Abstract
Background
Strategies for preventing hypoglycemia during exercise in children with T1D have not been well studied. DirecNet conducted a study to determine whether stopping basal insulin could reduce the frequency of hypoglycemia occurring during exercise.
Methods
Using a randomized, crossover design, 49 children 8–17y with T1D on insulin pump therapy were studied during structured exercise sessions on two days. On one day basal insulin was stopped during exercise and on the other day it was continued. Each exercise session, performed from approximately 4–5 p.m., consisted of four 15-minute treadmill cycles at a target heart rate of 140 beats/minute (interspersed with three 5-minute rest breaks over 75 minutes) followed by a 45 minute observation period. Frequently sampled glucose concentrations (measured in the DirecNet Central Laboratory) were measured prior to, during, and following the exercise.
Results
Hypoglycemia (≤70 mg/dL) during exercise occurred less frequently when the basal insulin was discontinued than when it was continued (16% vs. 43%; P=0.003). Hyperglycemia (increase from baseline of ≥20% to ≥200 mg/dL) 45 minutes after the completion of exercise was more frequent without basal insulin (27% vs. 4%; P=0.002). There were no cases of abnormal blood ketone levels.
Conclusion
Discontinuing basal insulin during exercise is an effective strategy for reducing hypoglycemia in children with T1D, but the risk of hyperglycemia is increased.
Although children and adolescents with type 1 diabetes (T1D) are encouraged to exercise regularly, plasma glucose concentrations are often difficult to manage during prolonged periods of physical activity. In patients maintained on fixed basal/bolus insulin regimens, exercise-induced increases in glucose utilization can lead to hypoglycemia (1, 2).
The examination of variables contributing to hypoglycemia during and after exercise in youth with diabetes has been the subject of increasing research (2–4). These studies illustrate that the type, duration and timing of exercise, as well as its temporal relation to meals and pre-meal insulin doses, may affect the glucose lowering effects of exercise in children with T1D. However, few recent studies have examined the most effective means to prevent hypoglycemia during exercise in children.
A previous DirecNet study of 50 children with T1D found that plasma glucose concentrations fell during moderate intensity, treadmill exercise in almost all patients, decreasing >25% in 83%, and 30% of subjects required treatment for hypoglycemia (2,5). Since the subjects received the same fixed basal insulin rates on both days, such maintenance of basal insulin may have contributed to the development of hypoglycemia.
The present study was undertaken to determine whether complete suspension of basal insulin infusion could effectively prevent hypoglycemia during exercise in children with T1D on insulin pump therapy.
Methods
Subjects
Consent Procedures
The DirecNet Data and Safety Monitoring Board and the Institutional Review Boards at each DirecNet center approved the study protocol, consent form and assent form. A parent or guardian and each subject gave written consent and assent, respectively.
Eligibility Criteria and Assessment
To be eligible for the study the subject had to have 1) age between 8 and 18 years, 2) clinical diagnosis of type 1 diabetes mellitus of ≥18 months duration, 3) stable insulin regimen using an insulin pump for at least one month prior, 4) HbA1c ≤10.0% measured with the DCA®2000 (Bayer Diagnostics, Tarrytown, NY), 5) body mass index (BMI) between the 5th and 95th percentile for age and gender (6), 6) body weight ≥39.5 kg, and 7) normal thyroid function. Subjects were not eligible if they 1) had asthma that was medically treated in the prior year, 2) were currently using glucocorticoids or beta blockers, 3) anticipated a significant change in exercise regimen between admissions, or 4) had a medical condition or were using a medication that in the judgment of the investigator could affect completion of the exercise protocol.
Study Procedures
The study consisted of two 75-minute exercise sessions in the late afternoon, separated by 6 to 36 days. For one of the visits (labeled “basal-stopped”), the insulin pump was turned off at the beginning of the exercise session and re-started 45 minutes post-exercise (approximately 2 hours total). For the other visit, the basal rate was continued during the exercise (labeled “basal-continued”). The order of the basal-stopped and basal-continued visits was determined at random using a crossover design.
Management of insulin and meals was as similar as possible during the basal-continued and basal-stopped visits. On both days, the subject arrived at the clinic’s clinical research center (CRC) prior to 12 p.m. and remained until approximately 6:30 p.m. Lunch was served in the CRC at approximately noon using the pre-lunch bolus and correction factor the subject usually used at home. Glucose was checked using a home glucose meter (HGM; see below) at 1 p.m., 2 p.m. and 3 p.m., with the goal of having the subject’s glucose concentration between 120 and 200 mg/dL just prior to the start of exercise (approximately 4 p.m.). An intravenous bolus of regular insulin (0.05 to 0.1 units/kg) was given because of its short half-life and duration of action if the investigator felt the subject would likely be >200 mg/dL at 4 p.m., or 15–30g of carbohydrate was given orally if the investigator felt the subject’s 4 p.m. glucose would likely be <120 mg/dL. If at 4 p.m., the subject’s glucose was not within 120 to 200 mg/dL or the subject had been given IV insulin within the previous hour, then the exercise session was delayed. The glucose was checked every 15 minutes and if not in range by 5:00 p.m. the visit was rescheduled (3 basal-stopped and 2 basal-continued visits were rescheduled).
Exercise Procedures and Post-exercise Period
The exercise session consisted of 15 minutes of brisk walking on a treadmill at a heart rate of approximately 140 beats/minute (estimated to be equivalent to 55% VO2max (7)) followed by a 5-minute rest period. A heart rate monitor was worn throughout exercise and treadmill speed and/or incline were adjusted as necessary to achieve the target heart rate. This cycle was repeated 3 more times for a total of four 15-minute exercise periods with 5-minute rest periods in between (75 minutes total).
Venous samples were used for HGM (FreeStyle® Flash™ (8)),“Freestyle”, Abbott Diabetes Care, Alameda, CA) and central laboratory (at the DirecNet Central Biochemistry Laboratory at the University of Minnesota using a hexokinase enzymatic method (9, 10)) glucose determinations prior to starting exercise, during each of the 3 rest periods, immediately following exercise, and at 15, 30 and 45 minutes following completion of the exercise session. All results are expressed as plasma glucose concentrations. If during exercise the HGM glucose dropped ≤65 mg/dL, further exercise was delayed until the glucose was >70 mg/dL. Prior to starting and at the completion of the exercise session, blood ketones were checked by fingerstick using a Precision Xtra™ meter (Abbott Diabetes Care, Alameda, CA).
Statistical Methods
The sample size was estimated to be 55 subjects to have 80% power with an alpha level of 5% to detect a halving (42% vs. 21%) of the hypoglycemia rate. During the study period of May to December 2005, 57 subjects were enrolled, but 7 dropped out before completing both exercise sessions because of scheduling conflicts (N=2), problems with the IV during the first visit (N=1), lost to follow-up (N=1), other reasons (N=3). These subjects did not differ from the others in terms of age, gender, HbA1c, duration of diabetes or BMI. One subject was excluded because the two visits were 116 days apart, leaving 49 subjects for analysis.
Hypoglycemia was considered to have occurred when a central laboratory glucose concentration was ≤70 mg/dL. Except where otherwise stated, the definition of hypoglycemia also included cases (1 basal-stopped and 1 basal-continued) in which hypoglycemia treatment was given based on a Freestyle meter glucose value but a confirmatory central laboratory glucose value ≤70 mg/dL was not present. Analyses using only central laboratory confirmed hypoglycemia cases are indicated as such.
The proportions of subjects developing hypoglycemia on the basal-continued visits were compared with those on the basal-stopped visits using generalized estimating equations (GEE) controlling for baseline glucose, period (1st vs. 2nd visit) and repeated measures from the same subject. Baseline glucose was treated as a continuous covariate.
Hyperglycemia was defined as a plasma glucose concentration ≥200 mg/dL that had increased from baseline by at least 20%. Development of hyperglycemia was analyzed using a similar GEE regression model.
Drop in glucose was defined as the baseline minus the nadir glucose concentration during exercise. Since subjects were treated when the glucose dropped ≤65 mg/dL, the nadir glucose was truncated at 65 mg/dL for calculation of the drop in glucose. The percent drop in glucose was defined as the drop divided by the baseline concentration. Both of these outcomes were analyzed using a repeated measures least squares regression model adjusting for baseline glucose and period effects.
Results
The mean age of the 49 subjects was 14.5 ± 2.0 years (range 8 to 17 years); 43% were female; 94% were Caucasian, 2% Hispanic, 2% Asian, and 2% reported more than one race. The mean body mass index of the subjects was 22.3 ± 3.0 kg/m2 (range 15.8 to 30.1) The mean duration of diabetes was 7.2 ± 3.8 years and the mean HbA1c was 7.5 ± 0.9%. A severe episode of hypoglycemia (resulting in seizure or loss of consciousness) in the 6 months prior to the study was reported by 3 subjects (6%). Twenty-six subjects completed the basal-continued visit first and 23 completed the basal-stopped visit first. The median time between the two visits was 14 days (25th, 75th percentiles 8, 20 days; range 6–36 days).
Completion of Exercise Protocol
The full exercise session was completed at 95 (97%) of the 98 visits. In 3 sessions (1 basal-continued and 2 basal-stopped), at least the first two cycles were completed but the subject could not complete the session due to hypoglycemia. On 6 of the other 95 visits (2 basal-continued and 4 basal-stopped), the subject failed to achieve the target heart rate of 140 beats/minute for one of the four cycles (heart rate achieved ranged from 128 to 137 beats/minute).
Pre-exercise Glucose Concentrations
Baseline glucose concentrations prior to the start of the exercise measured at the central laboratory ranged from 115 to 230 mg/dL (all but one of the Freestyle values were within the specified range of 120 to 200 mg/dL). Baseline values were similar on basal-continued and basal-stopped visits (mean ± SD = 156 ± 27 vs. 161 ± 24 mg/dL; P=0.30).
Drop in Plasma Glucose and Hypoglycemia
As seen in Figure 1 and the table, the drop in glucose from baseline during exercise was less during the basal-stopped visit than during the basal-continued visit (absolute change 44 ± 38 vs. 63 ± 33 mg/dL, P<0.001; relative change 28% ± 23% vs. 41% ± 19%, P<0.001), as was the frequency of hypoglycemia (16% versus 43%, P=0.003). Hypoglycemia occurred only on the basal-continued visit in 15 subjects (31%), only on the basal-stopped visit in 2 subjects (4%), on both visits in 6 (12%), and on neither visit in 26 (53%). For the two subjects with hypoglycemia on the basal-stopped days, one had similar baseline glucose concentrations on the two days (127 vs. 131 mg/dL) and one had a higher baseline glucose concentration on the basal-continued day (185 vs. 127 mg/dL). Four subjects who did not become hypoglycemic during the exercise became hypoglycemic within 45 minutes of exercise completion (all on the basal-continued visit). The lower incidence of hypoglycemia on the basal-stopped visits was consistent in subgroups based on HbA1c, age, gender, and frequency of exercise performed at home. As can be seen in Figure 2A, hypoglycemia during exercise was unusual in the basal-stopped but not the basal-continued visit, when the baseline glucose level was >130 mg/dL (9% vs. 46%). Hypoglycemia occurred more often on the first compared to the second visit, but the difference was not statistically significant (37% vs. 22%; P=0.12). There was no evidence of a treatment by period interaction (P=0.30).
Figure 1. Plasma Glucose Concentrations during/following Exercise (N=98 visits from 49 subjects).
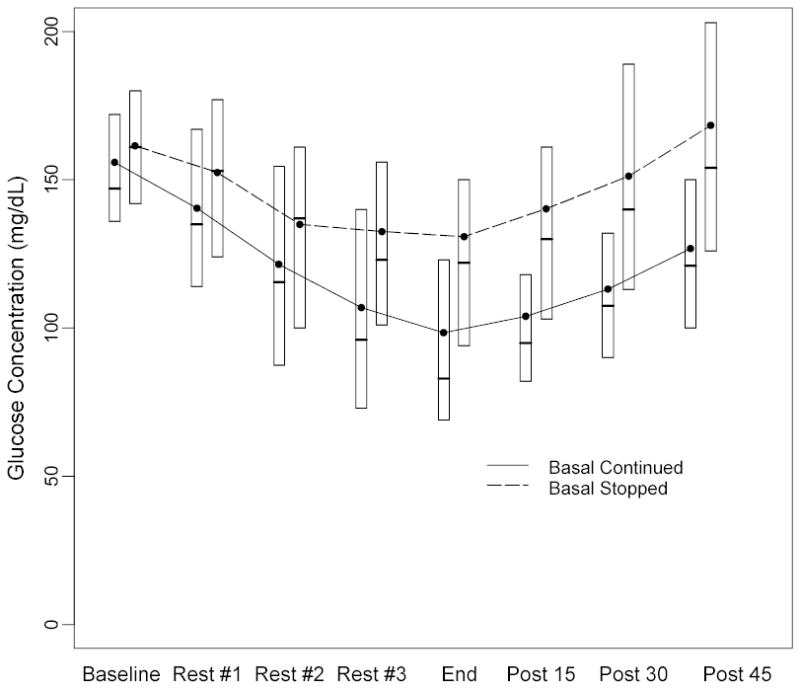
Black dots denote mean values and boxes denote median, 25th and 75th percentiles.
Table.
Change in Glucose and Development of Hypoglycemia During and Following Exercise*
| Basal-Continued (N=49) | Basal-Stopped (N=49) | P-value | |
|---|---|---|---|
| Baseline | 156 ± 27 | 161 ± 24 | 0.30 |
| During Exercise | |||
| Glucose Drop† | 63 ± 33 | 44 ± 38 | <0.001 |
| % Glucose Drop‡ | 41% ± 19% | 28% ± 23% | <0.001 |
| Hypoglycemia§|| | 21 (43%) | 8 (16%) | 0.003 |
| Hyperglycemia¶ | 2 (4%) | 6 (12%) | 0.11 |
| Additional Events Following Exercise# | |||
| Hypoglycemia§ | 4 | 0 | |
| Hyperglycemia¶ | 1 | 7 | |
| During or Following Exercise | |||
| Hypoglycemia§ | 25 (51%) | 8 (16%) | <0.001 |
| Hyperglycemia¶ | 3 (6%) | 13 (27%) | 0.008 |
Glucose values are mg/dL. Data are mean ± SD or N (%).
Baseline glucose minus nadir.
Glucose Drop divided by baseline glucose (expressed as a percentage).
Glucose ≤70 mg/dL.
Includes 2 visits (1 basal-stopped and 1 basal-continued) where treatment was given for hypoglycemia based on a meter glucose value, but the central laboratory value was >70 mg/dL (85 and 71 mg/dL). P =0.001 for analysis restricted to laboratory confirmed cases.
Glucose increased ≥20% from baseline to ≥200 mg/dL.
Glucose measured 15, 30 and 45 minutes following completion of exercise.
Figure 2. Nadir and Post-Exercise Plasma Glucose Concentrations by Baseline Level (N=98 visits from 49 subjects).
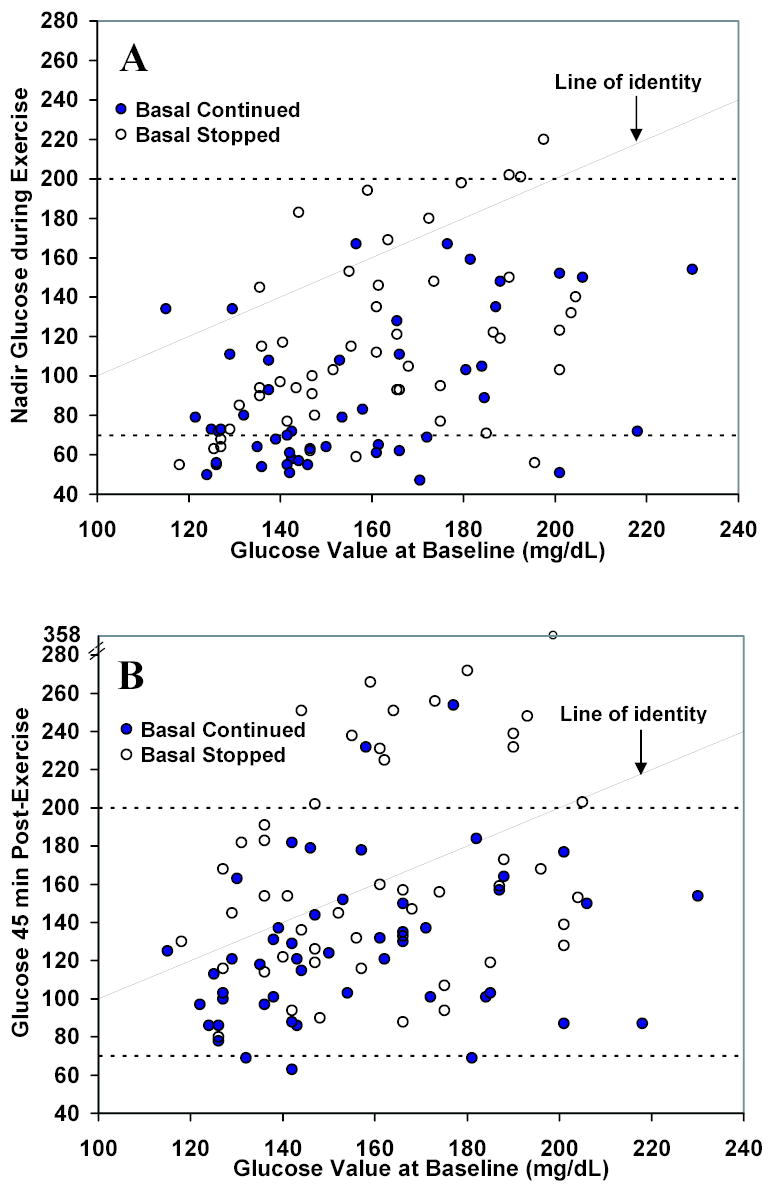
The nadir glucose concentration during exercise (A) and glucose concentration 45 minutes after completion of exercise (B) are shown by baseline level. Dashed lines denote the hypo- and hyperglycemia thresholds of 70 and 200 mg/dL, respectively. Note different scales on the horizontal and vertical axes.
Subjects were treated with 15–30g carbohydrates for hypoglycemia during or following exercise on 29 visits (22 basal-continued and 7 basal-stopped). A second treatment was necessary for 7 of these subjects (all basal-continued), two of whom required a third treatment and one required third and fourth treatments. Three subjects were still ≤70 mg/dL 45 minutes after exercise (63, 69 and 69 mg/dL). There were no cases of severe hypoglycemia during this study.
Hyperglycemia
During exercise, glucose concentrations increased from baseline by 20% or more to ≥200 mg/dL in 6 subjects on the basal-stopped visit and 2 subjects on the basal-continued visit (12% vs. 4%; P=0.11). The relationship between baseline and end of study (~120 minutes) glucose levels is shown in Figure 2B. At 120 minutes, plasma glucose levels had risen above baseline values in 21 (43%) of basal-stopped subjects, 13 (27%) of whom had become hyperglycemic (increased ≥20% from baseline to ≥200 mg/dL) compared with a rise in 8 (16%) basal-continued subjects, 2 (4%) of whom became hyperglycemic (P<0.001 and P=0.002). No abnormal blood ketone levels were observed during exercise (all values ≤0.4 mmol/L).
Discussion
In the present study, we demonstrated that stopping the basal insulin infusion at the start of a prolonged period of moderate aerobic exercise in the late afternoon was an effective strategy for reducing the risk of hypoglycemia during the exercise period. While this maneuver did not completely eliminate the risk of hypoglycemia, a fall in glucose that required treatment was infrequently observed if the pre-exercise plasma glucose level was >130 mg/dL. Moreover, the response to treatment of hypoglycemia with oral carbohydrate was more effective under basal-stopped conditions, since none of the subjects required more than one treatment with carbohydrate snacks compared with approximately one-third of the subjects during the basal-continued visit. Discontinuation of basal infusion was associated with a modest increased risk of hyperglycemia (12% vs. 4%, P=0.11) during exercise, but blood ketone levels remained suppressed.
Since children and adolescents with T1D may have periods of exercise and rest that extend beyond 75 minutes (e.g., an afternoon at the beach), we monitored the subjects for 45 minutes following exercise on both study days. During the basal-continued visit, 4 subjects who had not been hypoglycemic during exercise became hypoglycemic and one subject became hyperglycemic compared with no subjects becoming hypoglycemic and 7 becoming hyperglycemic during the basal-stopped visit. At the end of the study (approximately 120 minutes from the start of the study), a much greater rise in plasma glucose was observed on the basal-stopped day than on the basal-continued day, with hyperglycemia being present in 27% and 4% of subjects, respectively. The effect of delaying the restarting of insulin after exercise (as was done in the study) versus restarting it immediately following exercise warrants further study.
The duration and intensity of exercise in this study reflect the current national recommendation of at least 60 minutes of daily moderate to vigorous activity for children (11). We chose to study the effects of exercise at approximately 4 p.m., since this is when children and adolescents often engage in after school physical activities. Recent work using an objective monitoring system suggests that youth are most active from 3–7 p.m. (12). However, we recognize that a structured exercise program such as the one utilized in this study is not the same as real life exercise performed by children. We plan to conduct future studies with the use of accelerometry to better address the effect of “real life exercise” on glucose levels.
Since the last pre-meal bolus dose of insulin was given approximately 4 hours earlier, the subcutaneous depot of rapid-acting insulin analog was likely to be quite small in our subjects at baseline (13). While discontinuation of basal infusion was very effective in preventing episodes of exercise-induced hypoglycemia under these conditions, alterations in pre-meal bolus doses might be a more effective strategy to reduce the risk of hypoglycemia during bouts of exercise that occur shortly after a meal. This question was addressed by Schiffrin et al. in a study that pre-dated the introduction of insulin analogs (14). These investigators examined the effect of altering pre-meal insulin doses before a 45-minute exercise session in 7 adolescents on insulin pumps and 6 on multiple daily injections. Subjects were tested one time resting and four times during exercise after administering varied proportions of their usual insulin doses (0%, 50%, 67% and 100%). Under these conditions a 50% reduction in the pre-meal dose provided an effective means to reduce the risk of hypoglycemia. A recent study comparing 50% basal-insulin vs. basal-stopped during morning exercise in 10 adolescents with T1D found no difference in acute hypoglycemia or fall in BG (15). Hypoglycemia developed during 2 of the 10 basal-stopped sessions and during 2 of the 10 basal-continued sessions. The authors concluded that discontinuing the basal rate did not prevent hypoglycemia. A small sample size, shorter exercise session (40–45 minutes), timing of the exercise two hours after the breakfast meal and pre-meal bolus dose reduction in first study and 50% reduction in basal-insulin on the control visit in the latter study may explain why those results differ from the present study.
Because of its complexity, trial and error remains the principal method of regulating plasma glucose levels during exercise. However, the results of the present study can be used to guide recommendations for managing youth receiving insulin pump treatment during similar late afternoon exercise. The plasma glucose should be checked prior to exercise and 15–30 gms of carbohydrate should be taken if the glucose is <130 mg/dL or a small correction bolus should be given if the glucose is >200 mg/dL. While in most patients the pump can then be safely suspended or disconnected for up to 2 hours, glucose levels should be measured every 60–90 minutes during and after exercise and insulin administered when needed. The child and parents can also be informed that if hypoglycemia develops during exercise, it will be easier and more consistently treated with 15–30 gms of carbohydrate if the basal insulin infusion has been temporarily interrupted. The ability to suspend or reduce basal insulin during increased physical activity is another example of the flexibility of insulin pump therapy that distinguishes it from multiple daily injection regimens that utilize long-acting insulin analogs for basal insulin replacement.
Acknowledgments
Appreciation is expressed for the work performed by the CRC Nurses at the five clinical centers. This research has been supported by the following NIH/NICHD Grants: HD041919-01; HD041915-01; HD041890; HD041918-01; HD041908-01; and HD041906-01 and by Nemours Research Programs. Clinical Centers also received funding through the following GCRC Grant Numbers M01 RR00069; RR00059; RR 06022 and RR00070-41. Abbott Diabetes Care, Alameda, CA, provided the Freestyle® Blood Glucose Monitoring Systems and test strips and the Precision Xtra™ Ketone Monitoring Systems and test strips.
Appendix
Writing Committee
Lead authors: Eva Tsalikian, MD; Craig Kollman, PhD; William V. Tamborlane, MD; Additional writing committee members (alphabetical): Roy W. Beck, MD, PhD; Rosanna Fiallo-Scharer, MD; Larry Fox, MD; Kathleen F. Janz, EdD; Katrina J. Ruedy, MSPH; Darrell Wilson, MD; Dongyuan Xing, MPH; Stuart A. Weinzimer, MD
The DirecNet Study Group
Clinical Centers: (Listed in alphabetical order with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, MD (PI); Rosanna Fiallo-Scharer, MD (I); Laurel Messer, RN (C); Barbara Tallant, RN, MA (C); (2) Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Linda F. Larson, RN (C); Julie Coffey, MSN (C); (3) Nemours Children’s Clinic, Jacksonville, FL: Tim Wysocki, PhD, ABPP (PI); Nelly Mauras, MD (I); Larry A. Fox, MD (I); Keisha Bird, MSN (C); Kim Englert, RN (C); (4) Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Jennifer M. Block, RN, CDE (C); Paula Clinton, RD, CDE (C); (5) Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Elizabeth A. Doyle, MSN (C); Kristin Sikes, MSN (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Dongyuan Xing, MPH; Cynthia R. Stockdale; University of Minnesota Central Laboratory: Michael W. Steffes, MD, PhD; Jean M. Bucksa, CLS; Maren L. Nowicki, CLS; Carol A. Van Hale, CLS; Vicky Makky, CLS; National Institutes of Health: Gilman D. Grave, MD; Karen Teff, PhD; Karen K. Winer, MD; Data and Safety Monitoring Board: Dorothy M. Becker, MBBCh; Patricia Cleary, MS; Christopher M. Ryan, PhD; Neil H. White, MD, CDE; Perrin C. White, MD
Footnotes
This is an author-created, uncopyedited electronic version of an article accepted for publication in Diabetes Care (http://care.diabetesjournals.org). The American Diabetes Association (ADA), publisher of Diabetes Care, is not responsible for any errors or omissions in this version of the manuscript or any version derived from it by third parties. The definitive publisher-authenticated version is available online at [URL].
References
- 1.Stratton R, Wilson DP, Endres RK. Acute glycemic effects of exercise in adolescents with insulin-dependent diabetes mellitus. Phys Sportsmed. 1988;16:150–157. doi: 10.1080/00913847.1988.11709460. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Research in Children Network (DirecNet) Study Group. The effects of aerobic exercise on glucose and counter-regulatory hormone concentrations in children with type 1 diabetes. Diabetes Care. 2006;29:20–25. doi: 10.2337/diacare.29.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guelfi KJ, Jones TW, Fournier PA. Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2005;28:416–418. doi: 10.2337/diacare.28.2.416. [DOI] [PubMed] [Google Scholar]
- 4.Guelfi KJ, Jones TW, Fournier PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care. 2005;28:1289–1294. doi: 10.2337/diacare.28.6.1289. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Research in Children Network (DirecNet) Study Group. Impact of exercise on overnight glycemic control in children with type 1 diabetes. J Pediatr. 2005;147:528–534. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey. BMI-for-age charts, 2 to 20 years, LMS parameters and selected smoothed BMI percentiles, by sex and age, 2000. Available from: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm Accessed February 2003
- 7.Rowland TW. Exercise Testing. Developmental Exercise Physiology Champaign, IL: Human Kinetics Publishers; 1996:27–47
- 8.Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of newer-generation home blood glucose meters in a Diabetes Research in Children Network (DirecNet) inpatient exercise study. Diabetes Technol Ther. 2005;7:675–680. doi: 10.1089/dia.2005.7.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neese JW, Duncan P, Bayse D, Robinson M, Cooper T, Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national glucose reference method. Atlanta: Centers for Disease Control; 1976. HEW Publication No. 77–8330
- 10.Passey RB, Gillum RL, Fuller JB, Urry FM, Giles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974) Clin Chem. 1977;23:131–139. [PubMed] [Google Scholar]
- 11.President's Council on Physical Fitness and Sports. Physical activity for children: Current patterns and guidelines. Research Digest. 2004;5:1–8. [Google Scholar]
- 12.Jago R, Anderson CB, Baranowski T, Watson K. Adolescent patterns of physical activity: Differences by gender, day, and time of day. Am J Prev Med. 2005;28:447–452. doi: 10.1016/j.amepre.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43:396–402. doi: 10.2337/diab.43.3.396. [DOI] [PubMed] [Google Scholar]
- 14.Schiffrin A, Parikh S. Accommodating planned exercise in type 1 diabetic patients on intensive treatment. Diabetes Care. 1985;8:337–342. doi: 10.2337/diacare.8.4.337. [DOI] [PubMed] [Google Scholar]
- 15.Admon G, Weinstein Y, Falk B, Weintrob N, Benzaquen H, Ofan R, Fayman G, Zigel L, Constantini N, Phillip M. Exercise with and without an insulin pump among children and adolescents with type 1 diabetes mellitus. Pediatrics. 2005;116:348–355. doi: 10.1542/peds.2004-2428. [DOI] [PubMed] [Google Scholar]


