Abstract
To restrict infection by Legionella pneumophila, mouse macrophages require Naip5, a member of the nucleotide-binding oligomerization domain leucine-rich repeat family of pattern recognition receptors, which detect cytoplasmic microbial products. We report that mouse macrophages restricted L. pneumophila replication and initiated a proinflammatory program of cell death when flagellin contaminated their cytosol. Nuclear condensation, membrane permeability, and interleukin-1β secretion were triggered by type IV secretion-competent bacteria that encode flagellin. The macrophage response to L. pneumophila was independent of Toll-like receptor signaling but correlated with Naip5 function and required caspase 1 activity. The L. pneumophila type IV secretion system provided only pore-forming activity because listeriolysin O of Listeria monocytogenes could substitute for its contribution. Flagellin monomers appeared to trigger the macrophage response from perforated phagosomes: once heated to disassemble filaments, flagellin triggered cell death but native flagellar preparations did not. Flagellin made L. pneumophila vulnerable to innate immune mechanisms because Naip5+ macrophages restricted the growth of virulent microbes, but flagellin mutants replicated freely. Likewise, after intratracheal inoculation of Naip5+ mice, the yield of L. pneumophila in the lungs declined, whereas the burden of flagellin mutants increased. Accordingly, macrophages respond to cytosolic flagellin by a mechanism that requires Naip5 and caspase 1 to restrict bacterial replication and release proinflammatory cytokines that control L. pneumophila infection.
Macrophages are the guardians of the innate immune system, recognizing a broad array of pathogen-associated molecular patterns (PAMPs) to initiate immediate defenses and to recruit the adaptive branch of the immune system. Toll-like receptors (TLRs) detect extracellular microbial products, such as lipopolysaccharide, peptidoglycan, lipotechoic acid, and flagellin (1), whereas surveillance of the cytosol is the task of nucleotide-binding oligomerization domain (NOD) leucine-rich repeat (LRR) proteins. The best-characterized members of the NOD-LRR family are NOD1 and NOD2, which recognize distinct elements of bacterial cell wall peptidoglycan in the cytosol to mount or modulate a proinflammatory immune response or to promote apoptosis (2).
In mouse macrophages, the NOD-LRR protein Naip5 (Birc1e) restricts intracellular replication of the opportunistic human pathogen Legionella pneumophila (3–5). Naip5 is comprised of three modules: NH2-terminal baculoviral inhibitor of apoptosis repeats, a central NOD domain, and COOH-terminal LRRs (2). By analogy to other NOD-LRR proteins, the LRR region is thought to recognize microbial products, triggering oligomerization via the NOD domain and activation of a cellular response that is governed by various NH2-terminal effector-binding domains (2). Whereas virtually all mice are resistant to L. pneumophila, the A/J strain encodes a naip5 allele that confers susceptibility to infection (3). Whether the reduction in Naip5 protein (5) or a change in its function accounts for the failure of A/J macrophages to restrict L. pneumophila replication has not been unequivocally established.
The biochemical activity of mouse Naip5 is not yet known, but testable models can be drawn by analogy to related proteins. Human Naip/Birc1 inhibits apoptosis by binding effector caspases through its baculoviral inhibitor of apoptosis repeat domain (6, 7). Other NOD family members, including Ipaf and the NALPs, interact with the inflammasome, a caspase 1–containing complex that can be triggered by microbial products (8). In response to the intracellular pathogens Salmonella enterica, Shigella flexneri, or L. pneumophila, the inflammasome can initiate a caspase 1–dependent proinflammatory cell death (9–14).
Extensive inflammation, destruction of lung epithelium, and lysis of macrophages are clinical hallmarks of Legionnaires' disease pneumonia that are thought to result from cytotoxins (15–17). L. pneumophila can use its Dot/Icm type IV secretion system to insert pores into the membranes of either red or white blood cells (18, 19). However, to kill mouse macrophages, L. pneumophila requires not only type IV secretion but also flagellin (20), the major subunit of the flagellum. Flagellar genes are conserved in the species, as they equip L. pneumophila to build a flagellum and become motile, infect host cells efficiently, and avoid degradation (20, 21).
Both humans and mice detect L. pneumophila flagellin to mount an immune response. In humans, its recognition by TLR5 correlates with resistance to Legionnaires' disease (22). When injected into mice, L. pneumophila flagellin triggers a robust inflammatory response (23), which is a trait of other flagellins (24). Therefore, we tested the hypothesis that a Naip5- and caspase 1–dependent pathway equips mouse macrophages to detect cytosolic flagellin, induce a proinflammatory programmed death, and restrict the growth of intracellular L. pneumophila.
RESULTS
L. pneumophila requires flagellin to kill macrophages but not to perforate membranes
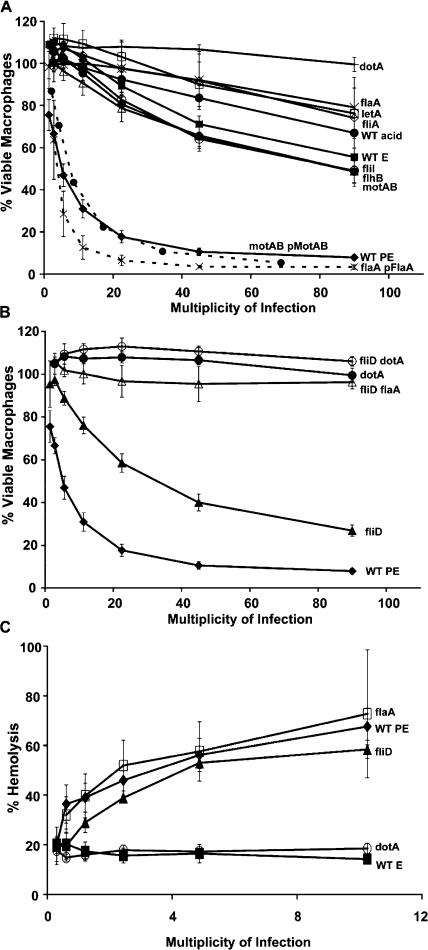
To test whether macrophages respond to L. pneumophila flagellin, a panel of previously characterized mutants (20) was analyzed for cytotoxicity to macrophages (Fig. 1 A). Compared with motile WT postexponential (PE) phase L. pneumophila, little macrophage toxicity was induced even by large numbers of the nonmotile strains that either lack flagellin or contain scant amounts (flagellin mutant flaA, MB534; regulatory mutant letA, MB413; and flagellar σ factor fliA, MB410). Flagellar mutants with intermediate amounts of flagellin induced corresponding levels of death (20). Flagellin on the surface of bacteria was implicated in cell death because a brief acid wash substantially reduced toxicity but not viability or intracellular multiplication of WT, motAB, and flhB microbes (Fig. 1 A and not depicted). Poor contact by nonmotile microbes did not account for the flagellin dependence of death, as flaA (flagellin mutant) and fliD (flagellin polymerization mutant MB552) bound macrophages to a similar extent (20), yet fliD mutants were substantially more toxic (Fig. 1). Flagellin also appeared more potent when nonpolymerized; compared with motAB motility motor mutants, fliD polymerization mutants have less total flagellin (20) but are more readily killed (40 vs. 65% viable bone marrow–derived macrophages; P < 0.05; multiplicity of infection [MOI] bin 30–60). As expected, the toxicity of fliD polymerization mutants required flagellin and type IV secretion (Fig. 1 B; flaA fliD, MB567; dotA fliD, MB569). Thus, by a process that requires type IV secretion, flagellin exported by L. pneumophila promoted macrophage death.
Figure 1.
L. pneumophila flagellin contributed to macrophage death but not pore formation. (A and B) After centrifugation with twofold dilutions of the strains indicated, A/J mouse macrophages and microbes were incubated for 1 h, and viability was determined by Alamar blue reduction. Mean percent viable macrophages ± SEM (error bars) are shown that were pooled from three or more experiments in MOI bins of twofold dilutions; the middle value for each bin is indicated. To facilitate comparisons between strains, the WT and dotA values are displayed in both A and B. (C) To quantify red blood cell lysis after incubation for 1 h with the microbes at each MOI indicated, soluble hemoglobin was measured spectrophotometrically. E, exponential phase (nonmotile); PE, postexponential phase (motile); acid, bacteria washed with acid to remove flagella; pFlaA and pMobAB, complementation plasmids carried by strains and described previously (20).
In stark contrast to its contribution to macrophage death, flagellin was dispensable for L. pneumophila to insert pores into red blood cells (15, 19). When equipped for Dot/Icm type IV secretion, nonmotile mutants were as hemolytic as motile WT whether or not they expressed flagellin (flaA, fliD, fliA, fliI, flhB, and motAB; Fig. 1 C and not depicted). Therefore, the ability to perforate eukaryotic membranes was not sufficient for L. pneumophila to kill macrophages rapidly; flagellin was also required.
Flagellin+ L. pneumophila triggers macrophage death independently of MyD88 TLR signaling but not Naip5
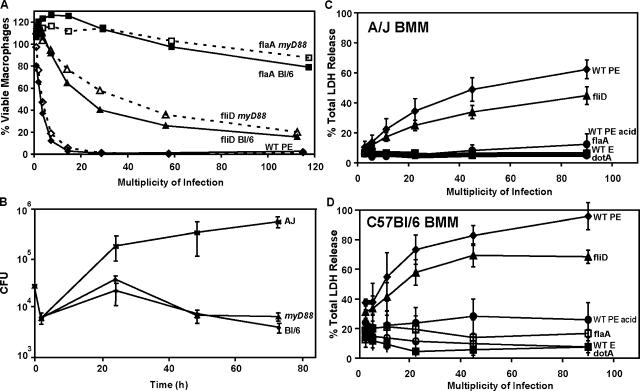
Extracellular flagellin is recognized by the human innate immune system through TLR5 (25). However, four observations discounted a role for TLR proteins in mouse macrophage intoxication by flagellin. Even in high concentrations, a crude flagellar preparation (CFP) was not toxic to macrophages (see Fig. 3 B; and not depicted). TLR5 is not detectable on mouse peritoneal or bone marrow–derived macrophages (26 and unpublished data). Macrophages that lack MyD88, the adaptor protein that mediates TLR5 and most other TLR signaling (1, 25), were as sensitive to flagellin-dependent death as the isogenic control cells (Fig. 2 A), and they also efficiently restricted L. pneumophila growth (Fig. 2 B).
Figure 3.
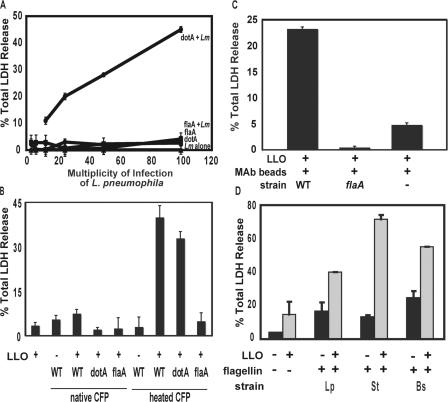
When present with a pore-forming activity, flagellin triggered macrophage death. (A) To test whether pore formation or substrates of the type IV secretion system are required for flagellin to stimulate death, LDH released by C57BL/6 cells incubated for 1 h with WT L. monocytogenes (Lm; constant MOI of 25) or dotA or flaA mutant L. pneumophila either alone or mixed (+) was quantified. To test whether cytosolic flagellin is toxic to macrophages, C57BL/6 macrophages were incubated with or without 1 ug/ml of the pore-forming toxin LLO for 2 h after an initial centrifugation with heat-treated or native CFPs (∼300 ng flagellin; B) or heat-treated flagellin (∼3 ng) that had been affinity purified and affixed to beads via a flagellin-specific monoclonal antibody (C). (D) To test whether macrophages responded to cytosolic flagellin from other microbes, C57BL/6 macrophages were incubated for 2 h without (black bars) or with 1 ug/ml LLO (gray bars) and 1.25 μg of either heated crude flagellin from L. pneumophila (Lp) or commercial preparations of S. typhimurium (St) or B. subtilis (Bs) flagellin. Means ± SD (error bars) of the percent total LDH released from permeable macrophages are shown, which were calculated from one experiment that is representative of at least two performed.
Figure 2.
Flagellin+L. pneumophila induced death by a mechanism independent of MyD88 but sensitive to Naip5. (A) To test whether TLR signaling induces death, the viability of C57BL/6 (solid lines, BL/6) or C57BL/6 myD88 −/− macrophages (dashed lines) was determined after infection for 1 h as shown and described in Fig. 1. (B) To test whether TLR signaling is required to restrict L. pneumophila growth, A/J, C57BL/6 (BL/6), or C57BL/6 myD88 −/− macrophages (myD88) were infected for the periods shown, and bacterial yield was determined by enumerating CFU. (C and D) To test whether Naip5 contributes to the host response, mean percent (± SEM) LDH released from naip5 A/J (B) or Naip5+ C57BL/6 (C) macrophages was quantified 1 h after the infections indicated in three or more experiments performed in triplicate, pooling results into MOI bins of twofold dilutions. E, exponential phase (nonmotile); PE, postexponential phase (motile); acid, bacteria washed with acid to remove flagella.
The cytosolic NOD-LRR protein Naip5 confers not only resistance of mice to L. pneumophila (3–5) but also susceptibility of infected macrophages to a caspase 1–dependent proinflammatory death (14). Therefore, we investigated whether Naip5 contributes to the detection of Flagellin+ microbes. Restrictive Naip5+ C57BL/6 macrophages were more sensitive than permissive naip5 mutant A/J cells to Flagellin+ L. pneumophila equipped for type IV secretion, as judged by lactate dehydrogenase (LDH) released at 1 or 6 h (Fig. 2, C and D; and not depicted). Compared with resistant cells, A/J macrophages express reduced levels of a Naip5 mutant protein that harbors 14 amino acid substitutions (3–5). Thus, the macrophage response to Flagellin+ pore-forming L. pneumophila correlated with the amount of Naip5 protein.
When present with a pore-forming activity, flagellin triggers macrophage death
To learn whether bona fide substrates of the type IV secretion are required for macrophage cytotoxicity, we exposed Flagellin+ L. pneumophila to the cytosol by another means. To perforate phagosomes, we exploited Listeria monocytogenes, a pathogen that escapes into the cytosol when listeriolysin O (LLO) forms pores in macrophage vacuoles. Like L. pneumophila that lack either type IV secretion (dotA) or flagellin (flaA), WT L. monocytogenes (1040S) did not rapidly cause considerable permeability of C57BL/6 macrophages, as measured by the release of cytosolic LDH (Fig. 3 A). However, macrophages released >30% of their LDH when coinfected with L. monocytogenes and either motile Flagellin+ dotA or nonmotile Flagellin+ dotA fliD L. pneumophila. The combination of pore formation and flagellin was required for the macrophage response because cells released little LDH when incubated either with a mixed suspension of L. monocytogenes and L. pneumophila flagellin-null mutants (flaA or dotA flaA MB600; Fig. 3 A and not depicted) or when coinfected with nonhemolytic llo − L. monocytogenes (DP-L2161) and Flagellin+ dotA L. pneumophila (not depicted). Thus, substrates of the L. pneumophila type IV secretion system were dispensable for macrophage cytotoxicity, whereas pore formation and flagellin were both required.
We investigated in more detail whether flagellin protein that has access to the cytosol triggers macrophage death. In either the presence or absence of the LLO toxin, CFPs of either WT, dotA, or flaA mutant L. pneumophila failed to trigger substantial LDH release from macrophages (Fig. 3 B). However, it is known that the flagellin epitopes recognized by TLR5 are buried within polymerized filaments (27), and we observed that L. pneumophila that secrete but cannot assemble flagellin protein are the most cytotoxic of the nonmotile mutants analyzed (Fig. 1, fliD). Therefore, to test whether cytoplasmic flagellin is toxic to macrophages when disassembled, the CFP was incubated at 78°C for 15 min, which is a treatment that promotes filament depolymerization (27). When exposed to heat-treated CFP and LLO, macrophages rapidly released LDH (Fig. 3 B).
Flagellin and not other microbial products appeared to trigger the macrophage response based on results of several control experiments. First, ∼3 ng of affinity-purified flagellin induced nearly the same amount of LDH release as either ∼30 or 300 ng of flagellin in CFP (Fig. 3 C and not depicted). Second, macrophages did not release substantial amounts of LDH when incubated with 1 μg/ml LLO protein that had been obtained from Escherichia coli lysates (Fig. 3, B and C). Third, when mixed with LLO, a mock flagellin preparation isolated from flaA mutant L. pneumophila was not toxic (Fig. 3, B and C). Fourth, either in the presence or absence of LLO, even 1 μg of native CFP did not induce considerable macrophage permeability (Fig. 3 B and not depicted). Fifth, proteinase K treatment substantially reduced the toxicity of heat-treated CFP (not depicted). Therefore, we postulate that macrophages are equipped with a cytosolic surveillance system that detects flagellin, specifically recognizing epitopes that are masked within L. pneumophila flagellar filaments.
The mouse macrophage response did not appear to be specific to cytosolic flagellin of L. pneumophila. Flagellin purified from the intracellular gram-negative pathogen Salmonella typhimurium or the soil gram-positive bacterium Bacillus subtilis also elicited LDH release from macrophages but only when LLO was present (Fig. 3 D). Heating had no effect on the potency of these commercial flagellins (unpublished data), which had already been treated with acid and heat. Thus, macrophages appear to recognize when their cytosol is contaminated with the flagellin from either L. pneumophila or at least two other microbes.
Flagellin+ L. pneumophila triggers a rapid, proinflammatory programmed cell death
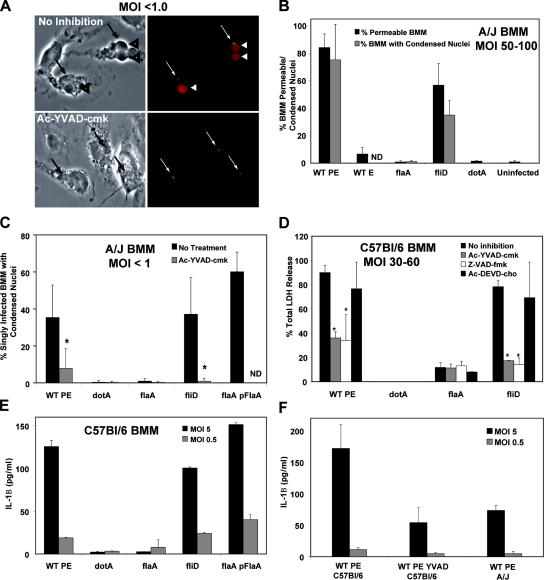
Because macrophages release cytosolic components soon after exposure to flagellin (Figs. 2 and 3), we postulated that this PAMP triggers pyroptosis, a rapid proinflammatory death that is accompanied by membrane permeability and nuclear condensation and requires caspase 1 (9, 12). After exposure for 1 h to Flagellin+ L. pneumophila, macrophages were permeable and had condensed nuclei (Fig. 4, A and B; WT or fliD). Both morphological changes were rare for macrophages infected by L. pneumophila that lack type IV secretion (dotA) or harbor little or no external flagellin (Fig. 4 B; WT E, flaA, and WT PE acid wash) even after prolonged incubations at a high MOI (Fig. 4 B and not depicted). A caspase 3 apoptosis inhibitor had little effect on LDH release (Fig. 4 D) or macrophage viability as measured by the reduction of Alamar blue (unpublished data). In contrast, macrophages were protected from flagellin-dependent toxicity by Ac-YVAD-CHO (Fig. 4; A, C, and D), a peptide inhibitor that exhibits a Ki > 200-fold lower for caspase 1 than caspases 2–10 (9, 28). Indeed, caspase 1 mutations render mice susceptible to infection by L. pneumophila, whereas mice that lack caspase 3 remain resistant (14). Even when infected for 1 h with one Flagellin+ bacterium, >35% of A/J macrophages exhibited caspase 1–dependent membrane permeability and nuclear condensation (Fig. 4, A and C).
Figure 4.
Pyroptosis was induced by pore-forming Flagellin+L. pneumophila. (A) After infection at an MOI of <1.0 for 1 h with WT L. pneumophila–expressing GFP, A/J macrophage permeability was analyzed by phase (left) and fluorescence microscopy (right). Arrows indicate single L. pneumophila; arrowheads indicate infected cells that have permeable membranes and phase dark condensed nuclei. (B) After infection with an MOI of 50–100 for 1 h as shown, mean percentages ± SD (error bars) of A/J macrophages that were permeable (black bars) or contained phase dark condensed nuclei (gray bars) were calculated from three or more independent experiments. (C) After infection at an MOI of <1.0 for 2 h as shown, mean percentages ± SD of A/J macrophages containing one bacterium that had phase dark nuclei was determined. (D) After infecting C57BL/6 macrophages for 1 h at an MOI of 30–60 as shown, mean percentages ± SD of LDH release was calculated from two to three experiments. (E and F) After infecting the macrophages shown for 1 h as indicated, secreted IL-1β was quantified. Results from one experiment representative of two to three others are shown. Where indicated, macrophages were treated for 1 h before and during the infection with 100 μM of inhibitors of caspase 1 (Ac-YVAD-cmk), pancaspases (Z-VAD-fmk), or caspase 3 (Ac-DEVD-cho). t test (*, P < 0.05) indicates significant differences ± caspase inhibitors. BMM, bone marrow–derived macrophage.
During pyroptosis, caspase 1 cleaves pro–IL-1β and pro–IL-18, which are discharged as active cytokines (12). After a 1-h exposure to Flagellin+ L. pneumophila, macrophages released IL-1β (Fig. 4 E; WT, fliD, or flaA pFlaA); by 6 h, IL-1β levels were approximately an additional fivefold higher (not depicted). Liberation of active IL-1β required not only L. pneumophila type IV secretion and flagellin (Fig. 4 E, flaA and dotA; and not depicted) but also macrophage caspase 1 activity (Fig. 4 F). Macrophages from mice that lack caspase 1 also fail to secrete IL-1β in response to L. pneumophila (14). Furthermore, Naip5+ C57BL/6 macrophages released more IL-1β than did naip5 mutant A/J macrophages (Fig. 4 F), which is consistent with their degree of permeability after exposure to Flagellin+ L. pneumophila (Fig. 2, B and C). Thus, when exposed to flagellin of type IV secretion-competent L. pneumophila, mouse macrophages initiated a rapid Naip5- and caspase 1–dependent proinflammatory death program.
Because L. pneumophila can induce apoptosis in numerous cell types (29–35), we tested whether flagellin triggers classic apoptosis in mouse macrophages. After a 5-h treatment with staurosporine, an inducer of apoptosis (34), 30% of the cells contained activated caspase 3, as judged by immunofluorescence microscopy (Fig. S1, A and B; available at http://www.jem.org/cgi/content/full/jem.20051659/DC1). In addition, a majority showed hallmarks of an apoptotic response, including chromatin condensation and nuclear blebbing, intact plasma membranes (as measured by LDH release and live/dead staining), and viability (as measured by Alamar blue reduction; unpublished data). A different pattern was observed for macrophages incubated with a high MOI of L. pneumophila: <5% contained appreciable activated caspase 3 even 5 h after infection (Fig. S1), and, by 1 h, ∼75% of the cells were permeable and had condensed nuclei (Figs. 4 B and S1 C). Thus, in mouse macrophages, L. pneumophila induces a proinflammatory death that is distinct from classic apoptosis, as judged by caspase 3 activation, nuclear morphology, plasma membrane permeability, sensitivity to caspase inhibitors and mutations (14), and the speed of the response.
Flagellin makes L. pneumophila vulnerable to innate immune defenses of mouse macrophages
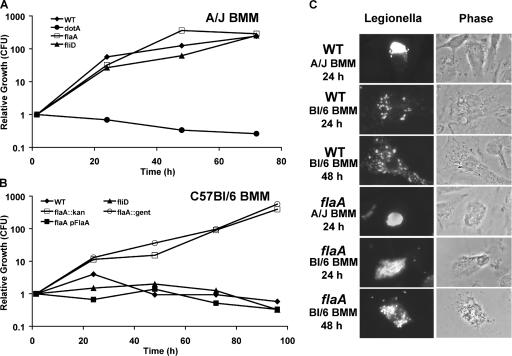
When infected by Flagellin+ L. pneumophila, Naip5+ mouse macrophages not only exhibit pyroptosis (Figs. 2–4 ) but also restrict bacterial replication (4, 5). Accordingly, we postulated that the Naip5 cytosolic surveillance pathway would be futile if the intracellular pathogens lack flagellin. During a 72-h incubation with permissive naip5 mutant A/J macrophages, all L. pneumophila strains equipped for type IV secretion replicated >100-fold regardless of motility, flagellin production, or assembly (WT, flaA, fliD, flhB, fliI, and motAB; Fig. 5 A and not depicted). As expected, Naip5+ C57BL/6 macrophages restricted the replication of WT L. pneumophila and other strains that encode flagellin (WT, fliD, flaA pFlaA, flhB, fliI, and motAB) to the level observed for type IV secretion mutants (dotA; Fig. 5 B and not depicted). In stark contrast, two independent flagellin-null mutant strains replicated freely in restrictive Naip5+ C57BL/6 or BALB/c macrophages (Fig. 5 B, MB534 flaA:kan and MB532 flaA:gent; and not depicted). A flagellin-null mutant of L. pneumophila strain Lp01 also escaped restriction by C57BL/6 macrophages (unpublished data).
Figure 5.
Naip5+ C57BL/6 macrophages restricted the growth of L. pneumophila that encode flagellin, in part, by degrading the intracellular progeny. Growth of the L. pneumophila strain shown in macrophages of permissive A/J mice (A) or restrictive C57BL/6 mice (B) was quantified in three or more experiments; representative data are shown. (C) The macrophages indicated were infected for 2 h with an MOI of <1 of WT or flaA mutant L. pneumophila, and at 24 or 48 h, the integrity of L. pneumophila and macrophages was analyzed by immunofluorescence (left) and phase-contrast (right) microscopy, respectively. BMM, bone marrow–derived macrophage.
Restrictive C57BL/6 cells exerted strong selective pressure against flagellin expression by intracellular L. pneumophila. In several experiments, 72–96 h after infecting macrophages with flaA mutants that carried the complementing plasmid pFlaA (MB557), a population of pFlaA-free microbes emerged, as quantified by loss of the plasmid's selectable marker (unpublished data). Enrichment for plasmid-cured bacteria was attributable to the flaA locus because flaA mutants maintained the pMMB vector (MB558) during replication in C57BL/6 macrophages, and they retained pFlaA (MB557) during growth in naip5 mutant A/J cells (unpublished data).
Microscopy provided additional insight into how Naip5+ mouse macrophages restrict L. pneumophila replication (Fig. 5 C). After a 2-h infection of either A/J or C57BL/6 macrophages, the majority of WT, flaA, and fliD microbes were intact, and <30% resided in LAMP-1–positive endosomal vacuoles, whereas >70% of dotA type IV secretion mutants were delivered to the endosomal pathway (20, 36). After a 24-h infection of permissive A/J macrophages, both WT and flaA microbes had replicated profusely and were mainly clustered in large LAMP-1–positive vacuoles as previously noted (37). A similar pattern was observed 24 h after Naip5+ C57BL/6 cells had ingested flaA mutants. In contrast, after 24 h in restrictive C57BL/6 cells, WT L. pneumophila had begun to replicate, but the progeny were less numerous, and they were dispersed throughout the cytoplasm in small LAMP-1–positive vacuoles (36). By 48 h, no further replication of WT microbes was evident; instead, a subpopulation of apparently healthy macrophages contained dispersed bacteria and bacterial debris. Thus, it was apparent that Naip5+ macrophages can restrict infection by Flagellin+ L. pneumophila either by committing pyroptosis or by slowly delivering microbes to degradative vacuoles.
Flagellin makes L. pneumophila vulnerable to innate immune defenses of mouse lungs
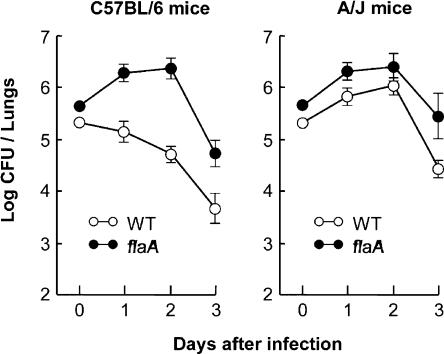
To evaluate whether the Naip5 surveillance pathway for cytosolic flagellin contributes to the control of L. pneumophila infections in lungs, the fates of WT and flaA mutant bacteria were compared after intratracheal inoculation of naip5 mutant A/J and Naip5+ C57BL/6 mice. As expected, within the lungs of restrictive Naip5+ mice, WT L. pneumophila failed to replicate; by the third day, the yield of CFU had decreased ∼50-fold (Fig. 6). In striking contrast, L. pneumophila that lack flagellin replicated in Naip5+ C57BL/6 mice. Their yield gradually increased for 2 d and then rapidly declined, a pattern similar to that of both WT and flaA mutant L. pneumophila within the lungs of naip5 mutant mice (Fig. 6). Thus, the detection of flagellin is critical to the robust mouse innate immune response that controls L. pneumophila infection.
Figure 6.
Naip5+ C57BL/6 mice restricted the growth of L. pneumophila that encode flagellin. C57BL/6 and A/J mice were infected via the trachea with L. pneumophila Lp01 or its flaA-deficient mutant, and the lung bacterial burden was quantified 1, 2, and 3 d later. Mean CFUs ± SD (error bars) are shown, each calculated from five animals.
DISCUSSION
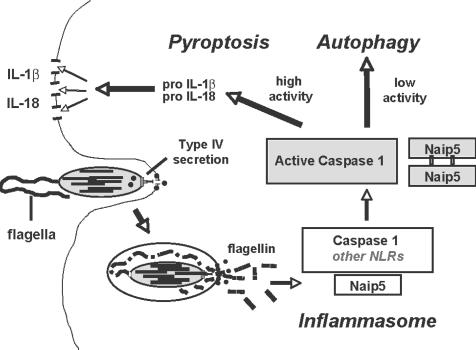
Cytosolic flagellin not only induced a rapid caspase 1–dependent proinflammatory macrophage death but also made L. pneumophila vulnerable to the innate immune system of mice that encode the NOD-LRR protein Naip5. Recent molecular genetic analysis of the signal transduction pathway that mediates the mouse response to L. pneumophila identified as critical components not only Naip5 but also the NOD-LRR proteins Ipaf and apoptosis-associated specklike protein (ASC; reference 14). Accordingly, we propose a model in which the macrophage response to Flagellin+ L. pneumophila is governed by Naip5 regulation of the inflammasome, a protein complex that contains the proinflammatory enzyme caspase 1 (Fig. 7; reference 38). During phagocytosis, the L. pneumophila type IV secretion system inserts pores into the macrophage membrane to deliver virulence effectors that perturb phagosome maturation (39). Flagellin protein that diffuses through these pores is detected by Naip5 either directly via its LRR region or indirectly by heterooligomerization with another NOD-LRR protein that binds flagellin (40). Consequently, Naip5 activates the inflammasome either directly or by interacting with the caspase 1 adaptor proteins Ipaf and ASC (14) and perhaps other NOD-LRRs. The activated inflammasome then coordinates the secretion of mature proinflammatory cytokines and the degradation of intracellular microbes to combat the infection (Figs. 4–6 ).
Figure 7.
Model for the induction of a caspase 1– and Naip5-dependent mouse macrophage innate immune response to cytosolic L. pneumophila flagellin. NLR, NOD-like receptor.
The inflammasome is a versatile sensor of infection whose specificity is conferred by adaptor proteins. Using mutant mice that lack particular components of the inflammasome, it was demonstrated that the detection of S. typhimurium requires the adaptor Ipaf. The response to L. monocytogenes, Staphylococcus aureus, or LPS in the presence of pore-forming agents requires cryopryin, whereas the mouse response to cytosolic Francisella tularenesis occurs independently of both (10, 41, 42). When macrophages are infected with L. pneumophila, Ipaf and Naip5 are required to restrict bacterial growth, whereas Naip5, Ipaf, and ASC coordinate the maximal secretion of IL-1β (14). In a transfected 293T cell model, the inflammasome can be activated by the muramyl dipeptide of peptidoglycan via cyropyrin (Nalp3) or Nalp1 or 2 (43). When incubated with bacterial RNA, macrophages release Il-1β by some mechanism that utilizes cryopyrin and caspase 1 (44). In this study, we implicate Naip5 as an adaptor that senses the cytosolic flagellin of L. pneumophila. Once activated, the inflammasome equips macrophages to combat intracellular pathogens by one or more methods: releasing inflammatory cytokines, degrading intracellular bacteria, and committing suicide (10, 14, 41, 42, 44).
In our model's simplest form, flagellin itself is detected by the macrophage cytosolic surveillance system. Formally, flagellin could instead mediate the release or translocation of another PAMP, such as peptidoglycan or LPS, which then activates the inflammasome (10, 45). For example, recent data indicate that Shigella flexneri delivers the LPS component lipid A to the cytosol, which induces a lytic death with features of pyroptosis, including nuclear condensation (13). However, we favor the model that flagellin protein is detected by the cytosolic surveillance system for several reasons. We have ruled out indirect contributions of flagellin to macrophage adherence (20) and type IV secretion. Macrophages are not intoxicated when exposed to native crude preparations of flagellin in the presence of the pore-forming LLO toxin but do respond to flagellin preparations that have been heated to disassemble filaments and affinity purified (Fig. 3). Most strikingly, the loss of flaA was sufficient to permit type IV secretion-competent L. pneumophila to replicate freely in restrictive mouse macrophages (Fig. 5) and also within lungs (Fig. 6). Whatever the exact mechanism, Naip5+ macrophages can efficiently restrict the replication of any microbe that harbors even minute quantities of flagellin (20).
How flagellin of type IV secretion-competent L. pneumophila is exposed to cytosolic NOD-LRR proteins remains to be determined. Because both the flagellar and type IV secretion systems are positioned at the bacterial pole (46) and become active exclusively in the PE phase of growth (20), sufficient flagellin may diffuse through the secretion channel into the cytoplasm to trigger the host response. Although flagellin does encode the two COOH-terminal leucine residue motifs that are common to known substrates of type IV secretion (47), L. pneumophila does not translocate flagellin as efficiently as bona fide effectors (46, 47), as judged by quantifying cAMP in macrophages infected with L. pneumophila expressing a CyaA-FlaA or a CyaA-RalF fusion protein (unpublished data). The type IV secretion system of Helicobacter pylori provides a conduit to the cytoplasm for peptidoglycan (48). Therefore, we favor the model that the L. pneumophila type IV secretion system inadvertently contaminates the macrophage cytosol with trace amounts of flagellin.
By analogy to TLR proteins, NOD-LRR proteins likely detect a variety of cytosolic PAMPs. Mice carry numerous tandem copies of closely related naip sequences and putative pseudogenes (3); presumably, these loci have diverged to detect distinct microbial components or fulfill distinct roles. Degradation products of bacterial peptidoglycan are detected by specific NOD-LRR family members (2), and our data indicate that flagellin is detected by a Naip5-dependent pathway. Compared with C57BL/6 cells, A/J macrophages contain reduced amounts of Naip5 protein (5), and they are less responsive to flagellin-mediated pyroptosis (Figs. 1 and 2), release less IL-1β (Fig. 4 F), and fail to restrict L. pneumophila replication in macrophages either in culture (Fig. 5) or in lungs (Fig. 6). Whether a component of the A/J mouse Naip5 pathway binds the flagellin-dependent PAMP less avidly or is misregulated requires further study. Although the detection of flagellin is one critical component of the mouse innate immune response to L. pneumophila infection, it is clear that other mechanisms also contribute: the burden of flagellin mutants does begin to decline after 3 d (Fig. 6 A). Unlike mice, humans encode a single Naip protein, so they may or may not use a similar mechanism to combat L. pneumophila infection.
Together with the TLR5 pathway, the Naip5 cytosolic surveillance system likely exerts selective pressure for intracellular pathogens equipped for flagellar motility to acquire sophisticated mechanisms in order to evade detection (24). S. enterica and L. monocytogenes repress flagellar expression in mammalian hosts (50), and not all species of flagellate microbes encode the epitope that is recognized by TLR5 (51). Because L. pneumophila coevolved with freshwater amoebae, perhaps this opportunistic human pathogen has not been subjected to selective pressures exerted by mammalian immune systems (52).
In addition to pyroptosis (Fig. 4), Naip5+ mouse macrophages also restrict infection by digesting intracellular L. pneumophila (36). During infections of S. enterica, an altered level of the NOD-LRR protein Ipaf affects whether host cells commit pyroptosis or restrict bacterial replication (45). Autophagy is a degradative pathway of macrophages whose activity correlates with naip5 status (53). Many microbes interact with the autophagy machinery (54), and there is regulatory cross talk between autophagy and programmed cell death (55). Accordingly, we have postulated that the degree of microbial contamination determines whether macrophages initiate autophagy as a cytoprotective measure or pyroptosis as a failsafe response to infection (56). By applying bacterial and mouse genetics, L. pneumophila infection of macrophages and lungs can be exploited to reveal how this cytosolic surveillance system detects and restricts infection by intracellular pathogens.
MATERIALS AND METHODS
Bacteria.
L. pneumophila (Table I) was cultured on CYET agar or in AYET broth to exponential or PE phase. The flagellar regulon is expressed exclusively in the PE phase (48). In synchronous PE broth cultures, >95% of strain Lp02 cells are motile, but only ∼10% of Lp01 bacteria are motile (20). Intracellular growth was calculated from duplicate wells as (total CFU)/(cell-associated CFU at 0 h) × 100.
Table I.
Bacterial strains
| Strain | Relevant genotype/phenotype | Reference |
|---|---|---|
| E. coli | ||
| DH5α | F-endA1 hsdR17 (r− m+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF)U16980dLacZΔM15λpirRK6 | M. Swanson lab |
| L. monocytogenes | ||
| 10403S | WT | 63 |
| DP-L2161 | LLO mutant | 64 |
| L. pneumophila | ||
| MB110 | Lp02 WT, thyA hsdR rpsL | |
| MB413 | Lp02 letA 22-3∷kan mutant (lpg2646) | 65 |
| MB416 | Lp02 letS 36∷kan mutant (lpg1912) | 65 |
| MB410 | Lp02 fliA 35∷kan mutant (lpg1782) | 65 |
| MB460 | Lp02 dotA∷gent mutant (lpg2646) | 57 |
| MB473 | Lp02 pMMBGentΔmob, vector control | 66 |
| MB552 | Lp02 fliD∷kan mutant (lpg1338) | 20 |
| MB553 | Lp02 fliD∷gent mutant | 20 |
| MB554 | Lp02 flhB∷gent mutant A (lpg1786) | 20 |
| MB556 | Lp02 flhB∷kan mutant | 20 |
| MB534 | Lp02 flaA∷kan mutant (lpg1340) | 20 |
| MB557 | Lp02 flaA∷kan mutant pMMBGent-flaA | 20 |
| MB558 | Lp02 flaA∷kan mutant pMMBGentΔmob vector | 20 |
| MB532 | Lp02 flaA∷gent mutant | 20 |
| MB559 | Lp02 pMMBGent-flaA | 20 |
| MB560 | Lp02 motAB∷gent mutant A (lpg1780-81) | 20 |
| MB562 | Lp02 motAB∷gent mutant A pMMB206-motAB | 20 |
| MB563 | Lp02 motAB∷gent mutant A pMMB206, vector control | 20 |
| MB564 | Lp02 fliI∷cam mutant (lpg1757) | 67 |
| MB567 | Lp02 flaA∷gent fliD∷kan double mutant A | 20 |
| MB569 | Lp02 dotA∷gent fliD∷kan double mutant A | 20 |
| MB593 | Lp02 dotA∷gent flaA∷kan double mutant A | This study |
| MB571 | Lp02 motAB∷gent fliD∷kan double mutant A | 20 |
| MB314 | Lp01 WT prototroph, hsdR rpsL | 68 |
| MB589 | Lp01 flaA∷kan mutant (lpg1340) | This study |
Macrophages.
Macrophages prepared from bone marrow of permissive A/J mice or restrictive BALb/C, C57BL/6J (The Jackson Laboratory), or C57BL/6 MyD88−/− mice (gift of C. Hogaboam, University of Michigan Medical School, Ann Arbor, MI) were cultured in RPMI+ 10% heat-inactivated FBS (20). Caspase activity was inhibited by 100 μM Ac-YVAD-cmk (caspase 1), Z-VAD-fmk (pancaspase), or Ac-DEVD-cho (caspase 3; Fisher Scientific).
Macrophage viability after a 1-h infection (Fig. 2 A) was quantified by the reduction of Alamar blue (AccuMed) after 6–12 h (20, 57). Macrophage permeability was indicated by LDH in supernatants using the CytoTox96 NonRadioactive Cytotoxicity kit (Promega; reference 19). Where noted, results were pooled by averaging percent viability in serial twofold MOI bins; mean percent viability ± SEM are shown. For coinfections, WT or LLO− L. monocytogenes (gift of M. O'Riordan, University of Michigan Medical School; Table I) at a final MOI of 25 was mixed with L. pneumophila at each MOI indicated (up to 100), centrifuged at 5,000 g for 5 min, gently resuspended in medium, and added to macrophages. Duplicate or triplicate wells were analyzed for LDH release; one experiment representative of more than three is shown.
Hemolysis.
A twofold dilution series of microbes in 100 μl of RPMI/FBS was distributed to 10 μl of 107 fresh washed sheep red blood cells (Becton Dickinson) in a 96-well plate. Samples were incubated at 37°C for 1 h and lysis assayed as described previously (19). Results were calculated for triplicate samples as percent hemoglobin released by detergent lysis prepared for a standard curve. Data were pooled in bins from three or more experiments.
Toxicity of cytosolic flagellin.
To eliminate motility but retain viability (Fig. 1 A), flagella were dissociated by treating PE bacteria with PBS, pH 2.0, for 5 min at 37°C (20). CFPs were obtained from WT, dotA, and flaA mutant L. pneumophila as described previously (20); protein concentration was determined by the Bradford assay. The protein concentration of the flaA mock flagellin preparation was adjusted to that of WT by the addition of BSA. To promote disassembly of flagellar filaments into monomers, CFPs were incubated at 78°C for 15 min (27). To affinity purify flagellin (Fig. 3 C), the rabbit monoclonal antibody 2A5 specific to L. pneumophila flagellin (58) was incubated overnight at 4°C with protein G carboxylate beads (Polysciences, Inc.) in PBS–BSA. After four washes with PBS, monoclonal antibody beads were incubated overnight at 4°C with or without heat-treated CFP. Bead preparations were washed four times and diluted into RPMI–FBS for macrophage infections. S. typhimurium and B. subtilis flagellin were purchased from InvivoGen.
To perforate macrophages, a recombinant His-tagged LLO protein was purified essentially as described previously (59) except that E. coli was lysed by the French press technique. The purity and concentration of the LLO toxin was determined by SDS-PAGE and Coomassie blue staining, and its activity was verified by hemolysis assay. To analyze the toxicity of cytosolic flagellin, LLO (1 μg/ml in RPMI–FBS) was incubated with either native or heat-treated CFP or affinity-purified flagellin bound to beads in RPMI–FBS at the concentration indicated, centrifuged onto 8 × 104 macrophages per well in 96-well plates, and incubated at 37°C for 2 h. Supernatants were then assayed for the cytosolic enzyme LDH.
Microscopy.
Duplicate coverslip cultures were stained with rabbit anti-Legionella, rat anti-LAMP1 antibody, and DAPI as described previously (20, 57). Macrophage permeability was quantified using the LIVE/DEAD Reduced Biohazard Cell Viability Kit (Invitrogen). After 1 h with a high (50–100) or low (<1) MOI, cells were incubated with dyes for 15 min and examined immediately. The percentage of total cells (high MOI) or percentage of singly infected cells (low MOI; phase contrast or SYTO-10 green stain) that was permeable (ethidium homodimer-2 red stain) was scored for >100 macrophages on duplicate coverslips; the means ± SD from three or more independent experiments are shown. To calculate the percent condensed nuclei ± SD in three or more experiments, duplicate samples were infected at the MOI indicated by centrifugation at 400 g for 10 min and were incubated for 1 or 2 h. After fixation and staining with DAPI, 100 macrophages from several fields were scored for phase dense, rounded, shrunken nuclei.
IL-1β.
4–5 × 105 macrophages were infected by centrifugation at 400 g for 10 min and incubated for 1 or 6 h. After centrifugation at 400 g for 5 min, supernatants were stored at −80°C until mouse IL-1β levels were determined in duplicate by the Quantikine ELISA (MLB00B; R&D Systems). Negligible IL-1β was detected when uninfected cells were lysed with 0.1% SDS, verifying that the mature form of IL-1β is not readily detected by this assay (60, 61). Means ± SD are shown for one experiment that is representative of two or three others.
Lung infections.
6–8-wk-old female A/J and C57BL/6 mice (Sankyo Laboratory) were cared for in the Toho University School of Medicine animal facility. Mouse infections were performed according to a protocol approved by the animal facility of the Toho University School of Medicine. After inducing anesthesia i.p. with 6 and 100 mg xylazine and ketamine per kilogram, respectively, mice were infected with PE L. pneumophila strain Lp01 as described previously (62). To quantify CFU, whole lungs were harvested and homogenized in 1.0 ml PBS using a tissue homogenizer (Biospec Products, Inc.), and 10-ml aliquots of a 1:10 dilution series in PBS were spread on CYE.
Online supplemental material.
Fig. S1 shows that L. pneumophila that express flagellin induced a macrophage death with features distinct from classical apoptosis, as judged by the lack of activated caspase 3 and the morphology of their nuclei. In Fig. S2, the purity and yield of the flagellin preparations were analyzed by SDS-PAGE. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20051659/DC1.
Supplemental Material
Acknowledgments
We thank Drs. M. O'Riordan, J. Swanson, and D. Friedman for advice and reagents, J.-D. Sauer for insightful discussions and data analysis, and P. Beemiller for the gift of purified LLO.
This work was supported by the University of Michigan Frederick G. Novy Fellowship (to A.B. Molofksy), the National Institutes of Health (grant 2 R01 AI040694 to A.B. Molofsky and M.S. Swanson), and the Naito Foundation, as well as grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to E.T. Fuse and K. Tateda).
The authors have no conflicting financial interests.
Note added in proof. Using independent genetic methods, Ren et al. also identified flagellin as a target of the mouse innate immune response to L. pneumophila (Ren, T., D.S. Zamboni, C.R. Roy, W.F. Dietrich, and R.E. Vance. 2006. PLoS Pathog. 2:e18).
Abbreviations used: ASC, apoptosis-associated specklike protein; CFP, crude flagellar preparation; LDH, lactate dehydrogenase; LLO, listeriolysin O; LRR, leucine-rich repeat; MOI, multiplicity of infection; NOD, nucleotide-binding oligomerization domain; PAMP, pathogen-associated molecular pattern; PE, postexponential; TLR, Toll-like receptor.
C.A. Madigan's present address is Program in Biological and Biomedical Sciences, Harvard Medical School, Boston, MA 02115.
References
- 1.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 2.Inohara, N., M. Chamaillard, C. McDonald, and G. Nunez. 2004. NOD-LRR Proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74:355–383. [DOI] [PubMed] [Google Scholar]
- 3.Fortier, A., E. Diez, and P. Gros. 2005. Naip5/Birc1e and susceptibility to Legionella pneumophila. Trends Microbiol. 13:328–335. [DOI] [PubMed] [Google Scholar]
- 4.Diez, E., S.H. Lee, S. Gauthier, Z. Yaraghi, M. Tremblay, S. Vidal, and P. Gros. 2003. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33:55–60. [DOI] [PubMed] [Google Scholar]
- 5.Wright, E.K., S.A. Goodart, J.D. Growney, V. Hadinoto, M.G. Endrizzi, E.M. Long, K. Sadigh, A.L. Abney, I. Bernstein-Hanley, and W.F. Dietrich. 2003. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13:27–36. [DOI] [PubMed] [Google Scholar]
- 6.Davoodi, J., L. Lin, J. Kelly, P. Liston, and A.E. MacKenzie. 2004. Neuronal apoptosis-inhibitory protein does not interact with Smac and requires ATP to bind caspase- 9. J. Biol. Chem. 279:40622–40628. [DOI] [PubMed] [Google Scholar]
- 7.Maier, J.K., Z. Lahoua, N.H. Gendron, R. Fetni, A. Johnston, J. Davoodi, D. Rasper, S. Roy, R.S. Slack, D.W. Nicholson, and A.E. MacKenzie. 2002. The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J. Neurosci. 22:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinon, F., and J. Tschopp. 2004. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 117:561–574. [DOI] [PubMed] [Google Scholar]
- 9.Fink, S.L., and B.T. Cookson. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73:1907–1916. [DOI] [PMC free article] [PubMed]
- 10.Mariathasan, S., K. Newton, D.M. Monack, D. Vucic, D.M. French, W.P. Lee, M. Roose-Girma, S. Erickson, and V.M. Dixit. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 430:213–218. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y., M.R. Smith, K. Thirumalai, and A. Zychlinsky. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan, M.A., and B.T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31–40. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki, T., K. Nakanishi, H. Tsutsui, H. Iwai, S. Akira, N. Inohara, M. Chamaillard, G. Nunez, and C. Sasakawa. 2005. A novel caspase-1/toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in infected macrophages. J. Biol. Chem. 280:14042–14050. [DOI] [PubMed] [Google Scholar]
- 14.Zamboni, D.S., K.S. Kobayashi, T. Kohlsdorf, Y. Ogura, E.M. Long, R.E. Vance, K. Kuida, S. Mariathasan, V.M. Dixit, R.A. Flavell, W.F. Dietrich, and C.R. Roy. 2006. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7:318–325. [DOI] [PubMed] [Google Scholar]
- 15.Alli, O.A., L.Y. Gao, L.L. Pedersen, S. Zink, M. Radulic, M. Doric, and Y. Abu Kwaik. 2000. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 68:6431–6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackmon, J.A., M.D. Hicklin, and F.W. Chandler. 1978. Legionnaires' disease. Pathological and historical aspects of a ‘new’ disease. Arch. Pathol. Lab. Med. 102:337–343. [PubMed] [Google Scholar]
- 17.Brieland, J., P. Freeman, R. Kunkel, C. Chrisp, M. Hurley, J. Fantone, and C. Engleberg. 1994. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am. J. Pathol. 145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel, J.P., H.L. Andrews, S.K. Wong, and R.R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 279:873–876. [DOI] [PubMed] [Google Scholar]
- 19.Kirby, J.E., J.P. Vogel, H.L. Andrews, and R.R. Isberg. 1998. Evidence of pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323–336. [DOI] [PubMed] [Google Scholar]
- 20.Molofsky, A.B., L.M. Shetron-Rama, and M.S. Swanson. 2005. Components of the Legionella pneumophila flagellar regulon contribute to multiple virulence traits, including lysosome avoidance and macrophage death. Infect. Immun. 73:5720–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuner, K., C. Dietrich, C. Skriwan, M. Steinert, and J. Hacker. 2002. Influence of the alternative sigma(28) factor on virulence and flagellum expression of Legionella pneumophila. Infect. Immun. 70:1604–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawn, T.R., A. Verbon, K.D. Lettinga, L.P. Zhao, S.S. Li, R.J. Laws, S.J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, et al. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J. Exp. Med. 198:1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricci, M.L., A. Torosantucci, M. Scaturro, P. Chiani, L. Baldassarri, and M.C. Pastoris. 2005. Induction of protective immunity by Legionella pneumophila flagellum in an A/J mouse model. Vaccine. 23:4811–4820. [DOI] [PubMed] [Google Scholar]
- 24.Ramos, H.C., M. Rumbo, and J.C. Sirard. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12:509–517. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi, F., K.D. Smith, A. Ozinsky, T.R. Hawn, E.C. Yi, D.R. Goodlett, J.K. Eng, S. Akira, D.M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410:1099–1103. [DOI] [PubMed] [Google Scholar]
- 26.Means, T.K., F. Hayashi, K.D. Smith, A. Aderem, and A.D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165–5175. [DOI] [PubMed] [Google Scholar]
- 27.Smith, K.D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M.A. Bergman, S.L. Barrett, B.T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4:1247–1253. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Calvo, M., E.P. Peterson, B. Leiting, R. Ruel, D.W. Nicholson, and N.A. Thornberry. 1998. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273:32608–32613. [DOI] [PubMed] [Google Scholar]
- 29.Muller, A., J. Hacker, and B. Brand. 1996. Evidence for apoptosis of human macrophage-like HL60 cells by Legionella pneumophila infection. Infect. Immun. 64:4900–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagele, S., J. Hacker, and B. Brand. 1998. Legionella pneumophila kills human phagocytes but not protozoan host cells by inducing apoptotic cell death. FEMS Microbiol. Lett. 169:51–58. [DOI] [PubMed] [Google Scholar]
- 31.Gao, L.Y., and Y. Abu Kwaik. 1999. Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect. Immun. 67:4886–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumeister, B., M. Faigle, K. Lauber, H. Northoff, and S. Wesselborg. 2002. Legionella pneumophila induces apoptosis via the mitochondrial death pathway. Microbiology. 148:3639–3650. [DOI] [PubMed] [Google Scholar]
- 33.Zink, S.D., L. Pedersen, N.P. Cianciotto, and Y. Abu-Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molmeret, M., S.D. Zink, L. Han, A. Abu-Zant, R. Asari, D.M. Bitar, and Y. Abu Kwaik. 2004. Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell. Microbiol. 6:33–48. [DOI] [PubMed] [Google Scholar]
- 35.Abu-Zant, A., M. Santic, M. Molmeret, S. Jones, J. Helbig, and Y. Abu Kwaik. 2005. Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila. Infect. Immun. 73:5339–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derre, I., and R.R. Isberg. 2004. Macrophages from mice with the restrictive Lgn1 allele exhibit multifactorial resistance to Legionella pneumophila. Infect. Immun. 72:6221–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturgill-Koszycki, S., and M.S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 10:417–426. [DOI] [PubMed] [Google Scholar]
- 39.Sexton, J.A., and J.P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic. 3:178–185. [DOI] [PubMed] [Google Scholar]
- 40.Damiano, J.S., V. Oliveira, K. Welsh, and J.C. Reed. 2004. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem. J. 381:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariathasan, S., D.S. Weiss, V.M. Dixit, and D.M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariathasan, S., D.S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W.P. Lee, Y. Weinrauch, D.M. Monack, and V.M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 440:228–232. [DOI] [PubMed] [Google Scholar]
- 43.Martinon, F., L. Agostini, E. Meylan, and J. Tschopp. 2004. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14:1929–1934. [DOI] [PubMed] [Google Scholar]
- 44.Kanneganti, T.D., N. Ozoren, M. Body-Malapel, A. Amer, J.H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, et al. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp 3. Nature. 440:233–236. [DOI] [PubMed]
- 45.Damiano, J.S., R.M. Newman, and J.C. Reed. 2004. Multiple roles of CLAN (caspase-associated recruitment domain, leucine-rich repeat, and NAIP CIIA HET-E, and TP1-containing protein) in the mammalian innate immune response. J. Immunol. 173:6338–6345. [DOI] [PubMed] [Google Scholar]
- 46.Bardill, J.P., J.L. Miller, and J.P. Vogel. 2005. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 56:90–103. [DOI] [PubMed] [Google Scholar]
- 47.Nagai, H., E.D. Cambronne, J.C. Kagan, J.C. Amor, R.A. Kahn, and C.R. Roy. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. USA. 102:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viala, J., C. Chaput, I.G. Boneca, A. Cardona, S.E. Girardin, A.P. Moran, R. Athman, S. Memet, M.R. Huerre, A.J. Coyle, et al. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166–1174. [DOI] [PubMed] [Google Scholar]
- 49.Bergman, M.A., L.A. Cummings, S.L. Barrett, K.D. Smith, J.C. Lara, A. Aderem, and B.T. Cookson. 2005. CD4+ T cells and toll-like receptors recognize Salmonella antigens expressed in bacterial surface organelles. Infect. Immun. 73:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grundling, A., L.S. Burrack, H.G. Bouwer, and D.E. Higgins. 2004. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc. Natl. Acad. Sci. USA. 101:12318–12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen-Nissen, E., K.D. Smith, K.L. Strobe, S.L. Barrett, B.T. Cookson, S.M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA. 102:9247–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molofsky, A.B., and M.S. Swanson. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29–40. [DOI] [PubMed] [Google Scholar]
- 53.Amer, A.O., and M.S. Swanson. 2005. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 7:765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkegaard, K., M.P. Taylor, and W.T. Jackson. 2004. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat. Rev. Microbiol. 2:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debnath, J., E. Baehrecke, and G. Kroemer. 2005. Does autophagy contribute to cell death? Autophagy. 1:66–74. [DOI] [PubMed] [Google Scholar]
- 56.Swanson, M.S., and A.B. Molofsky. 2005. Autophagy and inflammatory cell death, partners of innate immunity. Autophagy. 1:174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molofsky, A.B., and M.S. Swanson. 2003. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50:445–461. [DOI] [PubMed] [Google Scholar]
- 58.Byrne, B., and M.S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandal, M., and K.D. Lee. 2002. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim. Biophys. Acta. 1563:7–17. [DOI] [PubMed] [Google Scholar]
- 60.Herzyk, D.J., A.E. Berger, J.N. Allen, and M.D. Wewers. 1992. Sandwich ELISA formats designed to detect 17 kDa IL-1 beta significantly underestimate 35 kDa IL-1 beta. J. Immunol. Methods. 148:243–254. [DOI] [PubMed] [Google Scholar]
- 61.Dinarello, C.A. 1992. ELISA kits based on monoclonal antibodies do not measure total IL-1 beta synthesis. J. Immunol. Methods. 148:255–259. [DOI] [PubMed] [Google Scholar]
- 62.Tateda, K., T.A. Moore, J.C. Deng, M.W. Newstead, X. Zeng, A. Matsukawa, M.S. Swanson, K. Yamaguchi, and T.J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355–3361. [DOI] [PubMed] [Google Scholar]
- 63.Bishop, D.K., and D.J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005–2009. [PubMed] [Google Scholar]
- 64.Jones, S., and D. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammer, B.K., E.S. Tateda, and M.S. Swanson. 2002. A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol. Microbiol. 44:107–118. [DOI] [PubMed] [Google Scholar]
- 66.Hammer, B.K., and M.S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721–731. [DOI] [PubMed] [Google Scholar]
- 67.Merriam, J.J., R. Mathur, R. Maxfield-Boumil, and R.R. Isberg. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65:2497–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger, K.H., and R.R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.