Abstract
To evaluate the relevance of fragile histidine triad (FHIT) status in relation to drug treatment, we analyzed the sensitivity of the Fhit-negative non-small cell lung cancer (NSCLC) cell line NCI-H460 to different drugs, after treatment with an adenoviral vector expressing the FHIT transgene. Expression of Fhit resulted in reduced sensitivity to etoposide, doxorubicin, and topotecan. This feature was associated with Fhit-induced downregulation of DNA topoisomerases I and II. In contrast, expression of Fhit did not modulate sensitivity to Taxol, but produced a slight increase in sensitivity to cisplatin, as shown by colony-forming assays. Analysis of apoptosis revealed that, after cisplatin exposure, the number of apoptotic cells was two-fold higher in Fhit-expressing H460 cells. Moreover, it appeared that wild-type p53 was required for sensitization to cisplatin because the effect was marginal in A549 and Calu-1 cells, where the p53 pathway is altered and simultaneous restoration of p53 and Fhit in Calu-1 cells increased cisplatin sensitivity. Fhit could also partially restore sensitivity to cisplatin in Bcl-2- and Bcl-xL-overexpressing H460 cells that are normally resistant to this drug. Our results support the possible relevance of FHIT in cisplatin-based chemotherapy as well as in the reversal of drug resistance in NSCLC.
Keywords: Lung cancer, Fhit, cisplatin, chemosensitivity, apoptosis
Introduction
A major limitation to the curative potential of current chemotherapy is that many types of cancer, including lung cancer, are resistant to apoptotic stimuli of various antitumor agents and become progressively incurable [1,2]. The most effective chemotherapy for non-small cell lung cancer (NSCLC), the leading cause of death among human malignancies, has been cisplatin-based combination therapy for more than two decades; however, long-term survival rate remains unsatisfactory [3,4]. Understanding the molecular mechanism of apoptosis and the events that cause resistance to anticancer drugs is therefore crucial to developing new strategies for the therapeutic treatment of lung cancer, with the possibility of activating distinct or overlapping apoptotic pathways by combining different treatments to increase anti-tumor effects.
The tumor-suppressor gene FHIT, a member of the histidine triad gene family, might be a promising candidate for NSCLC therapy. The inactivation of Fhit protein is one of the earliest events in different types of human malignancies, including lung [5], esophageal [6], gastric [7], breast [8], and head and neck [9] tumors. Previous studies showed that the reintroduction of FHIT gene at low levels of expression in Fhit-negative lung cancer cell lines reduces cell proliferation and alters cell cycle profile, causing a G0/G1 arrest in a dose-dependent manner [10], whereas high levels of Fhit expression are required to efficiently induce apoptosis and to suppress tumorigenesis in vivo [11]. Furthermore, homozygous and heterozygous FHIT knockout mice exhibit high susceptibility to chemical carcinogens that can be prevented by treatment with Ad-Fhit, a Fhit-expressing adenoviral vector [12,13].
In the present study, to explore whether Fhit could modulate cell response to antitumor drugs, we used adenoviral-mediated gene transfer to restore Fhit expression in Fhit-negative lung cancer cells. We have previously reported that Fhit-induced apoptosis was associated with activation of the caspase-8 pathway and that Fhit-reexpressing cells were highly sensitive to external stimuli such as serum deprivation or Fas ligand treatment, suggesting an involvement of Fhit in regulating the apoptotic pathway at the cytoplasmic level [11]. In addition, by engineering various NSCLC cell lines to express different genes involved in apoptosis, we found that the apoptotic pathway triggered by Fhit is dependent on cytoplasmic mediators, whereas it is independent of mitochondrial mediators such as Bcl-2 and Bcl-xL. Nonetheless, early activation of caspase-8 leads to subsequent cleavage of Bid, promoting a connection between cytoplasmic and mitochondrial pathways to amplify the initial apoptotic stimulus mediated by caspase-8 [14].
Thus, because several lines of evidence support the notion that chemotherapeutic agents exert their action through a mitochondrial apoptotic pathway [15], we evaluated whether the FHIT gene is able to modulate the properties of antitumor drugs in NSCLC and we examined the effects of the simultaneous stimulation of Fhit-mediated extrinsic pathway and cisplatin-induced intrinsic pathway in different Fhit-negative lung cancer cell lines derived from different histologic subtypes: H460 (large cell carcinoma), A549 (adenocarcinoma), and Calu-1 (squamous cell carcinoma). Our data indicate that Fhit expression results in variable modulation of sensitivity to antitumor drugs, which can be explained at the molecular level with changes in drug target expression (e.g. DNA topoisomerases) or with cooperation between apoptotic pathways (e.g., cisplatin). The present study provides rational bases to combine Ad-Fhit and cisplatin to enhance apoptotic response in lung cancer cells.
Materials and Methods
Cell Lines
The human NSCLC cell lines H460, A549, and Calu-1 used in this study were purchased from ATCC (Manassas, VA). Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (Bio-Whittaker Europe, Verviers, Belgium). As previously described [15] for the generation of stable transfectants, H460 cells were transfected with 10 µg of pEFFLAGpGK DNA plasmid, in which Bcl-2 and Bcl-xL were subcloned, using Superfect reagent (Qiagen, Milan, Italy), and the selection was made using concentrations of puromycin (Sigma, St. Louis, MO) ranging from 1 to 2 µg/ml. Clones were tested by Western blot analysis for the expression of specific proteins. The ecdysone-inducible mammalian expression system (Invitrogen) was used to generate Calu-1 cells, which conditionally express FHIT and/or p53. The system uses the steroid hormone ecdysone analogue Ponasterone A (Pon A) to activate the expression of genes through a heterodimeric nuclear receptor. Clones selected from antibioticresistant cultures were tested for inducible p53 and FHIT expression after a 24-hour treatment with 7.5 µM Pon A.
Drugs
Different drugs were prepared as follows: cisplatin (Platinex; Bristol-Myers Squibb, New York, NY) was diluted in saline; Taxol was dissolved in dimethylsulfoxide and diluted in water; doxorubicin and topotecan (Hycamptin; Glaxo-SmithKline, Verona, Italy) were dissolved in water and diluted in saline; etoposide (Vepesid; Bristol-Meyers Squibb) was diluted in saline; and gemcitabine (Gemzar; Eli Lilly, Florence, Italy) was dissolved in saline.
Preparation of Adenoviral Vectors
The preparation of recombinant (E1- and E3-deleted) adenoviral vectors expressing the Fhit protein was performed in fetal kidney 293 cells (Microbix Biosystems, Inc., Toronto, Canada) using standard techniques, as described previously [11]. For Ad-Fhit, a 707-bp fragment of FHIT cDNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR) from human placental cDNA and cloned into an adenoviral shuttle vector (pQBI-AdCMV5) purchased from Qbiogene (Montreal, Canada). The adenovirus expressing LacZ (Ad-LacZ) protein was used as a control vector.
Western Blot Analysis
Western blot analysis was performed using standard techniques. From each sample, 25 µg of proteins lysates was separated on 8% to 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene difluoride membranes (Amersham Biosciences, Milan, Italy). Subsequently, membranes were incubated for 1 hour at room temperature in phosphate-buffered saline (PBS)-Tween supplemented with 5% nonfat dry milk. For immunodetection, the following antibodies were used: anti-Fhit polyclonal antibody 71-9000 (1:1000; Zymed, San Francisco, CA); anti-Bcl-2 monoclonal antibody clone 120 (1:250; Dako, Santa Barbara, CA); anti-Bcl-xL polyclonal antibody (1:1000; BD Pharmingen, San Diego, CA); topoisomerase I (1:500; BD Pharmingen); and topoisomerase IIα (1:500; Neomarkers, Fremont, CA). As controls, actin (1:5000; Sigma) and vinculin (1:100; Sigma) were used.
Sulforhodamine B (SRB) Assay
Cellular sensitivity to drugs of different classes was examined using the SRB assay [16]. Briefly, cells were treated with Ad-Fhit and Ad-LacZ for 48 hours and then harvested and seeded in 96-well microplates. Preliminary experiments were performed to determine the appropriate cell seeding number (H460 Ad-LacZ/H460 Ad-Fhit, 4000:6000 cells/well, respectively) after confirming the linear relationship between absorbance and the number of cells in the growth curve of each cell line. Cells were treated for 48 hours with cisplatin, Taxol, doxorubicin, etoposide, topotecan, or gemcitabine. The cells were fixed by adding cold trichloric acetic acid to medium. Plates were washed with spring water and air-dried, and SRB solution was added for 30 minutes. The plates were washed with 1% acetic acid, and cell-associated SRB was solubilized using 10 mM Tris (pH 10.5) [17]. IC50 is defined as the drug concentration causing a 50% decrease of absorbance at 550 nm over that of untreated control.
Colony-Forming Assay
Cell sensitivity to cisplatin was assessed by colony-forming assay, as previously described [17]. Cells were transduced with Ad-Fhit and Ad-LacZ constructs at a multiplicity of infection (MOI) = 5. Because Ad-Fhit infection alone resulted in a reduced number of outgrowing colonies compared with Ad-LacZ infection, 24 hours later, cells were harvested and seeded in 60-mm plates in triplicate (500 cells/dish for Ad-LacZ, and 1000 cells/dish for Ad-Fhit-transduced cells). After 24 hours, cells were exposed to 0.1 to 10 µM cisplatin for 1 hour or 24 hours. When the drug was removed, the cells were washed with saline and then incubated in drug-free medium until colonies in control samples have become evident (approximately 10 days later). Samples were stained with 1% crystal violet in methanol for 1 hour, and colonies of at least 30 cells were counted using an inverse microscope. IC50 is defined as the drug concentration producing a 50% decrease in cell survival.
TdT-Mediated dUTP Nick End Labeling (TUNEL) Assay
Analysis of apoptosis was performed using TUNEL. Cells were plated at 2 x 105 to 4 x 105 cells/six-well plate. On the following day, adenoviral vectors were added at an MOI = 5 in 1 ml of culture medium without serum. After 4 hours, fresh medium was added. Cisplatin treatment was performed 24 hours postinfection, and then the cells were cultured 24 to 48 hours before analysis. A total of 5 x 105 cells per sample were fixed with 2% paraformaldehyde in PBS (10 minutes on ice), washed three times with Tris-buffered saline (TBS; 50 mM Tris-HCl in saline solution, pH 7.5), permeabilized with ice-cold acetone (2 minutes on ice), and washed twice in TBS and once in distilled water. Staining was performed by incubating cells for 1 hour at 37°C in 30 µl (final volume) of TUNEL reaction mixture (In Situ Cell Death Detection Kit, Fluorescein; Roche, Milan, Italy). Cells with fragmented DNA display increased fluorescence, as measured using FACSCALIBUR flowcytometer (BD Biosciences, San Jose, CA). Apoptotic cells were defined based on negative controls represented by noninfected cells, cells infected with control adenoviruses (Ad-LacZ), and cells treated with TUNEL reaction mixture without the enzyme.
Quantitative Real-Time PCR
To evaluate the expression level of p21Waf1 mRNA in H460 infected with Ad-Fhit and Ad-LacZ and treated with cisplatin, quantitative real-time RT-PCR was performed. Total RNA (500 ng) from cells untreated or treated with cisplatin for 1 hour and then collected at different time points was reverse-transcribed. cDNA was then diluted 10 times, and 1 µl of the diluted sample was used in the PCR reaction performed with ready-to-use p21Waf1 Assay-on-Demand (Applied Biosystems, Milan, Italy). Hypoxanthine-guaninephosphoribosyl transferase was used as endogenous control for the normalization of different samples and for relative quantization of gene expression; the data were analyzed by comparative Ct method (ΔΔCt).
Results
Experimental Models
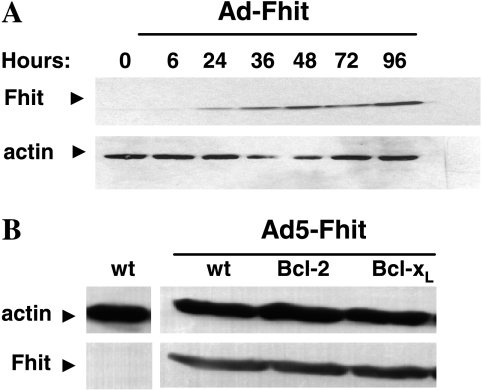
Adenoviral-mediated FHIT gene transfer was used to transduce the Fhit-negative NSCLC cell line H460. Cells were infected at an MOI = 5, and an adenoviral vector expressing the LacZ gene was used in all experiments as a control for possible alterations due to viral infection. In the experiments described below, we used experimental conditions under which 70% to 90% of target cells were transduced in the absence of vector-induced cytotoxicity. Our analysis included the parental H460 cell line as well as H460 clones stably transfected with antiapoptotic molecules Bcl-2 and Bcl-xL, which have been reported as a major determinant of resistance to anticancer agents [18,19]. Western blot analysis showed that Fhit protein expression was already detectable at 24 hours postinfection, increased at 36 hours, and then remained stable (Figure 1A). All cells were efficiently transduced and equally susceptible to adenoviral infection, as demonstrated by Western blot analysis (Figure 1B) and by a similar percentage of positive β-gal cells after infection with a control adenoviral vector carrying the LacZ reporter gene.
Figure 1.
Western blot analysis of Fhit expression in different cell systems. (A) Analysis of Fhit expression in Ad-Fhit-transduced NSCLC cells. H460 cells were infected at an MOI = 5 for Ad-Fhit, and Fhit expression was analyzed at different time points. The Fhit protein is already detectable at 24 hours, increases at 36 hours, and then remains stable. (B) Levels of expression of the Fhit protein in H460 clones stably transfected with the antiapoptotic proteins Bcl-2 and Bcl-xL.
Cellular Sensitivity of Ad-Fhit-Infected Cells to Antitumor Drugs of Different Classes
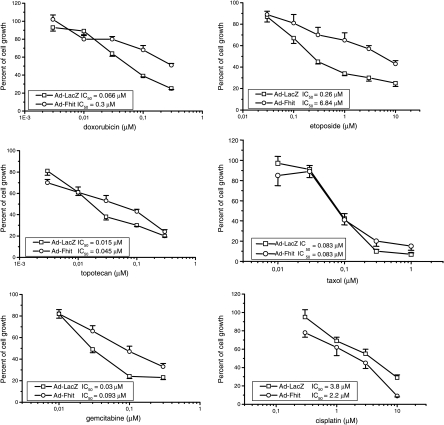
To define whether restoration of Fhit expression resulted in changes in cellular sensitivity to antitumor drugs of different classes, we used the SRB assay. H460 cells were infected with Ad-Fhit and Ad-LacZ at an MOI = 5. Cells were harvested 48 hours later and seeded in microtiter plates. Cells were treated for 48 hours with different concentrations of drugs, which act through different mechanisms of action (i.e., cisplatin, Taxol, etoposide, doxorubicin, gemcitabine, or topotecan). As shown in Figure 2, we found that adenovirus-mediated FHIT gene transfer resulted in reduced sensitivity to etoposide, doxorubicin, topotecan, and gemcitabine, but not to Taxol. Slightly increased sensitivity to cisplatin was observed with an IC50 = 2.2 µM for cells treated with Ad-Fhit compared to IC50 = 3.8 µM for cells treated with Ad-LacZ.
Figure 2.
Effect of Fhit expression on the sensitivity of H460 cell line to different antitumor drugs. Cell sensitivity was assessed by SRB assay after a 48-hour drug exposure. H460 cells were infected with Ad-Fhit and Ad-LacZ as controls at an MOI = 5 and were harvested 48 hours later. Cells were then seeded and exposed to drugs at indicated doses for 2 days. IC50 = concentration of a drug required to reduce absorbance by 50% at 550 nm.
DNA Topoisomerase Levels in Ad-Fhit-Infected Cells
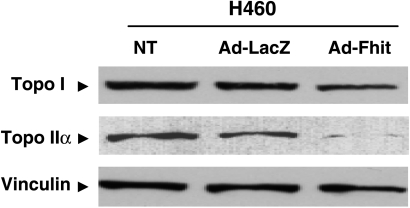
Because resistance to DNA topoisomerase I and II inhibitors has been associated with reduced levels of target [20,21], we performed a Western blot analysis of Ad-Fhit-infected cells to examine the modulation of topoisomerase expression. We found that expression of Fhit was associated with a decrease in topoisomerase II and I levels compared with Ad-LacZ-infected cells, with the extent being higher for the former enzyme (Figure 3).
Figure 3.
Western blot analysis of topoisomerase I and IIα expression. H460 cells were infected with an MOI = 5 of Ad-Fhit and Ad-LacZ, or treated with medium. The expression of Fhit was associated with a decrease in topoisomerase protein expression levels, which was more evident in topoisomerase IIα. Vinculin was used as a control.
H460 Sensitivity to Cisplatin Assessed by Colony-Forming Assay
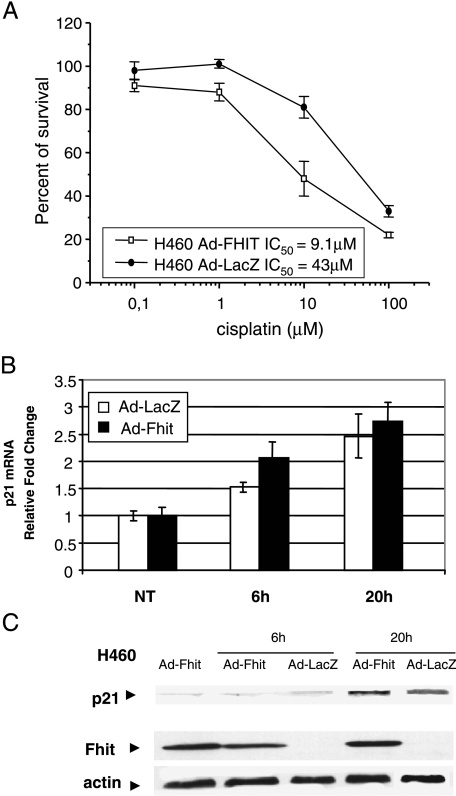
Because cisplatin is commonly used in the treatment of lung cancer, we performed further experiments to investigate whether the differences in sensitivity observed using the growth inhibition assay after combined treatment with cisplatin and Ad-Fhit were still evident using a clonogenic assay, which measures the long-term survival of tumor cells. The cells were infected with either Ad-Fhit or Ad-LacZ at an MOI = 5; after 24 hours, they were treated with different concentrations of cisplatin for 1 hour and then were tested for clonogenic potential. As shown in Figure 4A, reexpression of Fhit was associated with an increased sensitivity to cisplatin, with IC50 = 9.1 µM for cells treated with Ad-Fhit and with IC50 = 43 µM for cells treated with Ad-LacZ. Thus, Ad-Fhit cooperated with cisplatin to reduce the clonogenic survival of tumor cells.
Figure 4.
Antiproliferative effect of Ad-Fhit and cisplatin treatment. (A) Clonogenic survival of cisplatin-treated H460 cell line. H460 cells were infected with Ad-LacZ or Ad-Fhit for 24 hours and then treated with cisplatin for 1 hour before performing colony-forming assay. (B and C) Modulation of p21Waf1 expression. H460 cells were infected with Ad-Fhit and Ad-LacZ for 24 hours and treated with cisplatin. One hour later, the drug was removed; after 6 and 20 hours, p21Waf1 mRNA levels were assayed by real-time PCR (B). p21Waf1 protein expression was analyzed by Western blot analysis (C).
Previous studies have already reported the involvement of Fhit in cell growth control through increased expression levels of p21Waf1, a cell cycle regulator that binds and inhibits the activity of cyclin-dependent kinases [22]. To elucidate the molecular mechanisms underlying sensitization to cisplatin in H460 cells after FHIT gene transfer, we performed a realtime PCR to evaluate the levels of p21Waf1 transcript. H460 cells were infected with either Ad-Fhit and Ad-LacZ; after 24 hours, the cells were treated with cisplatin for 1 hour. The drug was removed; 6 and 20 hours later, cells were collected to evaluate the levels of p21Waf1. Real-time PCR analysis (Figure 4B) showed an increase of p21Waf1 transcript levels in Ad-Fhit-treated cells compared to controls (Ad-LacZ), suggesting that the synergistic effect between Fhit and cisplatin could be mediated by p21Waf1. The modulation of p21Waf1 transcript reflected changes also at protein levels because slightly higher levels of p21Waf1 protein were found in H460 cells under the combined Ad-Fhit and cisplatin treatment after 20 hours (Figure 4C). Recently, a synergistic oncosuppressive effect between Fhit and p53 has been reported. It has been suggested that this might be due to stabilization of p53, which is related to Fhit-mediated downregulation of MDM2 [23]. In our experimental conditions, we did not observe a change in p53 levels (data not shown); therefore, it seems more plausible that the higher sensitivity to cisplatin in Fhit-reexpressing cells might be explained by a convergence of the two pathways on a common mediator represented by p21Waf1.
Cisplatin-Induced Apoptosis in Ad-Fhit-Transduced Cells
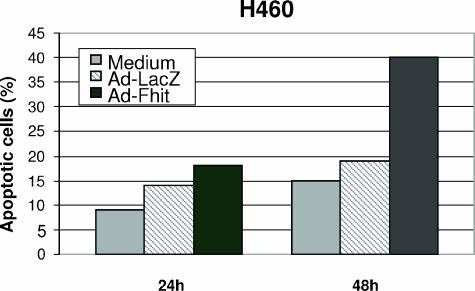
We have previously demonstrated that apoptosis induced by Fhit reexpression is modulated by a caspase-8-mediated cytoplasmic pathway, whereas cisplatin-induced apoptosis requires amplification by mitochondria for the activation of a downstream effector system [14]. Therefore, we performed an analysis of apoptosis induction using the TUNEL assay in H460 cells treated with cisplatin after transduction with Ad-Fhit or control Ad-LacZ. We used experimental conditions under which the infection rate was close to 70% without any cytotoxic effect induced by the vectors. The cells were then treated with 10 µM cisplatin, and TUNEL analysis was performed 24 or 48 hours after drug exposure. In the representative experiment shown, we found that, 24 hours after cisplatin exposure, the percentage of apoptotic cells was 18% in Ad-Fhit-infected cells compared to 14% in Ad-LacZ-infected cells and 9% in uninfected controls. TUNEL analysis performed 48 hours after cisplatin exposure revealed a marked difference in sensitivity, with the number of apoptotic cells being 40% in Ad-Fhit-infected cells, 19% in Ad-LacZ-infected cells, and 15% in uninfected controls (Figure 5). These data support the notion that restoration of Fhit protein in the Fhit-negative H460 cell line leads to an increased susceptibility to cisplatin through an enhancement at the apoptosis level.
Figure 5.
Apoptosis analysis. H460 cells were transduced with Ad-Fhit and Ad-LacZ at an MOI = 5, or treated with medium for 24 hours and then exposed to 10 µM cisplatin for 24 and 48 hours. Apoptosis was determined by TUNEL assay. The experiment is representative of assays, performed at least three times, that gave comparable results.
Effect of Ad-Fhit and Cisplatin Combination Treatment on Cisplatin-Resistant Clones
Bcl-2 and Bcl-xL are two important members of a family of proteins responsible for deregulation of apoptosis and prevention of death in cancer cells [24]. Their overexpression has been associated with cellular resistance to antitumor drugs [18,19]. H460 cells overexpressing Bcl-2 and Bcl-xL that were protected from cisplatin cytotoxicity remain sensitive to Fhit-induced apoptosis, thereby indicating that mitochondrial amplification is not required for Fhit activity [14]. To evaluate whether combined treatment with Ad-Fhit and cisplatin may result in a cooperative effect also in Bcl-2 and Bcl-xL H460-overexpressing cells, we examined clonogenic survival and apoptosis levels in these clones.
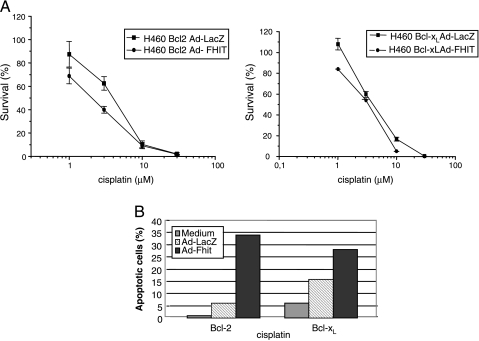
The clones overexpressing Bcl-2 and Bcl-xL were treated with Ad-Fhit, Ad-LacZ, or medium alone for 24 hours at an MOI = 5; under these conditions, the vectors did not show significant cytotoxicity. Transduced cells were then exposed to cisplatin for 1 hour. As shown in Figure 6A, the combination of Ad-Fhit and cisplatin moderately reduced colony formation, with the IC50 of Bcl-2-overexpressing cells transduced with Ad-Fhit being 2.07 µM and of cells transduced with Ad-LacZ being 4.21 µM. The effect was even less marked in Bcl-xL-overexpressing cells. Thus, the extent of modulation was less significant when compared to those obtained in the H460 parental cell line, probably due to the effect of Bcl-2 and Bcl-xL antiapoptotic molecules. We then evaluated the cooperation of cisplatin and Ad-Fhit to induce apoptosis in these clones by TUNEL assay. Cells were transduced with Ad-Fhit and Ad-LacZ for 24 hours at an MOI = 5 and treated with 16 µM cisplatin for 24 hours. Under these experimental conditions, the rate of apoptotic cells after Ad-Fhit/cisplatin combination was 34% in the Bcl-2-expressing clone and 28% in the Bcl-xL-expressing clone, compared with the Ad-LacZ/cisplatin combination that showed 6% and 16% of apoptotic cells. Cisplatin alone resulted in only 1% and 6% of apoptotic cells in Bcl-2 and Bcl-xL clones, respectively (Figure 6B). Thus, the combined treatment of Ad-Fhit and cisplatin was more effective in inducing cytotoxic effects compared with the combination treatment of cisplatin and Ad-LacZ, or with treatment with cisplatin alone, even in Bcl-2- or Bcl-xL-overexpressing clones.
Figure 6.
Effect of combination treatment with cisplatin and Ad-Fhit on Bcl-2-and Bcl-xL-overexpressing clones. (A) Clonogenic survival. Cells were infected with Ad-Fhit and Ad-LacZ for 24 hours at an MOI = 5 and were then exposed to cisplatin for 1 hour before clonogenic survival analysis. (B) Apoptosis analysis. Cells were transduced with Ad-Fhit and Ad-LacZ for 24 hours at an MOI = 5 and then treated with 16 µM cisplatin for 24 hours. The presence of apoptotic cells was assessed by TUNEL assay, and the experiment is representative of assays, performed at least three times, that gave comparable results.
Effect of Ad-Fhit and Cisplatin Combination Treatment on A549 and Calu-1 Cell Lines
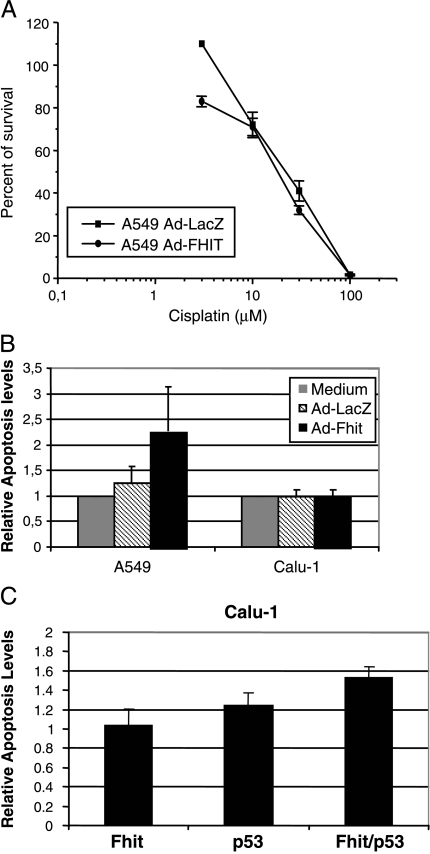
To further evaluate the possibility that sensitivity to cisplatin is modified by treatment with Ad-Fhit also in cell lines derived from other histologic subtypes of NSCLC, we extended our analyses to the A549 cell line (adenocarcinoma) and the Calu-1 cell line (squamous cell carcinoma). The A549 cell line was infected with Ad-Fhit and Ad-LacZ at an MOI = 5 for 24 hours and then treated with different concentrations of cisplatin for 24 hours. The results of the clonogenic assay are shown in Figure 7A. The value of IC50 in the A549 cell line was 17.8 µM for cells infected with Ad-Fhit and 23.4 µM for cells infected with Ad-LacZ, indicating no significant effect on sensitivity when compared to those observed in the H460 cell line.
Figure 7.
Sensitivity of A549 and Calu-1 cell lines to a combination treatment of cisplatin and Ad-Fhit. (A) Clonogenic survival in A549 cell line. A549 cells were infected with Ad-Fhit and Ad-LacZ at an MOI = 5 for 24 hours and then treated with different doses of cisplatin for 24 hours. (B) Apoptosis analysis in A549 and Calu-1 cell lines. Cells were infected with Ad-Fhit and Ad-LacZ or treated with medium as control for 24 hours and then treated with 32 and 64 µM cisplatin, respectively, for 24 hours. Results are expressed as percentages of apoptotic cells relative to medium-treated cells and represent the mean of three different independent analyses. (C) Calu-1 cells expressing inducible Fhit, p53, or both were incubated in the absence or in the presence of Pon A to induce transgene expression. After 24 hours, the cells were treated with 64 µM cisplatin for 24 hours, and the presence of apoptotic cells was assessed by TUNEL assay. The results are expressed as percentages of apoptotic cells relative to controls, represented by uninduced cells.
To evaluate whether the combined effect of Ad-Fhit and cisplatin results in an increased apoptotic response, we performed a TUNEL assay in the A549 and Calu-1 cell lines. Cells were transduced with Ad-Fhit and Ad-LacZ, or incubated with the medium for 24 hours. Under these conditions, the expression of Fhit protein was robust without any toxic effects. The A549 and Calu-1 cells were then treated with 32 and 64 µM cisplatin, respectively, and apoptosis was analyzed 24 hours after drug exposure. A slightly increased level of cisplatin-induced apoptosis was found in A549 cells, with a rate of apoptotic cells higher in Ad-Fhit-infected cells compared to that in Ad-LacZ-infected cells or untreated cells. No effects were observed in the Calu-1 cell line, where the combination of Ad-Fhit and cisplatin did not show a different apoptotic response when compared with the controls (Figure 7B). An interesting possibility is that the different response observed in the various cell lines could be related to underlying differences in the p53 pathway in H460 (p53-wt), A549 (p53-wt but ARF-null), and Calu-1 cells (p53-null).
To determine whether p53 status plays a role in modulating sensitivity to cisplatin in the presence of the FHIT gene, the Calu-1 cell line, which lacks the expression of FHIT and p53, was stably transfected with hormone-inducible vectors that allowed a tight modulation of transgene expression. The effect of the simultaneous restoration of Fhit and p53 on sensitivity to cisplatin was evaluated by comparing Calu-1 cells induced to express Fhit or p53 alone. Different cells were treated with 10 µM Pon A for 24 hours to activate transgene expression; 24 hours later, 64 µM cisplatin was added to assess the sensitivity to the drug. Apoptosis was analyzed 24 hours after drug exposure by TUNEL assay. Different levels of cisplatin-induced apoptosis were found, with the rate of apoptotic cells being higher in Calu-1 cells expressing both p53 and Fhit, compared with cells expressing either Fhit or p53. As expected, restoration of p53 alone also resulted in increased sensitivity but at lower levels compared to the expression of both genes. The results support the relationship of p53 gene status and sensitization to cisplatin in Fhit-expressing cells.
Discussion
Combination therapies have been shown to produce a response rate that is higher than those obtained with single-agent chemotherapy in many types of cancer, including NSCLC. However, the success of therapies is limited, and one of the reasons for this limitation is drug resistance. Because only a minority of patients survive for more than a year after treatment, new agents and novel approaches are needed to improve the response to conventional therapies in NSCLC patients [25]. Apoptosis has been regarded as a process that could be targeted for tumor cell killing because inadequacy in executing apoptotic programs has been associated with resistance to antitumor drugs [24]. Two major apoptotic pathways originating from different subcellular compartments have been identified (i.e., the extrinsic or receptor-mediated pathway, generated at the level of the cytoplasmic membrane, following the interaction of death receptors with their ligands, and the intrinsic or mitochondrial pathway originating from the mitochondria, whose impairment is induced by proapoptotic Bcl-2 family members) [26]. Recent studies have demonstrated that the overexpression of the FHIT gene in NSCLC by an adenoviral vector resulted in growth inhibition and increased apoptotic response in the presence of external apoptotic stimuli [11]. Furthermore, studies on H460 and other NSCLC cell lines overexpressing different molecules essential for apoptotic cascade delineated clearer evidence on the Fhit mechanism of action, which appears to be primarily mediated by caspase-8 activation rather than by mitochondrial mediators of apoptosis such as Bcl-2 and Bcl-xL [15]. On the other hand, some antitumor agents, including cisplatin, lead to cell death and cell cycle arrest through the formation of DNA lesions, which activate multiple pathways involving the mitochondria and p53 [3]. Based on this background, we investigated whether the simultaneous stimulation of the extrinsic pathway through Fhit transduction and of the intrinsic pathway using a cytotoxic agent (i.e., cisplatin) may result in increased cell death in NSCLC cell lines. A relevant finding of the present study is that FHIT gene transfer increases the sensitivity to cisplatin in NSCLC and that the pathways triggered by Fhit and cisplatin appear to cooperate in H460 cells with a mechanism underlying synergistic antiproliferative activity involving p21Waf1 upregulation. It has recently been suggested that Fhit can regulate p53 levels [23]; however, the fact that previous studies have shown that the oncosuppressive properties of Fhit and its ability to regulate p21Waf1 are p53-independent [10,27] and that we did not observe any p53 regulation in our system suggests that our results are due to a convergence of the Fhit and cisplatin-triggered pathways on p21Waf1 rather than on a Fhit-mediated increase in p53 levels.
Sensitization to cisplatin by Fhit reexpression was evident when clonogenic survival and apoptosis were assayed, whereas only a modest difference in IC50 values of Ad-LacZ-and Ad-Fhit-infected cells was observed using the SRB assay, which measures the inhibition of growth in a short-term assay. The apparent discrepancy between the former and the latter techniques further supports that the modulation of cisplatin sensitivity by Fhit occurs at the level of factors influencing survival and apoptotic cell death. Thus, a sufficient amount of time for the cells to die after they have been damaged by combined treatment with Ad-Fhit and cisplatin is critical. Moreover, in this context, it appears that wild-type p53 is required for sensitization to cisplatin because the effect was evident in H460 cells (wild-type p53) and was marginal in A549 cells in which the p53 function is altered due to the loss of ARF expression as a consequence of deletion or promoter methylation of the gene. This mechanism should result in a hyperactive MDM2 expression and enhanced p53 degradation. Again, no Fhit-mediated increase in cisplatin-induced apoptosis was observed in Calu-1 cells that carry a homozygous deletion of the p53 gene [29]. Thus, a hormone-inducible expression system was considered as an appropriate approach to clarify the effect of p53 and Fhit replacement on sensitization to cisplatin in Calu-1 cells. Indeed, Calu-1 cells became more sensitive to cisplatin-induced apoptosis on a regulated expression of p53 and Fhit obtained by the inducible system.
The increased sensitivity to cisplatin observed after adenoviral FHIT gene transfer in H460 cells was reduced in Bcl-2- and Bcl-xL-overexpressing clones compared with H460 parental cells when measured using clonogenic assay. However, marked sensitization to cisplatin was observed in terms of apoptosis. In fact, because the apoptotic mechanism triggered by the Fhit protein has been shown to be independent of mitochondrial mediators [14], we reasoned that restoration of Fhit could possibly modify the cisplatin sensitivity of Bcl-2- and Bcl-xL-expressing cells, especially with respect to apoptotic response. Colony-forming assay, on the other hand, measures the long-term response to cytotoxic stimuli [30]; under these experimental conditions (adenoviral infections), it has to be considered that the expression of the FHIT transgene is expected to decline over time (due to cell division), possibly resulting in insufficient counteraction of prosurvival signals (Bcl-2 and Bcl-xL). To further support this interpretation, it is worth noting that the antiproliferative effect of Fhit has recently been shown to be extremely dosedependent [10]. Apoptosis analysis was observed earlier than clones in a clonogenic assay, and it is likely that the results of the clonogenic assay also reflect the contribution of other factors involved in cell survival to the regulation of drug sensitivity. Most anticancer drugs act at the mitochondrial level, but alterations in this pathway are frequent in tumors and can negatively influence apoptotic response, leading to resistance of tumors to therapy. On the contrary, in a recent study, we showed that Fhit-induced apoptosis in H460 cells was not inhibited by the overexpression of Bcl-2 and Bcl-xL [14]. However, in those experiments, apoptosis was studied in peculiar experimental conditions (i.e., high level of infection in terms of MOI, days of infection, and serum starvation), which are useful in understanding molecular mechanisms but not in modulating drug sensitivity. Thus, in the present study, we maintained transduction levels of Fhit protein at around 60% to 70% to avoid vector toxicity, whereas sufficient expression levels of the Fhit protein were obtained. Our findings provide evidence that Ad-Fhit used in experimental conditions that usually do not cause apoptosis increased cell sensitivity to cisplatin, producing a synergistic effect on apoptosis in cells that are typically resistant to anticancer drugs.
An interesting observation of this study was the finding that the reexpression of Fhit in H460 cells resulted in reduced sensitivity to DNA topoisomerase I and II inhibitors and in no influence on cell response to Taxol. The acquisition of resistance to the abovementioned agents appeared dependent on the fact that Fhit expression produced a decrease on the level of target enzyme. The extent of downregulation is in keeping with the degree of resistance observed after Fhit expression. Indeed, resistance was higher in the case of etoposide (five-fold) and doxorubicin (around 20-fold) than for topotecan. The previously documented effect of Fhit on the slowdown of proliferation [11] could also confer resistance to DNA topoisomerase inhibitors and gemcitabine.
In conclusion, the therapeutic restoration of the FHIT gene in NSCLC is capable of modulating sensitivity to cisplatin in terms of colony formation and apoptosis in different cell systems. We also demonstrate that this therapeutic approach continues to be effective despite the overexpression of antiapoptotic molecules such as Bcl-2 and Bcl-xL. Combination therapy of Ad-Fhit and cisplatin could provide the basis for developing novel and effective therapeutic approaches that are able to enhance the efficacy of cisplatin-based chemotherapy as well as to reverse drug resistance in NSCLC.
Acknowledgements
We thank G. Giaccone (Division of Medical Oncology, University Hospital Vrije Universiteit, Amsterdam, The Netherlands) for providing NCI-H460 clones overexpressing Bcl-2 and Bcl-xL, and P. G. Petronini (University of Parma, Parma, Italy) for providing inducible Calu-1 cells.
Abbreviations
- FHIT
fragile histidine triad
- NSCLC
non-small cell lung cancer
- MOI
multiplicity of infection
- SRB
sulforhodamine B
- TUNEL
TdT-mediated dUTP nick end labeling
Footnotes
This work was partially supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) and the Fondazione Ermenegildo Zegna and Fenera holding. F.A. and L.R. were recipients of fellowships from AIRC.
G. Sozzi and L. Roz contributed equally as senior coauthors.
References
- 1.Ferreira CG, Huisman C, Giaccone G. Novel approaches to the treatment of non-small cell lung cancer. Crit Rev Oncol Hematol. 2002;41:57–77. doi: 10.1016/s1040-8428(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 2.Nishio K, Nakamura T, Koh Y, Suzuki T, Fukumoto H, Saijo N. Drug resistance in lung cancer. Curr Opin Oncol. 1999;11:109–115. doi: 10.1097/00001622-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Wang G, Reed E, Li QQ. Molecular basis of cellular response to cisplatin chemotherapy in non-small cell lung cancer. Oncol Rep. 2004;12:955–965. (Review) [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti MA, et al. Loss of FHIT function in lung cancer and preinvasive bronchial lesions. Cancer Res. 1998;58:5032–5037. [PubMed] [Google Scholar]
- 6.Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce CM. Altered expression of Fhit in carcinoma and precarcinomatous lesions of the esophagus. Cancer Res. 2000;60:1177–1182. [PubMed] [Google Scholar]
- 7.Capuzzi D, Santoro E, Hauck WW, Kovatich AJ, Rosato FE, Baffa R, Huebner K, McCue PA. Fhit expression in gastric adenocarcinoma: correlation with disease stage and survival. Cancer. 2000;88:24–34. [PubMed] [Google Scholar]
- 8.Campiglio M, Pekarsky Y, Menard S, Tagliabue E, Pilotti S, Croce CM. FHIT loss of function in human primary breast cancer correlates with advanced stage of the disease. Cancer Res. 1999;59:3866–3869. [PubMed] [Google Scholar]
- 9.van Heerden WF, Swart TJ, van Heerden MB, van Rensburg EJ, Engelbrecht S, Dreyer L, Huebner K. Immunohistochemical evaluation of Fhit protein expression in oral squamous cell carcinomas. J Oral Pathol Med. 1999;28:433–437. doi: 10.1111/j.1600-0714.1999.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 10.Cavazzoni A, Petronini PG, Galetti M, Roz L, Andriani F, Carbognani P, Rusca M, Fumarola C, Alfieri R, Sozzi G. Dose-dependent effect of FHIT-inducible expression in Calu-1 lung cancer cell line. Oncogene. 2004;23:8439–8446. doi: 10.1038/sj.onc.1207847. [DOI] [PubMed] [Google Scholar]
- 11.Roz L, Gramegna M, Ishii H, Croce CM, Sozzi G. Restoration of fragile histidine triad (FHIT) expression induces apoptosis and suppresses tumorigenicity in lung and cervical cancer cell lines. Proc Natl Acad Sci USA. 2002;99:3615–3620. doi: 10.1073/pnas.062030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumon KR, Ishii H, Fong LY, Zanesi N, Fidanza V, Mancini R, Vecchione A, Baffa R, Trapasso F, During MJ, et al. FHIT gene therapy prevents tumor development in Fhit-deficient mice. Proc Natl Acad Sci USA. 2001;98:3346–3351. doi: 10.1073/pnas.061020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii H, Zanesi N, Vecchione A, Trapasso F, Yendamuri S, Sarti M, Baffa R, During MJ, Huebner K, Fong LY, et al. Regression of upper gastric cancer in mice by FHIT gene delivery. FASEB J. 2003;17:1768–1770. doi: 10.1096/fj.03-0241fje. [DOI] [PubMed] [Google Scholar]
- 14.Roz L, Andriani F, Ferreira CG, Giaccone G, Sozzi G. The apoptotic pathway triggered by the Fhit protein in lung cancer cell lines is not affected by Bcl-2 or Bcl-x(L) overexpression. Oncogene. 2004;23:9102–9110. doi: 10.1038/sj.onc.1208142. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira CG, Span SW, Peters GJ, Kruyt FA, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 2000;60:7133–7141. [PubMed] [Google Scholar]
- 16.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 17.Perego P, Beretta G, Gatti L. Identification of determinants of sensitivity to antitumor drugs. In: P Michael Conn., editor. Handbook of Proteomic Methods. Totowa, NJ: Humana Press Inc.; 2003. pp. 319–331. [Google Scholar]
- 18.Perego P, Righetti SC, Supino R, Delia D, Caserini C, Carenini N, Bedogne B, Broome E, Krajewski S, Reed JC, et al. Role of apoptosis and apoptosis-related proteins in the cisplatin-resistant phenotype of human tumor cell lines. Apoptosis. 1997;2:540–548. doi: 10.1023/a:1026442716000. [DOI] [PubMed] [Google Scholar]
- 19.Pratesi G, Perego P, Zunino F. Role of Bcl-2 and its post-transcriptional modification in response to antitumor therapy. Biochem Pharmacol. 2001;61:381–386. doi: 10.1016/s0006-2952(00)00538-4. [DOI] [PubMed] [Google Scholar]
- 20.Dingemans AM, Pinedo HM, Giaccone G. Clinical resistance to topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:275–288. doi: 10.1016/s0167-4781(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 21.Larsen AK, Skladanowski A. Cellular resistance to topoisomerase-targeted drugs: from drug uptake to cell death. Biochim Biophys Acta. 1998;1400:257–274. doi: 10.1016/s0167-4781(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 22.Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo MP, Gramegna M, Croce CM, Pierotti MA, et al. The tumor-suppressor gene FHIT is involved in the regulation of apoptosis and in cell cycle control. Proc Natl Acad Sci USA. 1999;96:8489–8492. doi: 10.1073/pnas.96.15.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishizaki M, Sasaki J, Fang B, Atkinson EN, Minna JD, Roth JA, Ji L. Synergistic tumor suppression by coexpression of FHIT and p53 coincides with FHIT-mediated MDM2 inactivation and p53 stabilization in human non-small cell lung cancer cells. Cancer Res. 2004;64:5745–5752. doi: 10.1158/0008-5472.CAN-04-0195. [DOI] [PubMed] [Google Scholar]
- 24.Reed JC. Apoptosis mechanisms: implications for cancer drug discovery. Oncology (Williston Park, NY) 2004;18:11–20. [PubMed] [Google Scholar]
- 25.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 26.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 27.Ji L, Fang B, Yen N, Fong K, Minna JD, Roth JA. Induction of apoptosis and inhibition of tumorigenicity and tumor growth by adenovirus vector-mediated fragile histidine triad (FHIT) gene overexpression. Cancer Res. 1999;59:3333–3339. [PubMed] [Google Scholar]
- 28.Lu W, Lin J, Chen J. Expression of p14ARF overcomes tumor resistance to p53. Cancer Res. 2002;62:1305–1310. [PubMed] [Google Scholar]
- 29.Reiss M, Brash DE, Munoz-Antonia T, Simon JA, Ziegler A, Vellucci VF, Zhou ZL. Status of the p53 tumor suppressor gene in human squamous carcinoma cell lines. Oncol Res. 1992;4:349–357. [PubMed] [Google Scholar]
- 30.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]