Abstract
Apoptosis is a hallmark event observed upon infection with many viral pathogens, including influenza A virus. The apoptotic process is executed by a proteolytic system consisting of a family of cysteinyl proteases, termed caspases. Since the consequences of apoptosis induction and caspase activation for the outcome of an influenza virus infection are not clear, we have addressed this issue by interfering with expression or function of a major virus-induced apoptosis effector, caspase 3. Surprisingly, influenza virus propagation was strongly impaired in the presence of an inhibitor that blocks caspase 3 and in cells where caspase 3 was partially knocked down by small interfering RNAs. Consistent with these findings, poor replication efficiencies of influenza A viruses in cells deficient for caspase 3 could be boosted 30-fold by ectopic expression of the protein. Mechanistically, the block in virus propagation appeared to be due to retention of the viral RNP complexes in the nucleus, preventing formation of progeny virus particles. Our findings indicate that caspase 3 activation during the onset of apoptosis is a crucial event for efficient influenza virus propagation.
Keywords: apoptosis/caspase 3/influenza A virus/nuclear export
Introduction
Apoptosis, a morphologically and biochemically defined form of cell death (Kerr et al., 1972), has been demonstrated to play a role in a variety of diseases including virus infections (Razvi and Welsh, 1995). Apoptosis is mainly regarded to be a host cell defence against virus infections since many viruses express anti-apoptotic proteins to prevent this cellular response. The central component of the apoptotic machinery is a proteolytic system consisting of a family of cysteinyl proteases, termed caspases (for review, see Cohen, 1997; Thornberry and Lazebnik, 1998). Two groups of caspases can be distinguished: upstream initiator caspases such as caspase 8 or caspase 9, which cleave and activate other caspases, and downstream effector caspases, including caspase 3, 6 and 7, which cleave a variety of cellular substrates thereby disassembling cellular structures or inactivating enzymes (Thornberry and Lazebnik, 1998). Caspase 3 is the most intensively studied effector caspase. Work on MCF-7 breast carcinoma cells, which are deficient in caspase 3 due to a deletion in the Casp3 gene, has revealed the existence of a crucial caspase 3-driven feedback loop, which mediates the apoptotic process (Janicke et al., 1998; Slee et al., 1999). Thus, caspase 3 is a central player in apoptosis regulation and the level of procaspase 3 in the cell determines the impact of a given apoptotic stimulus.
Influenza A viruses are the prototype of the family Orthomyxoviridae and possess a genome of eight single-stranded RNA segments of negative polarity. It has long been known that influenza virus infection results in the induction of apoptosis both in cell culture and in vivo (Takizawa et al., 1993; Fesq et al., 1994; Hinshaw et al., 1994; Mori et al., 1995); however, the consequence of this activation for virus replication or host cell defence is less clear (Schultz-Cherry et al., 1998; Ludwig et al., 1999). Early studies demonstrated that overexpression of the anti-apoptotic protein Bcl-2 results in impaired virus production correlating with a misglycosylation of the viral surface protein hemagglutinin (Hinshaw et al., 1994; Olsen et al., 1996). Furthermore, it has been shown by the same group that the viral non-structural protein NS1 has pro-apoptotic features and induces apoptosis when ectopically expressed (Schultz-Cherry et al., 2001). These data have been challenged recently by the finding that a recombinant influenza virus lacking the same protein, the delta NS1 virus, is a stronger apoptosis inducer than the wild type, suggesting an anti-apoptotic function of NS1 (Zhirnov et al., 2002). These findings link viral apoptosis induction to the antiviral type I interferon (IFNα/β) response, since the NS1 protein was shown to be an efficient IFNα/β antagonist (Garcia-Sastre, 2001) and type I interferons are believed to be main inducers of influenza virus-induced apoptosis (Balachandran et al., 2000). Another finding in favour of an antiviral role of apoptosis is caspase-mediated cleavage of the influenza viral nucleoprotein (NP) (Zhirnov et al., 1999). The truncated form of the NP is not packaged into viral particles, suggesting that caspases act to limit amounts of virus protein for proper assembly. However, only the NPs of human virus strains are susceptible to this cleavage process (Zhirnov et al., 1999).
With the identification of PB1-F2, a new influenza virus protein expressed from a +1 reading frame of the PB1 polymerase gene segment, another pro-apoptotic influenza virus protein has been discovered (Chen et al., 2001). PB1-F2 induces apoptosis if added to cells, and infection with recombinant viruses lacking the protein results in reduced apoptotic rates in lymphocytes (Chen et al., 2001). However, most of the avian virus strains are lacking the reading frame for this protein and PB1-F2-deficient viruses do not affect apoptosis in a variety of other host cells (Chen et al., 2001). These results have let to the assumption that apoptosis induction by PB1-F2 may be required for the specific depletion of lymphocytes during an influenza virus infection, a process that is observed in infected animals (Van Campen et al., 1989a,b; Tumpey et al., 2000).
Others suggest an antiviral function of apoptosis by providing apoptotic cells or material to be efficiently phagocytosed by macrophages (Watanabe et al., 2002) or to be taken up by dendritic cells, inducing a cytotoxic T-cell response (Albert et al., 1998). However, no direct proof for each of the suggested functions has yet been given.
Here we show that influenza virus induces apoptosis in a variety of cell lines independent of a functional type I interferon response and that apoptotic activation of caspase 3 is required for efficient virus production by co-regulating an intrinsic viral export process.
Results
Switch of phosphatidylserine to the outer leaflet of cells is an early event in the process of apoptosis induction (Schlegel and Williamson, 2001) and is rapidly observed upon influenza A virus infection of different cell lines, as assessed in annexin V stainings (Figure 1A). As pointed out previously, type I interferons are believed to be major inducers of apoptosis in influenza virus-infected cells (Balachandran et al., 2000). Interestingly, we observed similar kinetics and levels of apoptosis induction in MDCK cells, A549 lung epithelial cells and Vero cells. The latter cell type is devoid of a type I interferon response due to a deletion in the IFNα/β genes (Diaz et al., 1988). This indicates that influenza viruses are capable of inducing apoptosis in the absence of a functional IFNα/β response.
Fig. 1. Influenza A virus induces apoptosis in various cell lines independent of type I interferons. MDCK, A549 and Vero cells were either mock infected or infected with replication-competent (A and B) or UV-inactivated (B) influenza A virus (m.o.i. = 1). (A) Cells were harvested at different time points as indicated and the switch of phosphatidylserine to the outer leaflet of the cell, an early marker of apoptosis, was determined with Annexin-V–Alexa568 staining according to the manufacturer’s protocol. Stained cells were visualized by FACS and quantified. The figure shows a mean ± SE of three individual experiments. (B) Protein lysates of cells were separated by SDS–PAGE, transferred to nitrocellulose membranes, before western blotting was performed with an antiserum detecting the cleaved and uncleaved form of PARP to monitor caspase 3 activity.
Caspases are the main executioners of the apoptotic response inside the cell. Consistent with earlier studies using various influenza virus strains and cell types (Takizawa et al., 1999; Zhirnov et al., 1999; Nichols et al., 2001; Lin et al., 2002) we observed proteolytic cleavage of poly(ADP-ribose) polymerase (PARP) (Tewari et al., 1995), a substrate of several caspases, including caspase 3, in MDCK, A549 and Vero cells (Figure 1B). Infection of all three cell lines with UV-inactivated virus did not result in any PARP cleavage (Figure 1B). This indicates that caspase activation and apoptosis induction require productive infection of the cell. Furthermore, the data helped to rule out the possibility that cytokines released to the medium from infected cells in which the virus was grown are responsible for the observed effects.
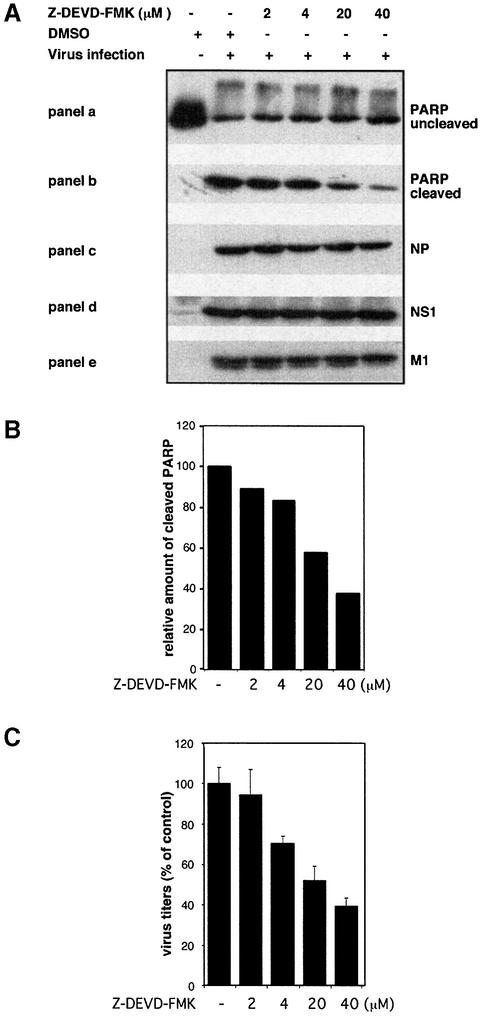
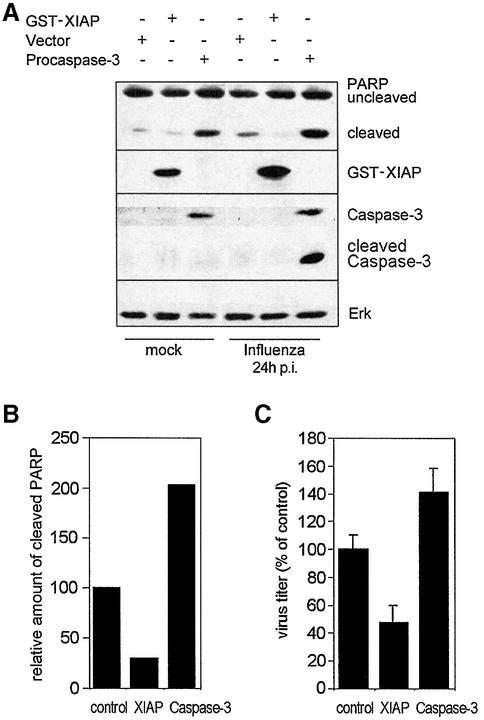
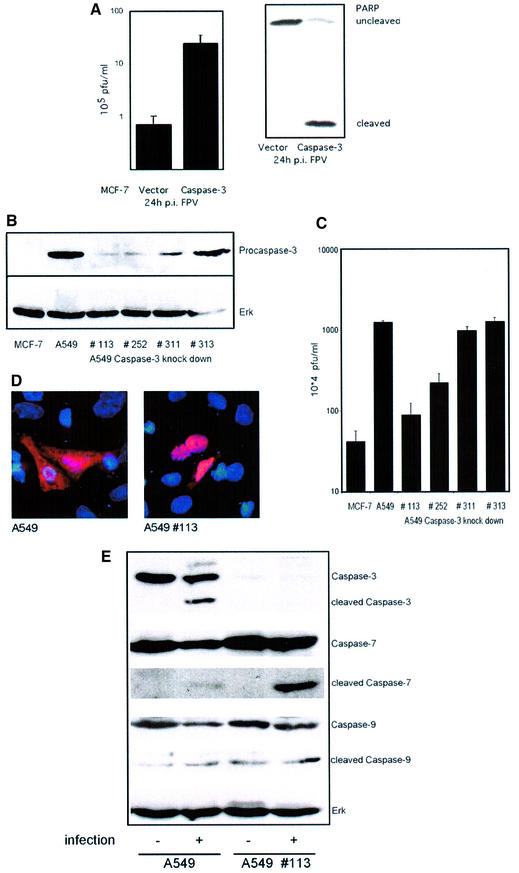
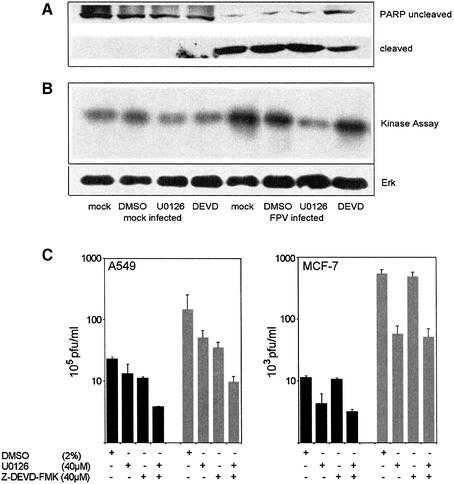
Virus-induced caspase activity could be efficiently suppressed by Z-DEVD-FMK, a cell-permeable, non-toxic inhibitor that binds irreversibly to activated caspase 3 and some other caspases in apoptotic cells (Figure 2A, a and b). When we compared titres of viruses grown in control cells to virus titres from cells treated with Z-DEVD-FMK we made a surprising observation. Virus titres fell to the same extent as caspase activity with increasing concentrations of the inhibitor (Figure 2B and C). This indicates that caspase activity is required for efficient influenza virus propagation. Interestingly, viral protein synthesis appears not to be affected by the caspase inhibitor. As assessed in western blots, neither the synthesis of the NP or the NS1, which are early viral proteins, nor the production of the matrix protein (M1), which is expressed late in the virus life cycle, was reduced (Figure 2A, c–e). This is consistent with earlier studies of Takizawa et al. (1999) showing that although virus-induced cell death could be inhibited by peptide caspase inhibitors, there was no effect on viral protein synthesis. Nevertheless, in the same samples efficient caspase inhibition and a concomitant reduction of viral titres were observed (Figure 2B and C). This strongly suggests that all the early events in the viral life cycle, such as virus entry, genome release, transcription and replication, as well as translation of early and late viral proteins, are not affected by caspase action. This is further supported by the finding that the full inhibitory effect on virus propagation is still seen when Z-DEVD-FMK was added as late as 4 h post-infection to the cell medium. In contrast, presence of the drug in the first 2 h of infection and subsequent washout did not result in any significant interference with virus production (Figure 3A). The central role for the caspases inhibited by Z-DEVD-FMK, including caspase 3, in the propagation of influenza virus is further highlighted by the observation that Z-VAD-FMK, a pan-caspase inhibitor, showed no enhanced inhibitory effect in comparison to Z-DEVD-FMK (Figure 3B), albeit that it additionally inhibits several other influenza virus-induced caspases (Takizawa et al., 1999). Moreover, inhibition of virus growth was also observed in cells transiently expressing XIAP, a protein that inhibits active caspases 3, 7 and 9 (Deveraux et al., 1997), while overexpression of procaspase 3 resulted in an enhanced virus production (Figure 4A and C). Again in these experiments the level of cellular caspase activity correlated directly with the amount of progeny virus produced in the respective host-cell culture (Figure 4B and C). This prompted us to analyse influenza virus propagation in a cell line that lacks caspase 3 due to a chromosomal deletion, the MCF-7 breast carcinoma cell line (Janicke et al., 1998). In wild-type MCF-7 cells where no caspase 3 activity is detectable influenza virus replicates only very poorly (Figure 5A). However, if these cells were re-supplied with the enzyme by transfection of a plasmid expressing procaspase 3, virus titres could be boosted by 30-fold (Figure 5A). As a further proof that the poor replication efficiency of influenza viruses in MCF-7 cells is due to the lack of caspase 3, we knocked down expression of the protein by stable expression of different caspase 3-specific small interfering RNAs (siRNAs). The different siRNAs showed a variable degree of knock-down efficiency (Figure 5B). This correlated perfectly with the decrease of viral titres observed in the different caspase 3 knock-down cell lines (Figure 5C). In the knock-down cell line with the lowest caspase 3 expression (113), viral titres were reduced ∼10-fold, while no reduction was observed in cells expressing a control construct with an insertion in the siRNA target sequence (Figure 5C). Interestingly, cells appear to compensate for the lack of caspase 3 by overexpression of caspase 7, while expression or activity of caspase 9 was not significantly altered (Figure 5E). However, the compensatory overexpression of caspase 7 did not rescue viral replication efficiencies. This clearly demonstrates that activation of the effector caspase 3 is most crucial for an efficient replication of influenza virus in cultured cells.
Fig. 2. Efficient influenza A virus replication requires caspase activity. MDCK cells were infected with influenza A virus (m.o.i. = 1) either in presence of the caspase inhibitor Z-DEVD-FMK or DMSO as a control. Twenty-four hours p.i., supernatants were collected and cells were lysed. (A) Protein lysates were separated by SDS–PAGE and transferred to nitrocellulose membranes, before western blot experiments were performed. Antibodies against the influenza viral proteins NP (c), NS1 (d) and M1 (e) were used to analyse effects on viral protein synthesis. (B) Relative band intensity of cleaved PARP in (b) was quantified using the TINA Software (Raytest) to estimate relative caspase activity. The DMSO-treated control was set to 100%. (C) Virus titres from supernatants of the samples described in (A) as determined in common plaque titrations. Data shown are from independently confirmed triplicate plaque titrations.
Fig. 3. Caspase activity is required at late steps during influenza virus replication in several cell types independent of the type I interferon response. (A) MDCK cells were infected with influenza A virus (m.o.i. = 1) and incubated in the presence of the solvent DMSO, the inactive inhibitor analogue Z-FA-FMK or the active caspase inhibitor Z-DEVD-FMK for the times indicated. (B) A549 or Vero cells were infected with influenza A virus (m.o.i. = 1) in the presence of the a broad-range caspase inhibitor Z-VAD-FMK or the more specific inhibitor Z-DEVD-FMK. The inactive inhibitor analogue Z-FA-FMK and the solvent DMSO served as a control. Twenty-four hours p.i., virus titres were determined by plaque assay. The DMSO control was arbitrarily set as 100%. The data shown are a mean ± SE of triplicate plaque titrations which were independently confirmed.
Fig. 4. Caspase activity is crucial for influenza A virus replication. MDCK cells were transfected with control vector or plasmids encoding the caspase inhibitory protein XIAP or procaspase 3. Transfection efficiency was determined upon parallel transfection of a GFP-expressing plasmid in FACS analysis to be 60%. Twenty-four hours post-transfection, cells were infected with influenza A virus (m.o.i. = 1). Supernatants were collected and cells were harvested 24 h p.i. (A) Proteins of cell lysates were separated by SDS–PAGE and blotted onto nitrocellulose. Membranes were incubated with an anti-PARP antibody to monitor caspase activity. Overexpression of GST–XIAP or procaspase 3 was verified by anti-GST or anti-caspase 3 antibodies (note that the caspase 3 antibody does not detect endogenous protein in MDCK cells). Equal loading of proteins was determined with an anti-ERK western blot. (B) Band intensity of cleaved PARP in (A) served as a marker for caspase activation levels. (C) Supernatants were assayed for virus content in common plaque titrations. Virus titres are shown relative to the DMSO control, which was arbitrarily set as 100%.
Fig. 5. Influenza virus propagation is strongly impaired in cells lacking caspase 3 or with reduced levels of caspase 3 expression. (A) MCF-7 human breast cancer cells were transfected with a vector encoding procaspase 3 or an empty control vector. Transfection efficiency was controlled with a GFP expression plasmid to be around 50%. Twenty-four hours later, cells were infected with influenza A virus (m.o.i. = 1). Twenty-four hours p.i., supernatants were assayed for virus titres, which are shown in a logarithmic scale (left). In parallel, cell lysates were analysed for caspase 3 activity as assessed by PARP cleavage in western blots (right). (B) A549 cells were stably transfected with pSUPER vectors expressing different caspase 3-specific RNAi target sequences. Clone 313 exhibited an insertion in the target sequence and served as a negative control. Procaspase 3 expression was detected with an anti-caspase 3 antiserum and equal protein loading of the gel was verified in an anti-ERK2 western blot. (C) MCF-7 cells, A549 cells or the different RNAi-expressing cell lines were infected with influenza A virus (m.o.i. = 1). Twenty-four hours p.i., supernatants were assayed for virus titres, which are shown in a logarithmic scale (left). (D) Parental A549 cells or caspase 3 knock-down cells (clone 113) were infected with influenza A virus (m.o.i. = 3) and were subjected to immunofluorescence studies 5 h p. i. using a specific anti-NP goat antiserum and an anti-goat Texas Red antibody. Cell nuclei were stained with DAPI. Stained cells were visualized with an inverse fluorescence microscope (magnification 40×). (E) Parental A549 or caspase 3 knock-down cells (clone 113) were either left uninfected or infected with influenza A virus. Twenty-four hours p.i., cell lysates were subjected to western blot analysis with antisera against cleaved and uncleaved caspases 3, 7 and 9. Equal loading of the gel was verified by western blotting with anti-ERK2.
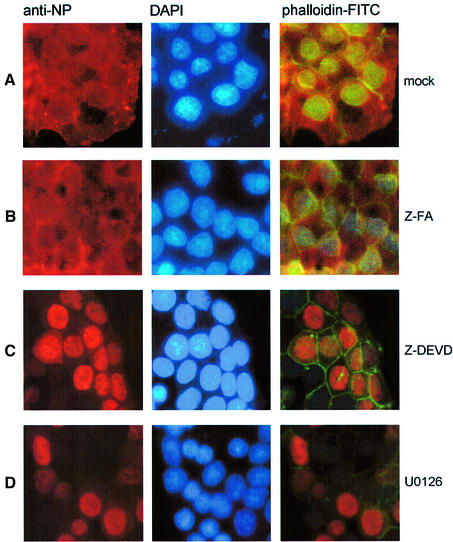
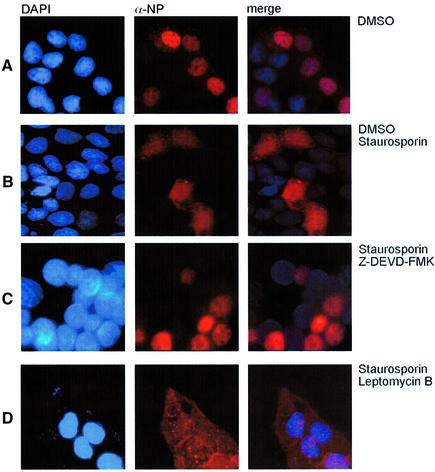
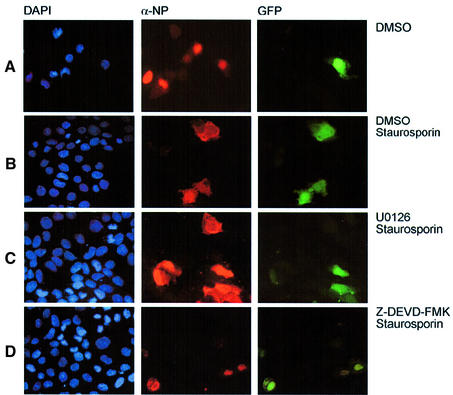
A remaining question to be addressed is: which late event in the virus life cycle is affected by caspase 3 inhibition. Influenza A viruses pursue a nuclear replication strategy. Thus, they have to export their genome in the form of ribonucleoprotein (RNP) complexes from the nucleus to the cytoplasm of the infected cell. With respect to this export process it was interesting to observe that in caspase 3 knock-down cells cytoplasmic accumulation of the RNPs appeared to be inhibited (Figure 5D). Efficient RNP migration to the cytoplasm was readily detected 5 h post-infection in wild-type A549 cells, whereas in knock-down cells RNPs were mainly nuclear, as assessed in immunofluorescence studies of the viral nucleoprotein, the major component of the RNPs (Figure 5D). The same efficient retention of the RNPs was observed when infected cells were treated with the active caspase inhibitor Z-DEVD-FMK (Figure 6C), while the inactive analogue Z-FA-FMK had no effect (Figure 6B). This finding was reminiscent of an earlier observation that inhibition of virus-induced signalling through the Raf/MEK/ERK kinase cascade by the specific MEK inhibitor U0126 leads to nuclear RNP retention (Figure 6D) (Pleschka et al., 2001). Thus, we analysed whether both signalling events may crosstalk to each other to result in the same outcome for viral replication. Although MEK inhibition had no effect on PARP cleavage (Figure 7A) and blockade of caspase 3 did not affect viral ERK activation (Figure 7B), both inhibitors act synergistically to impair influenza virus replication (Figure 7C, left panel). Moreover, inhibition of MEK still resulted in a reduction of virus titres in caspase 3-deficient MCF-7 cells (Figure 7C, right panel). Thus, both signalling events support viral RNP export; however, the underlying mechanisms are likely to be different. U0126 inhibits RNP export at least in part through interference with the activity of the viral nuclear export protein (NS2/NEP), which has been shown to interact with the cellular export machinery (O’Neill et al., 1998). However, it may well be that the virus uses additional strategies to optimize RNP export. It has recently been reported that active caspases directly or indirectly increase the diffusion limit of nuclear pores (Faleiro and Lazebnik, 2000). This increase allows molecules that could not pass through these pores in living cells to enter or leave the nucleus during apoptosis by diffusion. To analyse whether such a cell death-related passive diffusion would be a second alternative export mechanism of viral proteins, we employed a virus-free experimental setting. We transiently expressed the viral nucleoprotein that is nuclear in unstimulated cells (Figure 8A) (Neumann et al., 1997). If these NP-transfected cells were stimulated with the apoptosis inducer staurosporine, NP was detected in a speckled appearance both in the nucleus and in the cytoplasm of the cell (Figure 8B). This NP export appears to be caspase dependent since it could be at least partially prevented by Z-DEVD-FMK (Figure 8C). Moreover, cytoplasmic accumulation of the NP was not due to active nuclear export processes since it was not blocked by an inhibitor of the nuclear export machinery, leptomycin B. To analyse whether caspase-mediated passive diffusion is also allowed for large RNP complexes, we mimicked formation of these complexes with a plasmid-based system used for reverse genetic approaches to express the minimal components of a functionally active influenza viral RNP polymerase complex (Pleschka et al., 1996). To detect proper formation and function of the RNP complex, a template RNA that expresses green fluorescent protein (GFP) under the control of the influenza viral promoters was co-expressed. Figure 9 shows that GFP-positive RNP-containing cells also showed a cytoplasmic staining for RNPs upon apoptosis induction. This was inhibited by Z-DEVD-FMK but not by U0126, again arguing for a passive export process distinct form that controlled by the Raf/MEK/ERK cascade. Thus, apoptosis induction in infected cells, in particular activation of caspase 3, appears to be required for an efficient migration of the RNPs out of the nucleus to be packaged into progeny virions. Inhibition of caspase 3 activity results in nuclear RNP retention, which is most likely the molecular basis of subsequent reduction of virus titres.
Fig. 6. Viral RNP export requires caspase activity. MDCK cells were grown on chamber slides and infected with influenza A virus (m.o.i. = 5) in the presence of DMSO (A) Z-FA-FMK (B), Z-DEVD-FMK (C) or the MEK-inhibitor U0126 (D). Five hours p.i., cells were fixed with paraformaldehyde, permeabilized with acetone and stained for viral NP as described. To visualize cell nuclei or the cell borders, cells were stained with DAPI or phalloidin–FITC. Stained cells were visualized with an inverse fluorescence microscope (magnification 40×).
Fig. 7. Z-DEVD-FMK and U0126 showed no cross-inhibition on MEK or caspases, respectively, but act synergistically to inhibit influenza virus propagation in A549 cells. A549 (A–C) or MCF-7 cells (C) were infected with influenza A virus (m.o.i. = 1) and incubated in the presence of the solvent DMSO, the MEK inhibitor U0126 or Z-DEVD-FMK. (A) Twenty-four hours p.i., cells were lysed and subjected to an anti-PARP western blot. (B) The same lysates were subjected to an ERK2 immunocomplex kinase assay as desribed previously (Pleschka et al., 2001) using MBP as a substrate. (C) Supernatants of infected cells were analysed for progeny virus production 9 h p.i. (black bars) and 24 h p.i. (grey bars) as described.
Fig. 8. Caspase activity is critical for cytoplasmic accumulation of the NP upon apoptosis induction. MDCK cells were transfected with an expression plasmid encoding the influenza A virus NP protein. Sixteen hours post-transfection, cells were treated with solvent (DMSO) (A), 1 µM staurosporine and DMSO (B), 1 µM staurosporine and 40 µM Z-DEVD-FMK (C) or 1 µM staurosporine and 2 ng/ml leptomycin B (D) for 5 h. Cells were fixed with paraformaldehyde, permeabilized with acetone and stained for viral NP with a specific anti-NP goat antiserum and an anti-goat Texas Red antibody. Cell nuclei were counterstained with DAPI. Stained cells were visualized with an inverse fluorescence microscope (magnification 40×).
Fig. 9. Apoptosis induction promotes caspase-dependent cytoplasmic accumulation of influenza virus RNP complexes. MDCK cells were transfected with expression plasmids encoding the influenza A virus PB1, PB2, PA and NP proteins together with a plasmid expressing a template RNA with influenza-specific promoter regions flanking an antisense GFP coding region (Pleschka et al., 1996). GFP expression in green indicates formation of a functional viral RNP polymerase complex. Sixteen hours post-transfection, cells were treated with solvent (DMSO) (A), 1 µM staurosporine and DMSO (B), 1 µM staurosporine and 40 µM U0126 (C) or 1 µM staurosporine and 40 µM Z-DEVD-FMK (D) for 5 h. Cells were fixed with paraformaldehyde, permeabilized with 0.5% Triton X-100 and stained for viral NP with a specific anti-NP goat antiserum and an anti-goat Texas Red antibody. Cell nuclei were counterstained with DAPI. Stained cells were visualized with an inverse fluorescence microscope (magnification 40×).
Discussion
The question whether apoptosis in influenza virus-infected cells is beneficial for virus replication or for host-cell defence is still under debate. Here we have shown that apoptosis induction, in particular activation of caspase 3, is essential for efficient influenza virus propagation. On a molecular level, a caspase-dependent process appears to be required for migration of the viral RNPs from the nucleus to the cytoplasm of the infected cell.
Our findings are supported by several previous indications that apoptosis might be beneficial for virus growth. First of all, two viral proteins have been reported to act as apoptosis promoters: the NS1 protein and the recently identified PB1-F2 (Chen et al., 2001; Schultz-Cherry et al., 2001). PB1-F2 is only expressed in later phases of replication. This is in accordance with our finding that a later step in the virus life cycle requires caspase activity. In addition, it has been reported that in cells overexpressing the anti-apoptotic protein Bcl-2, virus replication is repressed (Olsen et al., 1996). Interestingly the authors have also observed that upon infection of Bcl-2-expressing cells, the viral RNP complexes were retained in the nucleus (Hinshaw et al., 1994). Although ectopic overexpression of an antiviral protein might be a more artificial situation compared with selective inhibition of a virus-induced activity, it is striking that in both cases inhibition of the apoptotic process results in the same molecular outcome.
Export of the RNPs has been demonstrated to be at least in part mediated by the active cellular export machinery involving activity of the viral nuclear export protein (NS2/NEP) (O’Neill et al., 1998). We have shown recently that the cellular Raf/MEK/ERK signalling pathway is activated upon influenza virus infection and supports active nuclear RNP export at least in part through NS2/NEP action (Pleschka et al., 2001). Caspase-dependent RNP migration most likely occurs through a different mechanism since caspase activation has never been linked to the function of the nuclear export machinery. The recent finding that active caspases directly or indirectly increase the diffusion limit of nuclear pores (Faleiro and Lazebnik, 2000) to allow passive diffusion of larger proteins suggests an alternative export strategy. Such a scenario is supported by our finding that isolated NPs or RNP complexes, which are nuclear when ectopically expressed, can partially translocate to the cytoplasm upon stimulation with an apoptosis inducer in a caspase 3-dependent manner (Figures 8 and 9).
It is puzzling that both a mainly anti-apoptotic acting signalling pathway such as the Raf cascade and pro-apoptotic mediators such as caspases should support the same step in the viral life cycle. The data presented here indicate that, although the two pathways do not influence each other, they act synergistically in virus propagation. Our findings are compatible with a model in which influenza virus has acquired the capability to take advantage of complementary host cell responses to support viral propagation. It is a common observation that viruses persue parallel strategies to optimize their replication. To this end it is interesting that, in the presence of MEK inhibitors, reduction of virus titres is not very effective and does not exceed one log phase (Pleschka et al., 2001). Thus, there is a certain degree of leakiness against MEK inhibition which was also observed on the level of RNP export in very late stages of the viral life cycle (our unpublished observation). This leakiness may be due to caspase-mediated passive diffusion of RNPs in late stages of the replication cycle, when integrity of the nuclear membrane may not be relevant for virus replication anymore. That would be a likely mechanism to further enhance RNP migration to the cytoplasm and thereby support virus replication.
It is a common belief that influenza virus-induced apoptosis is mainly caused by type I interferons acting in an autocrine and paracrine fashion via a caspase 8-dependent mechanism (Balachandran et al., 2000). Since IFNα/β are the most potent antiviral cytokines, this mechanism would argue for a role of apoptosis in host-cell defence. However, since we observed the same levels of phosphatidylserine switch and PARP cleavage both in normal cells and IFNα/β-deficient cells (Figure 1), the major mechanism of apoptosis induction appears to be independent of these cytokines. This is further supported by the finding that UV-inactivated virus in supernatants, which should still contain all secreted antiviral cytokines, did not induce apoptosis (Figure 1B). Thus, type I interferons may rather act as apoptosis modulators but not as cell death initiators during an influenza virus infection.
Although there is probably involvement of other upstream caspases in the observed effects, the data obtained with caspase 3 knock-down and MCF-7 cells clearly highlight a very prominent role of this enzyme for viral growth. Thus, our findings suggest that caspase 3 might be a good target for anti-viral intervention. However, although caspase 3 inhibition may reduce virus titres in cells of an infected tissue in the first place, it will also lead to a prolonged cell survival. That would mean that the remaining virus meets a greater number of cells to grow, which may result in higher end titres. Thus, the suitability of a caspase 3 inhibitor as an anti-influenza virus agent cannot be predicted yet and rigorous tests in animal models would have to precede any conclusive statement on that issue.
Beside its critical role in apoptosis, caspase 3 has been shown recently to act in other cellular decision processes independent of cell death induction (Fernando et al., 2002). Thus, one may hypothesize that influenza virus takes advantage of early events of apoptosis, such as caspase 3 activity, while the full execution of the apoptotic process may be an antiviral response. In support of such an assumption, cytoplasmic translocation of the NP or RNPs upon apoptosis induction with staurosporine is already observed in cells that do not exhibit late signs of apoptosis, such as nuclear condensation or membrane blebbing (Figures 8 or 9). Our findings are compatible with a scenario where cellular responses of the antiviral defence are employed by the virus to support its replication at some stage. This may even include complementary responses such as anti-apoptotic activation of the Raf pathway or pro-apoptotic activation of caspases. It is easier for a viral invader to take advantage of existing cellular activities than to develop strategies to actively induce these activities in the infected host cell. Thus, there appears to be no black or white situation. Cellular antiviral responses may also be supportive for the virus at some point. To fully evaluate the role of a given cellular component by interference with its function, it will always be necessary to assess either the net outcome on virus titres from cell culture or to monitor the disease progression in an infected animal.
Materials and methods
Viruses, cells and viral infections
Avian influenza virus A/Bratislava/79 (H7N7) (FPV) was taken from the strain collection of the Institute of Virology in Giessen, Germany, and used for infection of different cell lines. Madin-Darby canine kidney (MDCK) cells and the African green monkey epithelial cell line Vero were grown in minimal essential medium (MEM); the human alveolar epithelial cell line A549 was grown in Ham’s F12; the caspase 3-deficient human breast cancer cell line MCF-7 was grown in RPMI 1640 medium. All growth media contained 10% heat-inactivated fetal bovine serum and antibiotics. For infection, cells were washed with phosphate-buffered saline (PBS) and infected with FPV at the indicated multiplicity of infection (m.o.i.) in PBS/BA [PBS containing 0.2% bovine serum albumin (BSA), 1 mM MgCl2, 0.9 mM CaCl2, 100 U/ml penicillin, 0.1 mg/ml streptomycin] for 45 min at 37°C. The inoculum was aspirated and cells were incubated with RPMI 1640/BA (MCF-7), Ham’s F12/BA (A549) or minimal essential medium/BA (MDCK, Vero) (medium containing 0.2% BSA and antibiotics). The amount of infectious virus in cell supernatants was determined in common plaque assays as described previously (Ludwig et al., 2001; Pleschka et al., 2001).
Plasmids, antibodies and inhibitors
The pEBG-XIAP expression plasmid was kindly provided by B.W.M.Jordan (Jordan et al., 2001). The pcDNA3/YAMA (procaspase-3) construct was a kind gift of M.Tewari (Tewari et al., 1995). Plasmids expressing the minimal constituents of the influenza virus RNP polymerase complex (PB1, PB2, PA and NP), as well as a plasmid expressing a template viral RNA with influenza virus-specific promoter structures flanking an antisense coding region for GFP, were described previously (Pleschka et al., 1996; Ludwig et al., 2001). The anti-PARP monoclonal antibody was purchased from Transduction Laboratories. Antisera against the influenza virus proteins NP, NS1 and M1 were provided by Robert G.Webster, Memphis, TN, and Thorsten Wolff, Berlin, respectively. Antisera against cleaved caspase 3 (#9961), caspase 3 (#9662), cleaved caspase 7 (#9491), caspase 7 (#9492), cleaved caspase 9 (#9501) and caspase 9 (#9502) were purchased from Cell Signaling Technologies and all used at a 1:1000 dilution. The anti-GST antiserum was produced at the MSZ, Würzburg. The MEK inhibitor U0126 (Promega) was freshly dissolved in DMSO at a 10 mM stock concentration and used as described previously (Pleschka et al., 2001). The caspase inhibitor Z-DEVD-FMK, a pan-caspase inhibitor Z-VAD-FMK or an inactive inhibitor analogue Z-FA-FMK (all ‘ready-to-use’ at 2 mM in DMSO; Alexis Biochemicals) were added to the medium at the concentrations indicated after aspiration of the inoculum and maintained throughout the experiments unless otherwise indicated. Staurosporine and leptomycin B were purchased from Sigma-Aldrich.
Analysis of the apoptotic phosphatidylserine switch
MDCK, A549 and Vero cells were infected with FPV at a m.o.i. of 3. At the different time points indicated, cells were washed with PBS, detached from the culture plates with 0.25% trypsin and spun down. Cell pellets were washed twice with PBS. Annexin-V–Alexa568 (Roche Molecular Biochemicals) stain was used according to the manufacturer’s instructions to detect phosphatidylserine exposure at the outer leaflet of the cell membrane as an early apoptotic marker. Apoptotic cells were detected and quantified by FACS analysis.
Transient transfections and western blotting
MDCK and MCF-7 cells were transfected with LipofectAMINE 2000 (Life Technologies) according to the manufacturer’s instructions. Cells were lysed in Triton lysis buffer (20 mM Tris–HCl pH 7.4, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 50 mM sodium glycerolphosphate, 20 mM sodium pyrophosphate, 5 µg/ml aprotinin, 5 µg/ml leupeptin, 1 mM sodium vanadate, 5 mM benzamidine) on ice for 10–20 min. Cell lysates were then centrifuged and protein contents in supernatants were estimated employing a protein dye reagent (Bio-Rad Laboratories). Equal amounts of protein were separated by SDS–PAGE and blotted onto nitrocellulose membranes.
Indirect immunofluorescence analysis
MDCK cells were grown on chamber slides (Lab-Tak II Chamber Slide System; Nalge Nunc Int.). When 50% confluence was reached, cells were infected with FPV. Thirty minutes post-infection (p.i.), the inoculum was aspirated and medium/BA supplemented with DMSO or inhibitors was added. Five hours p.i., cells were washed twice with PBS, then fixed for 30 min with 3.7% paraformaldehyde (in PBS) at room temperature. After washing, cells were permeabilized with acetone or 0.5% Triton X-100, washed with PBS and blocked with 20% fetal bovine serum in PBS for 20 min at 37°C. After blocking, cells were incubated with goat antiserum against the viral NP (1:300) in PBS supplemented with 3% BSA for 1 h. After further washes, cells were incubated with Texas Red-labelled anti-rabbit IgG (1:100; Dianova) in PBS/3% BSA for 1 h. Phalloidin–FITC incubations were performed together with the secondary antibody. Finally, nuclei were stained with DAPI (5 µg/ml) in a 1:1000 dilution. Subsequently, cells were washed and mounted with Moviol (Sigma-Aldrich). Fluorescence was visualized using a Leitz DMRB fluorescence microscope.
siRNA mediated knock-down of caspase-3 in A549 cells
SiRNA target sequences of caspase-3 (DDBJ/EMBL/GenBank accession No. NM004346), TGACATCTCGGTCTGGTAC (nucleotides 417–435), CTGGACTGTGGCATTGAGA (nucleotides 734–755) and TACCAG TGGAGGCCGACTT (nucleotides 795–813) (clones 113, 252 and 311, respectively) were cloned into pSUPER vectors as described (Brummelkamp et al., 2002). Constructs were verified by sequencing, identifying an insertion in one of the clones (termed 313). This construct served as a negative control. The constructs were transfected together with the pCAGI-Puro vector into A549 cells. Twenty-four hours after transfection, cells were washed and incubated in growth medium, supplemented with 1 mg/ml puromycin. After 24 h, cells were extensively washed with PBS to get rid of dead or dying cells. This procedure was repeated over the following 7 days, in the presence of 0.6 µg/ml puromycin. After 7 days, cells were used for western blotting and viral replication studies.
Acknowledgments
Acknowledgements
We thank A.Avots and J.Troppmair for critical reading of the manuscript and helpful discussions. We greatfully appreciate the plasmids and antibodies provided by B.W.M.Jordan, M.Tewari, R.G.Webster and T.Wolff. This work was supported by the Deutsche Forschungs gemeinschaft (DFG), grant Lu477/4-3 to S.L. and by the Fonds der Chemischen Industrie (FdChI). This manuscript is dedicated to Rudolf Rott, a pioneer of influenza virology, who died in April 2003. His mentorship to S.L., O.P. and S.P. will be always warmly appreciated.
References
- Albert M.L., Sauter,B. and Bhardwaj,N. (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature, 392, 86–89. [DOI] [PubMed] [Google Scholar]
- Balachandran S., Roberts,P.C., Kipperman,T., Bhalla,K.N., Compans,R.W., Archer,D.R. and Barber,G.N. (2000) α/β interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol., 74, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp T.R., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. [DOI] [PubMed] [Google Scholar]
- Chen W. et al. (2001) A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med., 7, 1306–1312. [DOI] [PubMed] [Google Scholar]
- Cohen G.M. (1997) Caspases: the executioners of apoptosis. Biochem. J., 326, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q.L., Takahashi,R., Salvesen,G.S. and Reed,J.C. (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature, 388, 300–304. [DOI] [PubMed] [Google Scholar]
- Diaz M.O., Ziemin,S., Le Beau,M.M., Pitha,P., Smith,S.D., Chilcote,R.R. and Rowley,J.D. (1988) Homozygous deletion of the α- and β1-interferon genes in human leukemia and derived cell lines. Proc. Natl Acad. Sci. USA, 85, 5259–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faleiro L. and Lazebnik,Y. (2000) Caspases disrupt the nuclear-cytoplasmic barrier. J. Cell Biol., 151, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P., Kelly,J.F., Balazsi,K., Slack,R.S. and Megeney,L.A. (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl Acad. Sci. USA, 99, 11025–11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesq H., Bacher,M., Nain,M. and Gemsa,D. (1994) Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology, 190, 175–182. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A. (2001) Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology, 279, 375–384. [DOI] [PubMed] [Google Scholar]
- Hinshaw V.S., Olsen,C.W., Dybdahl-Sissoko,N. and Evans,D. (1994) Apoptosis: a mechanism of cell killing by influenza A and B viruses. J. Virol., 68, 3667–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke R.U., Sprengart,M.L., Wati,M.R. and Porter,A.G. (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem., 273, 9357–9360. [DOI] [PubMed] [Google Scholar]
- Jordan B.W., Dinev,D., LeMellay,V., Troppmair,J., Gotz,R., Wixler,L., Sendtner,M., Ludwig,S. and Rapp,U.R. (2001) Neurotrophin receptor-interacting mage homologue is an inducible inhibitor of apoptosis protein-interacting protein that augments cell death. J. Biol. Chem., 276, 39985–39989. [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie,A.H. and Currie,A.R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer, 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Holland,R.E.,Jr, Donofrio,J.C., McCoy,M.H., Tudor,L.R. and Chambers,T.M. (2002) Caspase activation in equine influenza virus induced apoptotic cell death. Vet. Microbiol., 84, 357–365. [DOI] [PubMed] [Google Scholar]
- Ludwig S., Pleschka,S. and Wolff,T. (1999) A fatal relationship—influenza virus interactions with the host cell. Viral Immunol., 12, 175–196. [DOI] [PubMed] [Google Scholar]
- Ludwig S., Ehrhardt,C., Neumeier,E.R., Kracht,M., Rapp,U.R. and Pleschka,S. (2001) Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem., 276, 10990–10998. [PubMed] [Google Scholar]
- Mori I., Komatsu,T., Takeuchi,K., Nakakuki,K., Sudo,M. and Kimura,Y. (1995) In vivo induction of apoptosis by influenza virus. J. Gen. Virol., 76, 2869–2873. [DOI] [PubMed] [Google Scholar]
- Neumann G., Castrucci,M.R. and Kawaoka,Y. (1997) Nuclear import and export of influenza virus nucleoprotein. J. Virol., 71, 9690–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J.E., Niles,J.A. and Roberts,N.J.,Jr (2001) Human lymphocyte apoptosis after exposure to influenza A virus. J. Virol., 75, 5921–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W., Kehren,J.C., Dybdahl-Sissoko,N.R. and Hinshaw,V.S. (1996) bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J. Virol., 70, 663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill R.E., Talon,J. and Palese,P. (1998) The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J., 17, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S., Jaskunas,R., Engelhardt,O.G., Zurcher,T., Palese,P. and Garcia-Sastre,A. (1996) A plasmid-based reverse genetics system for influenza A virus. J. Virol., 70, 4188–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S., Wolff,T., Ehrhardt,C., Hobom,G., Planz,O., Rapp,U.R. and Ludwig,S. (2001) Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol., 3, 301–305. [DOI] [PubMed] [Google Scholar]
- Razvi E.S. and Welsh,R.M. (1995) Apoptosis in viral infections. Adv. Virus Res., 45, 1–60. [DOI] [PubMed] [Google Scholar]
- Schlegel R.A. and Williamson,P. (2001) Phosphatidylserine, a death knell. Cell Death Differ., 8, 551–563. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S., Krug,R.M. and Hinshaw,V.S. (1998) Induction of apoptosis by influenza virus. Semin. Virol., 8, 491–495. [Google Scholar]
- Schultz-Cherry S., Dybdahl-Sissoko,N., Neumann,G., Kawaoka,Y. and Hinshaw,V.S. (2001) Influenza virus ns1 protein induces apoptosis in cultured cells. J. Virol., 75, 7875–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E.A., Adrain,C. and Martin,S.J. (1999) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ., 6, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Takizawa T., Matsukawa,S., Higuchi,Y., Nakamura,S., Nakanishi,Y. and Fukuda,R. (1993) Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol., 74, 2347–2355. [DOI] [PubMed] [Google Scholar]
- Takizawa T., Tatematsu,C., Ohashi,K. and Nakanishi,Y. (1999) Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol. Immunol., 43, 245–252. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan,L.T., O’Rourke,K., Desnoyers,S., Zeng,Z., Beidler,D.R., Poirier,G.G., Salvesen,G.S. and Dixit,V.M. (1995) Yama/CPP32 β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell, 81, 801–809. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A. and Lazebnik,Y. (1998) Caspases: enemies within. Science, 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Lu,X., Morken,T., Zaki,S.R. and Katz,J.M. (2000) Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol., 74, 6105–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Campen H., Easterday,B.C. and Hinshaw,V.S. (1989a) Destruction of lymphocytes by a virulent avian influenza A virus. J. Gen. Virol., 70, 467–472. [DOI] [PubMed] [Google Scholar]
- Van Campen H., Easterday,B.C. and Hinshaw,V.S. (1989b) Virulent avian influenza A viruses: their effect on avian lymphocytes and macrophages in vivo and in vitro. J. Gen. Virol., 70, 2887–2895. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Shiratsuchi,A., Shimizu,K., Takizawa,T. and Nakanishi,Y. (2002) Role of phosphatidylserine exposure and sugar chain desialylation at the surface of influenza virus-infected cells in efficient phagocytosis by macrophages. J. Biol. Chem., 277, 18222–18228. [DOI] [PubMed] [Google Scholar]
- Zhirnov O.P., Konakova,T.E., Garten,W. and Klenk,H. (1999) Caspase-dependent N-terminal cleavage of influenza virus nucleocapsid protein in infected cells. J. Virol., 73, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhirnov O.P., Konakova,T.E., Wolff,T. and Klenk,H.D. (2002) NS1 protein of influenza A virus down-regulates apoptosis. J. Virol., 76, 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]