results are shown in the presence of 1 mM pG and 30 μM d(AnTnGnAnCn)rU (a), in the absence of added hexamer (b), in the absence of pG and in the presence of 30 μM d(AnTnGnAnCn)rU (c), and in the absence of pG and d(AnTnGnAnCn)rU (d).
Fig. 3b shows results in the presence of 1 mM pG and absence of d(AnTnGnAnCn)rU. When [Mg2+] ≥ 4 mM, the fraction of 5′ exon–intron band is the same in the presence (Fig. 3a) and absence (Fig. 3b) of d(AnTnGnAnCn)rU. Thus, when [Mg2+] ≥ 4 mM, the 5′ exon–intron band is likely caused by hydrolysis at the intron–3′ exon junction. At 2 and 3 mM Mg2+, however, much more 5′ exon–intron product is formed in the presence of d(AnTnGnAnCn)rU, suggesting it results either from the trans-splicing reaction or from oligonucleotide-induced hydrolysis at the intron–3′ exon junction. Either mechanism results in the formation of oligonucleotide-dependent dead-end products. At 2 mM Mg2+, the fraction of completely excised intron decreases by a factor of 2.5 on adding 30 μM d(AnTnGnAnCn)rU (compare Fig. 3 a and b), suggesting that the dead-end products are, at least in part, being formed at the expense of completely excised intron. Surprisingly, the fraction of completely excised intron is almost 0.6 when [Mg2+] ≥ 5 mM in the presence of 30 μM d(AnTnGnAnCn)rU and 1 mM pG, while it is only 0.4 when pG is present in the absence of d(AnTnGnAnCn)rU. One possible reason for this is that d(AnTnGnAnCn)rU promotes hydrolysis at both the 5′ exon–intron and intron–3′ exon junctions, thus releasing intron. Fig. 3c shows that in the presence of d(AnTnGnAnCn)rU and absence of pG, the fraction of excised intron approaches 0.2 at high [Mg2+]. When added to the 0.4 fraction generated in the presence of pG and absence of d(AnTnGnAnCn)rU, this can account for the fraction observed in the presence of both pG and d(AnTnGnAnCn)rU.
Fig. 3c also shows that in the absence of pG and presence of 30 μM d(AnTnGnAnCn)rU, the 5′ exon–intron product maximizes at 2 mM Mg2+. Evidently, formation of this product does not depend on pG. At 3 mM ≤ [Mg2+] ≤ 7 mM in the presence of 1 mM pG and 30 μM d(AnTnGnAnCn)rU, completely excised intron product is generated at the expense of the 5′ exon–intron product (Fig. 3a).
Fig. 3d shows that in the absence of pG and d(AnTnGnAnCn)rU, hydrolytic production of the 5′ exon–intron band at 2 mM Mg2+ is about 1/10 that in the presence of d(AnTnGnAnCn)rU. This is further evidence that the large production of this band is not the result of simple hydrolysis at the intron–3′ exon splice junction.
The above results indicate that d(AnTnGnAnCn)rU interferes with self-splicing at 2–3 mM Mg2+ either by trans-splicing or by oligonucleotide-induced hydrolysis at the intron–3′ exon junction or both. An increase in the 5′ exon–intron product at 2 mM Mg2+ does not occur on adding up to 30 μM of the control hexamer d(CnAnGnTnAn)rU instead of d(AnTnGnAnCn)rU (data not shown), suggesting the effects are dependent on sequence complementarity between the oligonucleotide and the intron’s internal guide sequence (Fig. 2).
In contrast to the results at 37°C, at 50°C the 5′ exon–intron product predominates in H15Mg buffer (data not shown), suggesting the 5′ exon–intron product is favored under conditions that are expected to destabilize the group I intron structure.
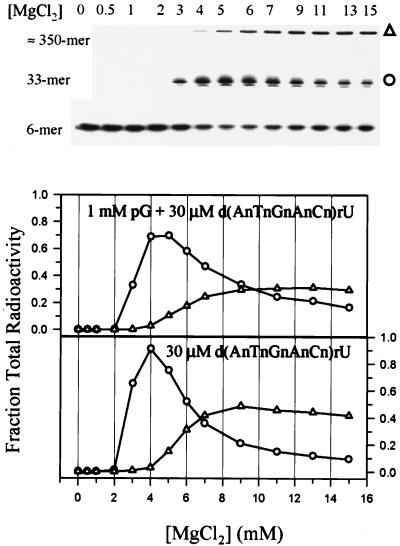
Reactivity of 5′ End Radiolabeled Hexamer as a Function of [Mg2+].
To directly monitor the trans-splicing product, 5′ radiolabeled d(AnTnGnAnCn)rU or d(CnAnGnUnAn)rU was added to solutions of unlabeled precursor. Fig. 4 shows that the 5′ exon mimic, d(AnTnGnAnCn)rU, is incorporated into two products; one is 33 nt in length corresponding to the expected trans-spliced product, and one is ≈350 nt. Formation of the trans-spliced product in the presence of pG is maximal at 4–5 mM Mg2+ and then gradually decreases with increasing Mg2+ (Fig. 4, Upper plot), which corresponds to a gradual increase in formation of the 350-mer product. This trend also holds when the assay is conducted in the absence of pG (Fig. 4, Lower plot), indicating that pG is not required for formation of either product. The control, d(CnAnGnTnAn)rU, is not reactive in 0, 2, 3, or 15 mM Mg2+, as expected, showing that the reactions are sequence-dependent.
Figure 4.
Magnesium dependence of trans-splicing with 4 nM 5′ end-labeled d(AnTnGnAnCn)rU and 150 nM unlabeled precursor in the presence (Upper) and absence (Lower) of pG. The gel corresponds to the Upper plot. Reactions were run for 1 h in HxMg buffer, consisting of 50 mM Hepes (25 mM Na+) at pH 7.5, 135 mM KCl, and x mM MgCl2, where x is listed below the plot and above the corresponding lanes of the gel. ○ represents the hexamer-3′ exon trans-spliced product at 33 nt, and ▵ represents the unidentified ≈350-nt product.
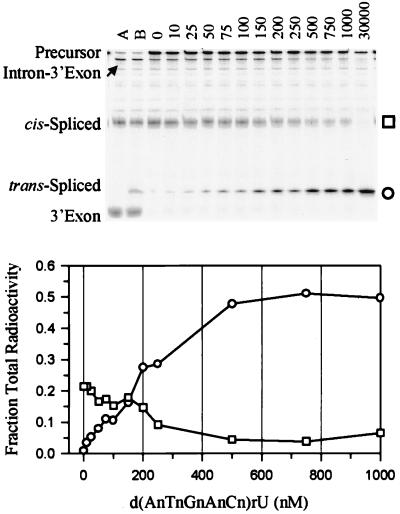
Reactivity of 3′ End Radiolabeled Precursor as a Function of d(AnTnGnAnCn)rU Concentration at 4 mM Mg2+.
To directly monitor both cis- and trans-splicing products, a 3′ end radiolabeled precursor was used. With 3′ end radiolabeled precursor, Mg2+-dependent trans-splicing reaches a plateau at 4 mM Mg2+ (data not shown). Therefore, the dependence of d(AnTnGnAnCn)rU concentration on trans-splicing with 3′ end radiolabeled precursor was analyzed at 4 mM Mg2+ (Fig. 5). The second-fastest migrating band in Fig. 5 is the 33-mer trans-spliced product, and its formation depends on oligonucleotide concentration, as expected. Note that the 27-mer 3′ exon hydrolysis product is distinguishable from the 33-mer trans-spliced product, and its formation is independent of oligonucleotide concentration. The trans-spliced product plateaus at about 500 nM d(AnTnGnAnCn)rU, and the amount of trans-spliced product is greater than the amount of properly spliced product at concentrations of d(AnTnGnAnCn)rU as low as 200 nM. Because the Kd for d(AnTnGnAnCn)rU binding to the internal guide mimic, r(GGUCAU), is 34 μM (incorrectly reported as 340 μM in Table 1 of ref. 11) under the more stabilizing conditions of H15Mg buffer, the exon mimic is likely binding to the precursor at least partially through tertiary interactions. A trans-spliced product is not formed with up to 30 μM of the control oligonucleotide, d(CnAnGnTnAn)rU, indicating that the reaction is sequence-specific (data not shown).
Figure 5.
Oligonucleotide concentration dependence of self-splicing (cis-splicing) and trans-splicing. The gel corresponds to the plot. Reactions consisted of approximately 6 nM 3′ end radiolabeled precursor, 1 mM pG, H4Mg buffer, and various concentrations of d(AnTnGnAnCn)rU (listed in nM above each corresponding lane of the gel). ○ represents the hexamer-3′ exon trans-spliced product and squares represent the 5′ exon–3′ exon cis-spliced product. The fractions of the trans-spliced and cis-spliced products are 0.74 and 0.01 at 30 μM d(AnTnGnAnCn)rU. Lane A contains the same assay as the other lanes, but conducted in H15Mg buffer and 3 mM pG, conditions that promote hydrolytic cleavage of the 3′ exon. Lane B additionally contains 1 μM d(AnTnGnAnCn)rU. Note that the 3′ exon (27-mer), which is a product of hydrolysis at the intron–3′exon junction, is distinguishable from the trans-spliced product (33-mer) on the gel.
Table 1.
McCl2 dependence of binding d(AnTnGnAnCn)rU and P-8/4x
| [MgCl2], mM | kd, nM |
|---|---|
| 3 | 175 |
| 4 | 94 |
| 5 | 99 |
| 15 | 31 (16)* |
Assays were run in HxMg buffer, consisting of 50 mM Hepes (25 mM Na+) at pH 7.5, 135 mM KCl, and x mM MgCl2. Each reported value is the average of at least two independent assays. The dissociation constant, Kd, was determined by direct band-shift gel electrophoresis.
Kd was determined by competition band-shift gel electrophoresis (11).
Mg2+ Dependence of d(AnTnGnAnCn)rU Binding to the P-8/4x Ribozyme.
To examine the effects of Mg2+ concentration on the binding of d(AnTnGnAnCn)rU to the catalytic core, binding was measured to the P-8/4x ribozyme, which is precursor truncated to remove 5′ and 3′ splice sites (11). The dissociation constant at 3 mM Mg2+ is roughly 2-fold and 6-fold larger than those at 4 or 5 and 15 mM Mg2+, respectively (Table 1).
DISCUSSION
P. carinii is one of a class of mammalian opportunistic pathogens that contain conserved group I introns (21). The functional importance of introns combined with their absence in mammalian hosts makes them potentially important targets for pharmacological intervention (17, 22). Hexamers that mimic the 5′ exon of a P. carinii ribosomal RNA group I intron bind tightly to a derived ribozyme through base pairing and tertiary interactions (10, 11). The ribozyme, however, lacks the 5′ exon sequence that is endogenous to the P. carinii ribosomal RNA precursor. Therefore, it was unknown whether mimics can compete with the endogenous 5′ exon for the 5′ exon-binding pocket (see Fig. 2).
The reactivity of the exogenous 5′ exon mimic d(AnTnGnAnCn)rU with precursor RNA indicates that the mimic binds to the catalytic core of the group I intron in the presence of the endogenous exons at about 2 mM Mg2+ for the assay with internally labeled precursor and about 4 mM Mg2+ for the other assays. Such Mg2+ concentrations are likely near physiological (23, 24). The difference in the Mg2+ dependence of trans-splicing between assays may be the result of structural heterogeneity of the precursor that differs because of different protocols for preparing unlabeled, internally radiolabeled, and 3′ end radiolabeled precursor. Such structural heterogeneity is commonly seen for pre-rRNA transcripts and ribozymes (21, 25–28). Attempts to produce more homogeneous solutions by using published protocols (26, 27) did not alter these results (data not shown). Nevertheless, at low Mg2+ concentrations, d(AnTnGnAnCn)rU is spliced in trans to the endogenous 3′ exon of the precursor in a reaction that mimics the second step of splicing (Figs. 2, 4, and 5), and this reduces the amount of properly spliced product. Thus, this hexamer is a suicide inhibitor of the self-splicing reaction. Formation of the trans-spliced product in vivo would result in an inability of the P. carinii ribosomal RNA and the ribosome to mature, and hence would be disruptive to P. carinii protein synthesis and propagation. Thus, d(AnTnGnAnCn)rU is a potential lead therapeutic against P. carinii pneumonia propagation.
As shown in Fig. 5, the fraction of precursor RNA that is trans-spliced is half-maximal at 200 nM d(AnTnGnAnCn)rU in 4 mM Mg2+. Evidently, at 4 mM Mg2+, d(AnTnGnAnCn)rU effectively competes with the intramolecular 5′ exon sequence for binding in the catalytic pocket. This is surprising because intramolecular binding has a considerable effective concentration advantage over bimolecular binding (29).
It was previously reported that the dinucleotide monophosphate CU in 5 mM Mg2+ at 30°C (30) and CCCCN (where N is U, C, or A) in 10 mM Mg2+ at 42°C (31) trans-splice with the natural Tetrahymena thermophila ribosomal RNA precursor in the absence of pG. Moreover, in 5 mM Mg2+ at 30°C in the presence of pG, the 3′ terminal end of the 5′ exon of the group I intron from T. thermophilia can base pair with upstream exon sequences, allowing exogenous 5′ exon mimics to bind the internal guide sequence and act as trans-splicing substrates (32). The 3′ end of the P. carinii 5′ exon can also form such an upstream structure, but the predicted thermodynamics suggest that it is significantly weaker than the structure with the 3′ end of the 5′ exon base pairing to the internal guide sequence (−7 vs. −3 kcal/mol; 1 cal = 4.18 J). Indeed, by using internally radiolabeled precursor, when [Mg2+] > 3 mM, the completely excised intron product predominates in the presence of pG and d(AnTnGnAnCn)rU, suggesting that under these conditions, formation of the intramolecular 5′ exon–internal guide sequence helix is favored (Figs. 2 and 3). With internally labeled precursor at 2–3 mM Mg2+, however, the internal guide sequence appears more accessible because more 5′ exon–intron product is formed than completely excised intron, as expected if trans-splicing predominates.
The internal guide sequence may be more accessible at low Mg2+ concentrations because the intron may not be completely or properly folded. Intracellular Mg2+ concentrations are often less than 2 mM (23, 24), and cases are known where proteins are required to stabilize or catalyze proper folding of group I introns (33). Thus, there may be windows of opportunity in the cell for exogenous oligonucleotides to bind the intron during transcription or before a chaperone/folding protein has trapped the intron into its active three-dimensional structure.
The N3′ → P5′ phosphoramidate linkages (Fig. 1) in d(AnTnGnAnCn)rU are resistant to chemical and nuclease degradation, a requirement for an effective therapeutic (20, 34–36). The results show that the oxygen to amino and 2′ OH to 2′ H functional group modifications (Fig. 1) permit both binding and trans-splicing. Moreover, the oligonucleotide effectively competes with the 5′ exon for binding the catalytic core at low Mg2+ concentrations. Therefore, N3′ → P5′ phosphoramidate linkages are potentially useful for designing oligonucleotides that target base pairing, tertiary interactions, and transition states inherent in functional RNAs. Other phosphoramidate oligonucleotides are known to bind tightly to RNA binding proteins (37). Evidently, phosphoramidates are able to mimic many of the properties of RNA that are important for molecular recognition.
Implications for Antisense Development.
These results show that the N3′ → P5′ phosphoramidate hexamer, d(AnTnGnAnCn)rU, binds in a sequence-specific manner to the P. carinii group I intron precursor and is an effective suicide inhibitor of the self-splicing reaction in vitro. The irreversibility of forming the dead-end inhibition products results in an increase in specificity relative to oligonucleotides that reversibly bind to the target.
Targeting group I introns as a general therapeutic strategy is attractive because of the advantages mentioned above and because these intron inhibitors require a minimum amount of design; only knowledge of the 5′ exon sequence is required, and then modifications for nuclease stability must be analyzed. Also, there is a need for more effective treatments against a variety of intron-containing pathogens (15), including the fungi Candida albicans (38) and Aspergillus nidulans (39). Furthermore, there are other functionally important RNA structures that might be targeted by exploiting tertiary interactions and reactivity with therapeutic oligonucleotides or with even simpler compounds containing a reactive group such as the cis-diol in d(AnTnGnAnCn)rU. Key elements in the success of such strategies are identification of functional structures, assessment of their specific biological activities, and understanding their structures to at least a low level of resolution. The enormous amount of sequence data being generated for RNAs will greatly expedite this process.
One potential limitation of this specific example is the relatively large amount of therapeutic that probably must be delivered to ribosomal RNA precursors, because this will be an abundant RNA. Also, designing suicide inhibitors requires catalytic potential between the RNA target and its bound therapeutic. Both of these limitations are likely to be obviated in coming years as new RNAs are identified as potential targets.
Acknowledgments
The authors thank Thomas W. Barnes, III and Matthew Disney for helpful discussions. This work was supported by National Institutes of Health Grant GM22939 to D.H.T. and National Institutes of Health postdoctoral fellowship GM17985 to S.M.T.
References
- 1.Gates M, Tschudi G. J Am Chem Soc. 1952;74:1109–1110. [Google Scholar]
- 2.Wipf P, Lim S. J Am Chem Soc. 1995;117:558–559. [Google Scholar]
- 3.Nicolaou K C, Yang Z, Shi G-Q, Gunzner J L, Agrios K A, Gartner P. Nature (London) 1998;392:264–269. doi: 10.1038/32623. [DOI] [PubMed] [Google Scholar]
- 4.Gait M J, Karn J. Trends Biotechnol. 1995;13:430–438. doi: 10.1016/S0167-7799(00)88998-2. [DOI] [PubMed] [Google Scholar]
- 5.Skulnick H I, Skulnick H I, Johnson P D, Aristoff P A, Morris J K, Lovasz K D, Howe W J, Watenpaugh K D, Janakiraman M N, Anderson D J, Reischer R J, et al. J Med Chem. 1997;40:1149–1164. doi: 10.1021/jm960441m. [DOI] [PubMed] [Google Scholar]
- 6.Chrissey L. Antisense Res Dev. 1991;1:65–113. [PubMed] [Google Scholar]
- 7.Baserga R, Denhardt D T, editors. Antisense Strategies; Annals of the New York Academy of Sciences. Vol. 660. New York: New York Acad. Sci.; 1992. [Google Scholar]
- 8.Wagner R W, Matteucci M D, Grant D, Huang T, Froehler B C. Nat Biotechnol. 1996;14:840–844. doi: 10.1038/nbt0796-840. [DOI] [PubMed] [Google Scholar]
- 9.Herschlag D. Proc Natl Acad Sci USA. 1991;88:6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testa S M, Haidaris C G, Gigliotti F, Turner D H. Biochemistry. 1997;36:15303–15314. doi: 10.1021/bi9713097. [DOI] [PubMed] [Google Scholar]
- 11.Testa S M, Gryaznov S M, Turner D H. Biochemistry. 1998;37:9379–9385. doi: 10.1021/bi980092t. [DOI] [PubMed] [Google Scholar]
- 12.Bloch K. Acc Chem Res. 1969;2:193–200. [Google Scholar]
- 13.Abeles R H, Maycock A L. Acc Chem Res. 1976;9:313–319. [Google Scholar]
- 14.Hughes W T. Annu Rev Med. 1991;42:287–295. doi: 10.1146/annurev.me.42.020191.001443. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg S. Science. 1994;266:1632–1634. doi: 10.1126/science.7702654. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Rocourt M, Pan S, Liu C, Leibowitz M J. Nucleic Acids Res. 1992;20:3763–3772. doi: 10.1093/nar/20.14.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mei H-Y, Cui M, Lemrow S M, Czarnik A W. Bioorg Med Chem. 1997;5:1185–1195. doi: 10.1016/s0968-0896(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 18.Nikolcheva T, Woodson S A. RNA. 1997;3:1016–1027. [PMC free article] [PubMed] [Google Scholar]
- 19.Gryaznov S M, Letsinger R L. Nucleic Acids Res. 1992;20:3403–3409. doi: 10.1093/nar/20.13.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gryaznov S M, Chen J-K. J Am Chem Soc. 1994;116:3143–3144. [Google Scholar]
- 21.Lin H, Niu M T, Yoganathan T, Buck G A. Gene. 1992;119:163–173. doi: 10.1016/0378-1119(92)90268-t. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Leibowitz M J. Nucleic Acids Res. 1995;23:1284–1290. doi: 10.1093/nar/23.8.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison P M, Hoare R J. Metals in Biochemistry. New York: Chapman & Hall; 1980. pp. 8–9. [Google Scholar]
- 24.Maquire M E. In: Metals in Biological Systems. Sigel H, Sigel A, editors. Vol. 26. New York: Dekker; 1990. pp. 135–153. [Google Scholar]
- 25.Bevilacqua P C, Turner D H. Biochemistry. 1991;30:10632–10640. doi: 10.1021/bi00108a005. [DOI] [PubMed] [Google Scholar]
- 26.Emerick V L, Woodson S A. Biochemistry. 1993;32:14062–14067. doi: 10.1021/bi00213a040. [DOI] [PubMed] [Google Scholar]
- 27.Uhlenbeck O C. RNA. 1995;1:4–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Pan J, Thirumalai D, Woodson S A. J Mol Biol. 1997;276:7–13. doi: 10.1006/jmbi.1997.1311. [DOI] [PubMed] [Google Scholar]
- 29.Jencks W P. Catalysis in Chemistry and Enzymology. New York: Dover; 1987. [Google Scholar]
- 30.Inoue T, Sullivan F X, Cech T R. Cell. 1985;43:431–437. doi: 10.1016/0092-8674(85)90173-4. [DOI] [PubMed] [Google Scholar]
- 31.Barfod E T, Cech T R. Mol Cell Biol. 1989;9:3657–3666. doi: 10.1128/mcb.9.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodson S A, Cech T R. Biochemistry. 1991;30:2042–2050. doi: 10.1021/bi00222a006. [DOI] [PubMed] [Google Scholar]
- 33.Weeks K M, Cech T R. Cell. 1995;82:221–230. doi: 10.1016/0092-8674(95)90309-7. [DOI] [PubMed] [Google Scholar]
- 34.Gryaznov S M, Skorski T, Cucco C, Nieborowska-Skorska M, Chiu C Y, Lloyd D, Chen J-K, Koziolkiewicz M, Calabretta B. Nucleic Acids Res. 1996;24:1508–1514. doi: 10.1093/nar/24.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escudé C, Giovannangeli C, Sun J-S, Lloyd D H, Chen J-K, Gryaznov S M, Garestier T, Helene C. Proc Natl Acad Sci USA. 1996;93:4365–4369. doi: 10.1073/pnas.93.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skorski T, Perotti D, Nieborowska-Skorski M, Gryaznov S M, Calabretta B. Proc Natl Acad Sci USA. 1997;94:3966–3971. doi: 10.1073/pnas.94.8.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigl C T, Lloyd D H, Tsou D S, Gryaznov S M, Wilson D W. Biochemistry. 1997;36:650–659. doi: 10.1021/bi961980w. [DOI] [PubMed] [Google Scholar]
- 38.Mercure S, Montplaisir S, Lemay G. Nucleic Acids Res. 1993;21:6020–6027. doi: 10.1093/nar/21.25.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Netzker R, Köchel H G, Basak N, Küntzel H. Nucleic Acids Res. 1982;10:4783–4790. doi: 10.1093/nar/10.15.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]