Abstract
The objective of this investigation was to determine the efficacy of several novel agents in preventing lung tumorigenesis in mice. We evaluated polyphenon E, red ginseng, and rapamycin in A/J mice treated with the tobacco-specific carcinogen benzo(a)pyrene for their ability to inhibit pulmonary adenoma formation and growth. We found that treatment with polyphenon E exhibited a significant reduction on both tumor multiplicity and tumor load (tumor multiplicity x tumor volume) in a dose-dependent fashion. Polyphenon E (2% wt/wt) in the diet reduced tumor multiplicity by 46% and tumor load by 94%. This result provided key evidence in support of a phase II clinical chemoprevention trial of lung cancer. Administration of red ginseng in drinking water decreased tumor multiplicity by 36% and tumor load by 70%. The mammalian target of rapamycin inhibitor rapamycin showed significant efficacy against lung tumor growth in the tumor progression protocol and reduced tumor load by 84%. The results of these investigations demonstrate that polyphenon E, red ginseng, and rapamycin significantly inhibit pulmonary adenoma formation and growth in A/J mice.
Keywords: Chemoprevention, lung tumor, polyphenon E, red ginseng, rapamycin
Introduction
Lung cancer is the leading cause of cancer deaths of men and women in the United States and western Europe [1]. Epidemiological and laboratory animal model studies have demonstrated that smoking and environmental exposure to carcinogens are closely linked to increased lung cancer risk [2–5]. Tobacco exposure has been implicated in 90% of lung carcinomas, and smokers have a 20-fold greater risk of developing lung cancer compared to persons who have never smoked [6–8]. Chemoprevention is a potentially important approach used to reduce the large number of tobacco-caused cancer deaths for both current and former smokers. Chemoprevention is the use of pharmacologic or natural agents to inhibit the development of cancer. Numerous studies have found that chemoprevention can prevent a wide variety of cancers in multiple animal models.
Because of similarities in the histopathology and tumor progression stages between mouse and human lung adenocarcinomas, the mouse lung tumor model has been used extensively to evaluate the efficacy of putative lung cancer chemopreventive agents [3,10]. Among the more than 50 different agents tested, several groups of chemicals have shown significant efficacy against mouse lung tumor development, including glucocorticoids and isothiocyanates. Glucocorticoids have proven to be successful during the progression stage of tumor development, whereas isothiocyanates have proven particularly effective in blocking carcinogenesis [3,11]. The present investigation is a continuing effort to develop an effective chemoprevention of carcinogenesis of the lungs. We evaluated several novel agents, including polyphenon E, ginseng, and rapamycin, in A/J mice treated with the tobacco-specific carcinogen benzo(a)pyrene (BP) for their ability to inhibit pulmonary adenoma formation and/or growth.
Polyphenon E is a well-standardized decaffeinated green tea catechin mixture containing five different catechins: epicatechin, gallocatechin gallate, epigallocatechin, epicatechin gallate, and epigallocatechin gallate (EGCG), with EGCG being the most abundant [12]. Because of the fact that its formulation is highly reproducible, polyphenon E is the recommended form of green tea for clinical chemoprevention trials and has been used in several clinical studies, including an ongoing clinical phase II study of lung cancer [13,14]. Previously, green tea in drinking water or topical application has been shown to be a potent inhibitor of carcinogenesis of the skin, lung, forestomach, esophagus, liver, colon, and mammary glands in rodent models [15,16]. Green tea in drinking water inhibited carcinogen-induced mouse lung tumorigenesis by 63% [15,16]. Furthermore, green tea was also found to inhibit the growth—or to even cause the regression—of established benign skin tumors, suggesting that it may be preventive against multiple stages of carcinogenesis [16]. However, the effect of the administration of polyphenon E in diets on lung tumorigenesis has not yet been determined.
Ginseng has long been used as a traditional medicine in Asia to improve physical condition and to prolong life [17]. Ginseng appears to accomplish this by preventing various diseases such as cancer and diabetes [17]. Epidemiological studies suggest that ginseng is a chemopreventive agent against a variety of cancer types (cancer of the lips, oral cavity, pharynx, esophagus, stomach, colon, liver, pancreas, larynx, lung, and ovary) [18–22]. One study showed that users of ginseng extract had a decreased cancer risk compared to nonusers, and that the decrease was greatly dependent on the frequency of ginseng intake [18]. Another large cohort study with 4634 (2362 men, 2272 women) adults over 40 years old showed that ginseng users had a decreased risk (risk ratio = 0.40; 95% CI = 0.28–0.56) of developing several types of cancers compared to nonusers [21,22]. The general conclusion from these studies is that ginseng has nontoxic and non-organ-specific preventive effects against several types of cancers, including lung cancer. Since 1980, several researcher groups [23–27] at the Korea Cancer Center Hospital (Seoul, South Korea) have performed a series of rodent studies using ginseng and have shown that ginseng is a potent inhibitor of lung carcinogenesis in a mouse lung tumor model using urethane, 7,12-dimethylbenz(a)anthracene (DMBA), or aflatoxin B1 as a carcinogen. Thus, we sought to test the efficacy of ginseng in our standard BP-induced lung tumorigenesis in A/J mice.
Recently, mammalian target of rapamycin (mTOR) inhibitors have received much attention as another group of anticancer drugs [28]. Rapamycin, a natural product derived from Streptomyces hygroscopicus, is the first defined inhibitor of mTOR [28]. Rapamycin has a strong antitumor activity through inhibition of mTOR and blocking of cell cycle progression from G1 to S [28]. One of the mTOR pathway's upstream regulators is Akt, which can be activated by phosphatidylinositol 3-kinase (PI3K), which in turn is controlled by PTEN [29]. As a result, tumors with increased mTOR activity through Akt activation or PTEN mutation are highly susceptible to mTOR inhibition [29]. The significant efficacy of rapamycin's antitumor activity has been reported in a variety of human tumor cell lines and animal models of human xenografts [30,31]. For example, rapamycin and its derivatives exhibited significant growth inhibition of breast cancer, prostate cancer, leukemia, melanoma, renal cell cancer, glioblastoma, and pancreatic cancer cells, and in xenografts [30,31]. Because of the activation of the PI3K/Akt/mTOR pathway in human and mouse lung tumors, we tested the efficacy of rapamycin against mouse lung tumor progression.
The goal of this investigation was to discover and develop effective chemopreventive agents against lung tumorigenesis. We determined the capacity of the three agents to prevent pulmonary adenoma formation and growth, and found that polyphenon E, red ginseng, and rapamycin are potent inhibitors of lung tumorigenesis in A/J mice.
Materials and Methods
Reagents and Animals
BP and tricaprylin were purchased from Sigma Chemical Co. (St. Louis, MO). The chemopreventive agent polyphenon E was obtained from Tokyo Food Techno Co., Ltd. (Tokyo, Japan). Red ginseng extract was obtained from the Korea Cancer Center Hospital. Rapamycin was purchased from Wyeth (Philadelphia, PA). Female A/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 6 weeks of age. Animals were quarantined for 1 week and housed with wood chip bedding in environmentally controlled, clean-air rooms with a 12-hour light-dark cycle and a relative humidity of 50%. Drinking water and diet were supplied ad libitum. The study was approved by the Washington University's Institutional Animal Care and Use Committee.
Animal Experiments
Mice were randomly divided into control and treatment groups with approximately 10 mice per group. All groups received a single dose of BP (100 mg/kg body weight) in 0.2 ml of tricaprylin through intraperitoneal injection. One week after the initiation of BP, all mice (except control group) were treated with polyphenon E for 20 weeks. Mice were fed 0%. 0.5%, 1.0%, 1.5%, or 2.0% (wt/wt) polyphenon E in AIN-76A purified powder diet (Dyets, Inc., Bethlehem, PA). Because mice were somewhat adverse to eating diets containing polyphenon E, we added 3% (wt/wt) sugar to the diet in all groups, including the control group. All foods were prepared weekly, and feeding jars were cleaned weekly. Foods were prepared once a week with a powerful KitchenAid (St. Joseph, MI) mixer running for at least 1 hour. Drinking water was available ad libitum. The body weights of mice were measured every 2 weeks for the duration of the study.
Mice were randomly divided into one control group and two treatment groups. For red ginseng, the first treatment group was given 10 mg/ml in drinking water, and the second treatment group was administered 2 mg/ml in drinking water. The control group received water as control. The red ginseng in drinking water was changed every other day and was prepared just before water change. Water (control and red ginseng) was available ad libitum. Treatments were initiated 1 week before the introduction of carcinogen. After 1 week of treatment, all groups received a single intraperitoneal dose of BP (100 mg/kg body weight) in 0.2 ml of tricaprylin. The body weights of mice were measured every 2 weeks.
For the testing of rapamycin, both control and treatment groups received a single dose of BP (100 mg/kg body weight) in 0.2 ml of tricaprylin through intraperitoneal injection. Twelve weeks after the initiation of BP, all mice (except for the control group) were treated with rapamycin by intraperitoneal injection at a dose of 2 mg/kg body weight, five times a week, for 14 weeks. During the entire assay period, drinking water was available ad libitum, and the body weights of mice were measured every 2 weeks.
We monitored the consumption of food and water by measuring them daily to control the dose of these chemopreventive agents. During the study duration, the health conditions of mice were monitored everyday, and the body weights were measured every 2 weeks. We did not observe any sign of toxicity and loss of body weight. Mice were sacrificed 20 to 26 weeks after carcinogen treatment by CO2 asphyxiation. For the phenotyping of lung tumors in all bioassays described above, lung tissues were fixed in Tellyesniczky's solution overnight, followed by 75% EtOH. Lung tumor development was estimated by two investigators using a Leica MZ75 dissecting microscope to measure number (N), volume (V), and total tumor load (NV), as reported previously [32]. Volume calculation was based on the formula: V (mm3) = 4/3πr3. Histopathological examinations were performed to determine the diagnosis of the lung tumors.
Statistical Analysis
We hypothesized that BP-induced lung tumors are more likely to occur in the carcinogen control group than in the treatment groups. To test this hypothesis, Student's t test was used. The data were obtained from the BP control groups and different treatment groups in each experiment. We applied square root transformation of tumor numbers because the original data did not follow normal distribution. The transformed data were of normal distribution (data not shown). Accordingly, Student's t test was used to test the differences between the control group and the treatment groups.
Results
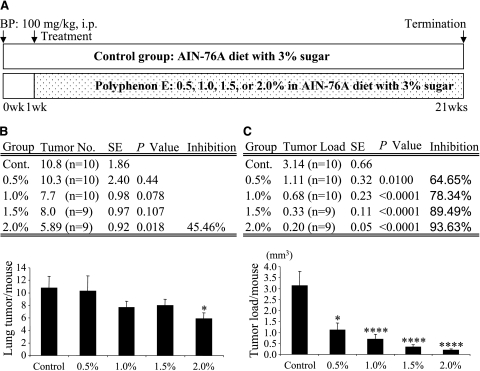
The effect of feeding mice polyphenon E during lung carcinogenesis was determined. Beginning 1 week following initiation by BP, polyphenon E was administered in the diet at concentrations ranging from 0.5% to 2.0%. Doses of 0.5%, 1.0%, 1.5%, and 2% polyphenon E did not cause any gross toxicity or significant body weight loss. The incidence of lung tumor in all BP-treated groups was 100%. BP induced an average of 10.80 ± 1.86 (n = 10) tumors per mouse in the control group. Mice treated with 2.0% polyphenon E showed a significant decrease in tumor multiplicity [46%; 5.89 ± 0.92 (n = 9)] when compared to the control group (P < .05) (Figure 1B), whereas treatments with 0.5%, 1.0%, and 1.5% polyphenon E were less effective in decreasing tumor multiplicities [10.30 ± 2.39 (n = 10), 7.7 ±0.98 (n = 10), and 8.0 ± 0.97 (n = 9), respectively]. In contrast, mice in all treatment groups showed significant reduction in total tumor load [number of tumors x volume of tumors] (ranging from P < .01 to P < .0001). The tumor load in the control group was 3.14 ± 0.66 (mm3; n = 10), whereas the tumor load was 1.11 ±0.32, 0.68 ± 0.23, 0.33 ± 0.11, and 0.20 ± 0.05 mm3 in groups treated with 0.5%, 1.0%, 1.5%, and 2.0% polyphenon E, respectively (Figure 1C). We conducted detailed histopathological examinations to determine the degree of lung tumor progression related to the effect of the chemopreventive agent on tumor development. All of the lung nodules were diagnosed as lung adenomas. Lung adenomas are characterized by a monomorphic growth pattern and are generally comprised of well-differentiated cells but sometimes exhibited a small degree of pleomorphism in slightly larger adenomas (data not shown).
Figure 1.
Effects of polyphenon E on BP-induced lung tumorigenesis in A/J mice. (A) Experimental design. We used the postinitiation protocol. All groups received a single intraperitoneal dose of BP (100 mg/kg body weight) in 0.2 ml of tricaprylin. One week after the initiation of BP, the control group received AIN-76A diet with 3% sugar. Mice in treatment groups were fed 0.5%, 1.0%, 1.5%, or 2.0% (wt/wt) polyphenon E in AIN-76A diet with 3% sugar for 20 weeks. (B) Effect of polyphenon E on lung tumor multiplicity. In the 2.0% group, polyphenon E decreased tumor multiplicity by 46%. (C) Effect of polyphenon E on lung tumor load. Polyphenon E decreased tumor load by 65% (in the 0.5% group), 78% (in the 1.0% group), 90% (in the 1.5% group), and 94% (in the 2.0% group). Error bars indicate standard error. *P < .05 and ****P < .0001, compared to the control group.
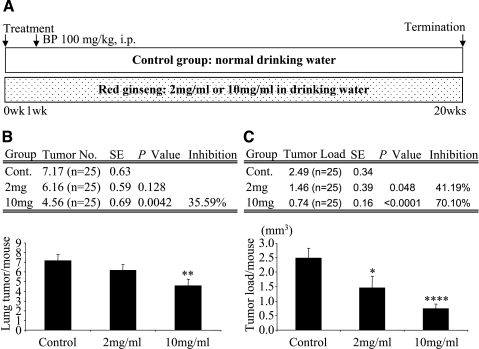
Next, we showed that red ginseng in drinking water reduced pulmonary tumors. During bioassay, no significant loss of body weight or signs of gross toxicity were observed in either treatment group. The incidence of lung tumor was 100% (25/25) in the control group, 96% (24/25) in the low-dose group (2 mg/ml), and 84% (21/25) in the high-dose group (10 mg/ml) (Figure 2). BP produced an average of 7.17 ± 0.63 tumors per mouse in A/J mice (n = 25). The average tumor multiplicity was 6.16 ± 0.59 in the low-dose group (n = 25) and 4.56 ± 0.69 in the high-dose group (n = 25). The treatment of red ginseng in the high-dose group decreased tumor number by 36% (P < .01) (Figure 2B). There was a significant decrease in tumor size in both red ginseng treatment groups. Tumor load was decreased by 41% (P < .05, n = 25) in the low-dose group (1.46 mm3) and by 70% (P < .0001, n = 25) in the high-dose group (0.74 mm3) when compared to the control group (2.49 mm3) (Figure 2C). All of the lung nodules found in experiment 2 were diagnosed as lung adenomas.
Figure 2.
Effects of red ginseng extract on BP-induced lung tumorigenesis in A/J mice. (A) Experimental design. We used the complete protocol. After 1 week of red ginseng treatment, all groups received a single intraperitoneal dose of BP (100 mg/kg body weight). Treatment with red ginseng extract continued for 20 weeks. (B) Effect of red ginseng extract on lung tumor multiplicity. In the 10-mg/ml group, treatment decreased tumor number by 36%. (C) Effect of red ginseng extract on lung tumor load. Red ginseng decreased tumor load in a dose-dependent manner (41 % and 70% in the 2- and 10-mg/ml groups, respectively). Error bars indicate standard error. *P < .05, **P < .01, and ****P < .0001, compared to the control group.
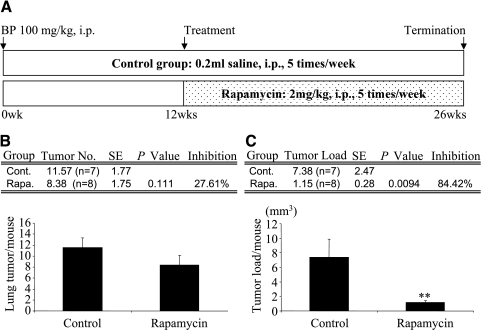
Finally, we found that rapamycin is a chemopreventive agent in the mouse lung tumor carcinogenesis model. As shown in Figure 3A, 6-week-old A/J mice were randomized into two groups. The treatment group was given rapamycin (2 mg/kg, i.p., five times a week) for 12 weeks after carcinogen treatment, and this continued for 14 more weeks. The experiment was terminated 26 weeks after exposure to BP. We observed that administration of rapamycin reduced both lung tumor multiplicity and tumor volume in A/J mice (Figure 3, B and C). Tumor multiplicity for control animals treated with BP was 11.57 tumors per mouse and decreased to 8.38 tumors per mouse in mice treated with rapamycin (Figure 3B). Tumor volume for control animals treated with BP was 7.38 mm3 and decreased to 1.15 mm3 with rapamycin treatment (P < .01; Figure 3C). Therefore, administration of rapamycin decreased tumor load in mouse lungs by 84% compared to the control group. All of the lung nodules found were diagnosed as lung adenomas.
Figure 3.
Effects of rapamycin on BP-induced lung tumorigenesis in A/J mice. (A) Experimental design. We used the progression protocol. After 12 weeks of initiation with a single dose of BP (100 mg/kg body weight), rapamycin was administered with a dose of 2 mg/kg body weight, i.p., for 14 weeks. (B) Effect of rapamycin on lung tumor multiplicity. As expected, rapamycin did not show significant inhibitory effects on tumor multiplicity (P > .05). (C) Effect of rapamycin on lung tumor load. Rapamycin decreased tumor load by 84% (P < .01). Error bars indicate standard error. **P < .01, compared to the control group.
Discussion
Effective chemoprevention of lung cancer, the principal cause of cancer deaths in the United States, has not been achieved. Characterization and use of effective chemopreventive agents have become important issues in the prevention and control of this deadly disease, particularly in the case of former smokers who are known to remain at high risk. Aiming to identify novel chemopreventive agents for lung cancer, we have determined the efficacy of several agents, including polyphenon E, ginseng, and rapamycin, in preventing lung tumorigenesis in mice and found that these three agents can significantly inhibit lung tumorigenesis in A/J mice.
Green tea has been shown to be chemopreventive in several animal models [32–36]. However, the effect of the administration of polyphenon E in the diet on lung tumorigenesis has not yet been determined. Polyphenon E is a well-standardized decaffeinated green tea catechin mixture containing five different catechins: epicatechin, gallocatechin gallate, epigallocatechin, epicatechin gallate, and EGCG [12]; it is the recommended form of green tea for clinical chemoprevention trials [13,14]. In this study, we treated the mice with different concentrations of polyphenon E (0.5%, 1.0%, 1.5%, and 2%) in AIN-76A diet. We initiated the polyphenon E treatment after BP treatment because green tea in drinking water was already known to be effective, and because this post-initiation schedule made the most sense in modeling clinical trials in former smokers. We found that polyphenon E (2% in diet) reduced tumor multiplicity by 46% (Figure 1B) and tumor load by 94% (Figure 1C). The latter result implies that, cumulatively, polyphenon E has a profound effect on tumor growth. Because we have shown that the development of adenocarcinomas in this model is highly dependent on tumor size [32], one would readily hypothesize that this treatment should profoundly decrease adenocarcinoma formation. We believe that this is the first report of the efficacy of polyphenon E given in the diet against lung tumorigenesis in A/J mice, and this lends strong support to the planned clinical phase II chemoprevention study of lung cancer.
The results of administering polyphenon E in the diet are consistent with many previously reported studies that used green tea in the drinking water, which showed the inhibition of carcinogenesis in many rodent models, including the skin, lung, forestomach, esophagus, liver, colon, and mammary glands [15,16]. Green tea and one of its components (EGCG), when given in drinking water, have been shown to inhibit 4-(N-methylnitrosamino)-1-(3-pyridyl)-butanone (NNK)-induced mouse lung tumorigenesis by 63% and 28%, respectively [37]. Green tea has also been found to inhibit the growth—and to cause the regression—of established benign skin tumors, suggesting that it may be preventive at all stages of carcinogenesis [16]. Tea polyphenols have various biologic activities, including antioxidation, modulation of enzyme systems for metabolizing chemical carcinogens, inhibition of nitrosation reactions, scavenging of activated metabolites of chemical carcinogens, and inhibition of tumor promotion [11,38,39]. Recently, several lines of evidence have suggested that green tea is a potent inducer of apoptosis in tumor cell lines [38,39]. It is likely that some degree of apoptosis and inhibition of cell proliferation may contribute to achieving striking decreases in tumor load, which we have observed.
Ginseng has been used for thousands of years in Asia. It has antioxidant, antitumor promotion, and anti-inflammatory properties [40]. Ginseng contains more than 30 different ginsenosides of protopanaxadiol, protopanaxatriol, or oleanane [22]. Ginseng is a potent inhibitor of carcinogenesis in many rodent models, including the lung, skin, liver, mammary gland, uterine cervix, brain, and colon [23–27,41]. The chemopreventive efficacy of ginseng has been demonstrated in a mouse lung tumor model using urethane, DMBA, or aflatoxin B1 as a carcinogen [23–27]—a result that we have readily confirmed in this standard BP model of lung carcinogenesis. Furthermore, three specific ginsenosides Rg3, Rg5, and Rh2 showed a reduction of lung tumor incidence in mice [17,41]. In a two-stage mouse skin model with DMBA, ginseng exhibited inhibitory effects against the development of skin papillomas in a dose-dependent manner [42]. When ginseng was given in the diet, it suppressed preneoplastic lesions [aberrant crypt foci (ACF)] induced by 1,2-dimethylhydrazine or azoxymethane [43]. More significantly, ginseng appears to be effective against ACF development at the postinitiation stage of colon carcinogenesis [44]. There are some reports on the mechanisms of the chemopreventive activity of ginseng. These studies suggest that the possible actions of ginseng are antioxidation, antitumor promotion, induction of p21 or p27, activation of NF-κB, activation of ERK, inhibition of cell proliferation, and induction of apoptosis [45,46]. In this study, red ginseng treatment in drinking water did not cause any gross toxicity and did not affect body weight. Treatment with red ginseng significantly inhibited both lung tumor multiplicity and tumor load. Our results demonstrated that red ginseng was a potent chemopreventive agent for the prevention of lung tumorigenesis in A/J mice.
Rapamycin is an antifungal agent originally purified from S. hygroscopicus [28]. It is a drug clinically used to suppress immune response after organ transplantation [28]. Rapamycin has also been used to inhibit restenosis due to smooth muscle cell overgrowth after angioplasty. The cellular target of rapamycin has been well characterized [31]. Rapamycin forms a tight complex with FKBP12, and the rapamycin-FKBP12 complex binds to and inhibits mTOR, which is a protein kinase [31]. mTOR plays a central role in the regulation of cell growth and has been shown to directly phosphorylate S6K and 4EBP1 [31]. S6K stimulates cell growth by phosphorylating the ribosomal protein S6 and by stimulating translation [47]. S6K activity is stimulated by mitogens, nutrients, and energy sufficiency [48]. The immunosuppressant drug rapamycin inhibits S6K activation, which demonstrates that mTOR plays an important role in S6K regulation [49]. mTOR is a member of the phosphoinositide kinase-related family of protein kinases. It has been reported that mTOR phosphorylates S6K on T389 and activates S6K in vitro [50]. T389 is therefore the primary rapamycin-sensitive phosphorylation site in vivo [49,50]. The rationale for testing the chemopreventive efficacy of rapamycin in the mouse lung tumor model is the observation that the PI3K/Akt/mTOR pathway was activated in mouse lung tumors [51]. A recent study has performed an immunohistochemical analysis of paraffin-embedded lung tissues using phosphospecific antibodies against serine 473 of Akt, serine 2448 of mTOR, and serine 9 of GSK3β [51]. It was shown that Akt and mTOR (but not GSK3β) were activated in lung tumors induced by NNK in A/J mice [51]. Another line of evidence is the finding that blocking an enzyme (mTOR) that acts further down the PI3K/Akt/mTOR pathway sensitizes tumors to killing by conventional chemotherapy agents [52]. In this study, we showed that rapamycin is chemopreventive in the mouse lung tumor carcinogenesis model of A/J mice induced by BP. Administration of rapamycin reduced both lung tumor multiplicity and tumor load. This is the first attempt, based on our knowledge, to test rapamycin in animal models of lung carcinogenesis to establish the efficacy of this compound as a chemopreventive agent of lung cancer. Thus, the activation of the PI3K/Akt/mTOR pathway in lung tumors is a new target for chemoprevention, especially by agents that act on this pathway, namely rapamycin.
In summary, the results of this study show that polyphenon E, red ginseng, and rapamycin are novel lung chemopreventive agents that are effective in A/J mice. Interestingly, the effects were substantially greater on tumor volume than on multiplicity in mice treated with polyphenon E, ginseng, or rapamycin, suggesting that the major effect of these agents is on later stages of the carcinogenic process. To follow up on these observations, we are actively working on potential mechanisms by which these agents may prevent lung tumorigenesis by conducting microarray studies and real-time polymerase chain reactions of genes that are relevant to their mechanisms of action, which are reported to be separate once they are completed. Finding new and effective agents that can prevent lung cancer is urgently needed because cancer of the lungs remains the principal cause of cancer deaths in the United States and because effective chemoprevention of this cancer type remains elusive. For example, carotene, retinol, and vitamin E/C have been shown to have little—or a negative—effect on human lung cancer development in smokers [11]. Thus, polyphenon E, red ginseng, and rapamycin are among the more promising new preventive agents for lung cancer and should be considered for further studies in animal models and clinical trials.
Footnotes
This work was supported, in part, by grants from the National Cancer Institute, National Institutes of Health (R01 CA058554 and P01 CA9696401).
References
- 1.Beckett WS. Epidemiology and etiology of lung cancer. Clin Chest Med. 1993;14:1–15. [PubMed] [Google Scholar]
- 2.Fielding JE. Smoking: health effects and control (1) N Engl J Med. 1985;313:491–498. doi: 10.1056/NEJM198508223130807. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CR, Lubet RA, You M. Genetic alterations in mouse lung tumors: implications for cancer chemoprevention. J Cell Biochem Suppl. 1997;29:49–63. [PubMed] [Google Scholar]
- 4.Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]
- 5.Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997;18:575–586. doi: 10.1093/carcin/18.3.575. [DOI] [PubMed] [Google Scholar]
- 6.Shopland DR, Eyre HJ, Pechacek TF. Smoking-attributable cancer mortality in 1991: is lung cancer now the leading cause of death among smokers in the United States? J Natl Cancer Inst. 1991;83:1142–1148. doi: 10.1093/jnci/83.16.1142. [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Hill AB. A study of the aetiology of carcinoma of the lung. Br Med J. 1952;2:1271–1286. doi: 10.1136/bmj.2.4797.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minna JD. The molecular biology of lung cancer pathogenesis. Chest. 1993;103:449S–456S. doi: 10.1378/chest.103.4_supplement.449s. [DOI] [PubMed] [Google Scholar]
- 9.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 10.Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52:2670s–2676s. [PubMed] [Google Scholar]
- 11.You M, Bergman G. Preclinical and clinical models of lung cancer chemoprevention. Hematol Oncol Clin North Am. 1998;12:1037–1053. doi: 10.1016/s0889-8588(05)70040-x. [DOI] [PubMed] [Google Scholar]
- 12.Namiki M, Osawa T. Antioxidants/antimutagens in foods. Basic Life Sci. 1986;39:131–142. doi: 10.1007/978-1-4684-5182-5_11. [DOI] [PubMed] [Google Scholar]
- 13.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 14.Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, Bae SM, Lee IP. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- 16.Wang ZY, Huang MT, Ho CT, Chang R, Ma W, Ferraro T, Reuhl KR, Yang CS, Conney AH. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52:6657–6665. [PubMed] [Google Scholar]
- 17.Yun TK, Lee YS, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C. A. Meyer and identification of active compounds. J Korean Med Sci. 2001;16:S6–S8. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun TK, Choi SY. A case-control study of ginseng intake and cancer. Int J Epidemiol. 1990;19:871–876. doi: 10.1093/ije/19.4.871. [DOI] [PubMed] [Google Scholar]
- 19.Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomark Prev. 1995;4:401–408. [PubMed] [Google Scholar]
- 20.Yun TK, Choi SY, Lee YS. Nontoxic and nonorgan specific cancer preventive effect of Panax ginseng C. A. Meyer. In: Shibamoto T, Terao J, Osawa T, editors. Function Foods for Disease Prevention II: Medicinal Plants and Other Foods. Washington DC: American Chemical Society; 1997. pp. 162–177. [Google Scholar]
- 21.Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol. 1998;27:359–364. doi: 10.1093/ije/27.3.359. [DOI] [PubMed] [Google Scholar]
- 22.Yun TK. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 23.Yun TK, Yun YS, Han IW. Anticarcinogenic effect of longterm oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detect Prev. 1983;6:515–525. [PubMed] [Google Scholar]
- 24.Yun TK, Kim SH, Oh YR. Medium-term (9 weeks) method for assay of preventive agents against tumor. J Korean Cancer Assoc. 1987;19:1–7. [Google Scholar]
- 25.Yun TK, Kim SH. Inhibition of development of benzo(a)-pyrene-induced mouse pulmonary adenoma by natural products in medium-term bioassay system. J Korean Cancer Assoc. 1988;20:133–142. [Google Scholar]
- 26.Yun TK. Usefulness of medium-term bioassay determining formations of pulmonary adenoma in NIH(GP) mice for finding anticarcinogenic agents from natural products. J Toxicol Sci. 1991;1:53–62. doi: 10.2131/jts.16.supplementi_53. [DOI] [PubMed] [Google Scholar]
- 27.Yun TK, Kim SH, Lee YS. Trial of a new medium-term model using benzo(a)pyrene induced lung tumor in newborn mice. Anticancer Res. 1995;15:839–845. [PubMed] [Google Scholar]
- 28.Dutcher JP. Mammalian target of rapamycin inhibition. Clin Cancer Res. 2004;10:6382S–6387S. doi: 10.1158/1078-0432.CCR-050008. [DOI] [PubMed] [Google Scholar]
- 29.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 30.Rao RD, Buckner JC, Sarkaria JN. Mammalian target of rapamycin (mTOR) inhibitors as anti-cancer agents. Curr Cancer Drug Targets. 2004;4:621–635. doi: 10.2174/1568009043332718. [DOI] [PubMed] [Google Scholar]
- 31.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Liu Q, Lantry LE, Wang Y, Kelloff GJ, Anderson MW, Wiseman RW, Lubet RA, You M. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 2000;60:901–907. [PubMed] [Google Scholar]
- 33.Yang CS, Yang GY, Landau JM, Kim S, Liao J. Tea and tea polyphenols inhibit cell hyperproliferation, lung tumorigenesis, and tumor progression. Exp Lung Res. 1998;24:629–639. doi: 10.3109/01902149809087391. [DOI] [PubMed] [Google Scholar]
- 34.Cao J, Xu Y, Chen J, Klaunig JE. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Fundam Appl Toxicol. 1996;29:244–250. doi: 10.1006/faat.1996.0028. [DOI] [PubMed] [Google Scholar]
- 35.Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- 36.Mimoto J, Kiura K, Matsuo K, Yoshino T, Takata I, Ueoka H, Kataoka M, Harada M. (-)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis. 2000;21:915–919. doi: 10.1093/carcin/21.5.915. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 38.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang CS, Chung JY, Yang GY, Li C, Meng X, Lee MJ. Mechanisms of inhibition of carcinogenesis by tea. Biofactors. 2000;13:73–79. doi: 10.1002/biof.5520130113. [DOI] [PubMed] [Google Scholar]
- 40.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;6:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yun TK. Experimental and epidemiological evidence on nonorgan specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 42.Chen XG, Liu HY, Lei XH, Zhaodi F, Yan L, Lihua T, Rui H. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60:71–78. doi: 10.1016/s0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Wanibuchi H, Salim EI, Wei M, Yamamoto S, Nishino H, Fukushima S. Inhibition by ginseng of 1,2-dimethylhydrazine induction of aberrant crypt foci in the rat colon. Nutr Cancer. 2000;36:66–73. doi: 10.1207/S15327914NC3601_10. [DOI] [PubMed] [Google Scholar]
- 44.Wargovich MJ. Colon cancer chemoprevention with ginseng and other botanicals. J Korean Med Sci. 2001;16:S81–S86. doi: 10.3346/jkms.2001.16.S.S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 46.Surh YJ, Na HK, Lee JY, Keum YS. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C. A. Meyer. J Korean Med Sci. 2001;16:S38–S41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 48.Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- 49.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–451. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 52.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]