Abstract
Endothelin (ET) 1 is important in the growth of prostate cancer cells through the activation of the endothelin A (ETA) receptor. ET receptor blockade is a new therapeutic target in treating advanced prostate cancer. This study investigates the impact of the combination of the ETA antagonist atrasentan (ABT-627) and taxane chemotherapy on prostate cancer cell survival in vitro and on the delay of prostate cancer in a xenograft mouse model. In vitro, PPC-1 cells transfected with an ETA-overexpressing vector were treated with ABT-627, paclitaxel/docetaxel, or both. Clonogenic viability and cell death assays were used to determine cell survival and apoptosis, respectively. ABT-627 and docetaxel combination treatment was used in vivo to treat mice with established ETA-overexpressing PPC-1 xenograft tumors, and tumor growth rates were assessed. Cell proliferation and vascularity were determined with Ki-67 and CD31 staining, respectively. Cells treated with combination therapy had significantly fewer viable cells and more programmed cell death than cells given monotherapy. Xenograft tumor growth rates were significantly lower in mice treated with combination therapy than in animals given a single agent. Ki-67 immunostaining demonstrated significantly fewer proliferative cells following combination therapy than following monotherapy. This study demonstrates ABT-627 to have additive antitumor effects when used in combination with taxane drugs both in vitro and in vivo.
Keywords: ABT-627, endothelin, prostate cancer, atrasentan, taxane
Introduction
Prostate cancer remains the second leading cause of cancer-related deaths among males in the United States. An average male carries an 18% lifetime risk of developing a clinically apparent disease [1]. If detected early, localized therapy, such as prostatectomy or radiation, can be curative; however, approximately 25% of patients with localized disease experience biochemical recurrence following treatment [2]. Initially, such men are treated with androgen deprivation to induce apoptotic pathways, but the effects are typically shortlived as patients develop androgen-refractory prostate cancer (ARPC), followed by painful bone metastasis and death at a median of 20 months following recurrence [3]. Unfortunately, very little is known about the molecular mechanisms behind advanced prostate cancer and the loss of androgen sensitivity. As such, no curative therapy exists for ARPC.
In the treatment of advanced prostate cancer, one promising target is the potent vasoconstrictor, endothelin (ET) 1. ET peptides, which include ET-1, ET-2, and ET-3, work by binding to endothelin A (ETA) and endothelin B (ETB) G-protein-coupled seven-transmembrane receptors. Through interaction with these receptors, ET peptides have been implicated in several cancer-related processes, including nociception, tumor invasion, neovascularization, osteogenesis, mitogenesis, and apoptosis [4,5], in many solid organ malignancies, including prostate cancer [6–14].
Accordingly, investigators have turned their attention to the selective blockade of ETA receptors as a possible therapeutic approach toward treating advanced prostate cancer. The orally available atrasentan (ABT-627) potently (Ki = 34 pM) and selectively (1862-fold) blocks the ETA receptor [15]. Although prior studies have demonstrated a significant effect of ABT-627 monotherapy on prostate cancer [16], the ETA antagonist is likely to find its therapeutic niche as an agent best used in combination with other chemotherapeutics, specifically the cytotoxic taxanes paclitaxel and docetaxel. Currently the most effective Food and Drug Administration-approved treatment for advanced prostate cancer, the taxanes inhibit microtubular depolymerization, thereby arresting cells in the G2M phase of the cell cycle [1]. Preliminary xenograft studies demonstrated that paclitaxel + ABT-627 combination therapy significantly reduces tumor growth compared to monotherapy alone [17]. In an ovarian cancer model, Rosano et al. [18] described a significant additive effect of taxane + ABT-627 on the reduction of tumor growth, apoptosis, and angiogenesis both in vivo and in vitro, further supporting the potential of this combinational therapy. Given these encouraging preliminary results, we set out to further investigate the role of taxane + ABT-627 combination therapy in a prostate cancer model.
Materials and Methods
Cell Lines
Wild-type PPC-1 prostate cells transfected previously with an ETA-overexpressing vector (pCMV-ETA) were grown in RPMI 1640 supplemented with 10% fetal bovine serum (GibcoBRL, Grand Island, NY) and 1% penicillin/streptomycin. As negative controls, PPC-1 cells transfected with the pCMV vector alone were grown under the same conditions.
Clonogenic Viability Assay
PPC-1-ETA and PPC-1-pCMV cells were each plated at a density of 1.0 x 103 cells/well in six-well plates. Cells were allowed to adhere overnight and were then pretreated with either serum-free medium, ET-1 (10-7 M), ET-1 (10-7 M) + ABT-627 ETA antagonist (10-6 M; Abbott Laboratories, Abbott Park, IL), ET-1 (10-7 M) + A192621 ETB antagonist (10-6 M; Abbott Laboratories), or ABT-627 (10-6 M) alone for 2 hours. Following pretreatment, serum-free medium, paclitaxel (10-7 M), or docetaxel (10-7 M) was added to each of the conditions for 24 hours. Afterward, cells were given fresh growth media and allowed to grow under normal conditions. After 4 weeks, cells were washed with serum-free medium, fixed, and stained with 0.1% crystal violet dissolved in 20% methanol for 15 minutes. Plates were rinsed and allowed to dry overnight. Colonies were counted in a blinded fashion by three individuals and averaged; the results were graphed and analyzed using GraphPad Prism3 (San Diego, CA). Clonogenic assay was replicated in three separate experiments for both docetaxel and paclitaxel.
Cell Death Detection
PPC-1-ETA cells were plated at a density of 2 x 104 cells/well in 100-mm3 dishes. Cells adhered overnight and were then pretreated with ET-1 (10-7M), serum-free medium, or ABT-627 (10-6 M) for 2 hours. Afterward, cells were treated for 24 hours with pretreatment conditions plus either paclitaxel (10-7 and 10-8 M), docetaxel (10-7 and 10-8 M), or vehicle. Spectrophotometric enzyme-linked immunosorbent assay (ELISA) was used to quantify histone-associated DNA fragments present in cell lysates, according to the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN). Briefly, the standard solution and samples were added to the wells of a 96-well plate coated with a monoclonal antibody. After incubation, the plate was washed, and an enzyme-labeled antibody was added. After further incubation, the plate was washed again and treated with the substrate, and optical density was determined at 405 nm using an absorption spectrophotometer (Benchmark 680; BioRad, Hercules, CA). Apoptosis assays were repeated in three independent experiments.
Xenograft Studies
PPC-1-ETA cells were grown in culture in the above conditions. When cells had reached 80% confluency, cells were dissociated with trypsin and washed twice in Hank's balanced salt solution (HBSS), and 40 male athymic nu/nu mice (Harlan Laboratories, Indianapolis, IN) received subcutaneous flank injections of 5 x 105 cells per 100 µl of HBSS. When the average tumor size had reached 0.05 cm3, the mice were given one of four drug treatments: 1) vehicle (ethanol, i.p., every 3 days); 2) ABT-627 (20 mg/kg per day in drinking water); 3) docetaxel (5 mg/kg, i.p., every 3 days); or 4) ABT-627 (20 mg/kg per day in drinking water) + docetaxel (5 mg/kg, i.p., every 3 days). Once the largest tumor burden had reached 2 cm3, the mice were sacrificed and the tumors were removed, measured, weighed, fixed in 4% paraformaldehyde, and embedded in paraffin for further analysis. Given ET's known role in angiogenesis, serum was also collected for the quantification of vascular endothelial growth factor (VEGF) by ELISA, in accordance with the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Immunohistochemistry
Five-micron sections of paraffin-embedded tumors were quenched in 3% hydrogen peroxide for 15 minutes and stained following citrate-steam antigen retrieval with TEC-3 Ki-67 (M7249; Dako, Caripinteria, CA) and CD31 (SC-1506; Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies to determine cell proliferation and angiogenesis, respectively. ETA and ETB polyclonal antibodies (Abbott Laboratories) were also used to verify ET receptor expression in xenograft tumors. A biotinylated secondary antibody was used, followed by streptavidin-conjugated horseradish peroxidase and 3,3′-diaminobenzidine chromogen (K0690; Dako). Slides were scored by three separate individuals in a blinded fashion.
Tumor Pharmacokinetic Studies
Tumors were removed 12 hours after the administration of docetaxel alone and in combination with ABT-627. Tumor samples were obtained, weighed, frozen in liquid nitrogen, and stored at -80°C until analysis. Tumor samples were processed using a liquid-liquid extraction procedure followed by solid-phase extraction, as previously described [19]. Docetaxel concentrations in tumors were determined by a liquid chromatography-mass spectrometry assay [20].
Statistical Analysis
All experimental data are representative of experiments performed at least in triplicate. For statistical comparisons of groups, Graph Pad Prism software was used (Graph Pad Software, San Diego, CA). For comparisons of values between groups, Student's t test was used. Data are presented as mean ± SE, and the level of significance was set at < .05 for all analyses.
Statistical Modeling
Tumor growth patterns in xenograft mice were assessed using an exponential growth model (Y = baseline x eα x time), with the determination of doubling time for each treatment (Doubling Time = 0.693/α). This growth pattern served as a template for determining growth parameters by nonlinear mixed-effects modeling (NONMEM). Each treatment group was assessed while preserving within-individual and between-individual variances present in measurements of tumor growth over time. In addition, the mixed-effects modeling approach used every data point to determine growth parameters, both as a mean and as a variance across individuals. Therefore, balance in the groups was not as critical (weighting across individual contributions to this model is based on data contributed, as well as on the informativeness of data to a particular parameter). In mixed-effects modeling, population average and variance were calculated. In the case of an exponential model, population average and variance were based on the population average baseline tumor size (S0) and a population average growth rate parameter (α). Each treatment group was tested as a covariate in the growth model to examine whether that group exhibited unique growth characteristics. The threshold for determining significance was based on the objective function returned by the NONMEM software program, which is equal to -2 times the log likelihood. The difference between nested models of this objective function follows a chi-square distribution and thus provides a means of statistically comparing growth descriptions across models. The α level for significance was set at .05, corresponding to an objective function change of 3.84 points for 1 df.
Results
Combination Therapy Yields Fewer Viable Cells Than Does Monotherapy
Clonogenic studies of PPC-1-ETA cells demonstrated that treatment with 10-7 M paclitaxel significantly decreased cell viability (data not shown) (P = .0033). Figure 1D illustrates that pretreatment of cells with 10-7 M ET-1 counteracted the effects of paclitaxel. The addition of 10-6 M ABT-627, an ETA antagonist, to ET-1 pretreatment blocked the ability of ET-1 to protect cells from paclitaxel, significantly decreasing the average number of colonies (P = .0438). Cells pretreated with the ETB antagonist A192621, along with ET-1, remained viable after paclitaxel administration (P = .4520) (data not shown), suggesting that ET-1 mediates its protective effect through the ETA receptor. This corroborates our earlier findings that ETB receptor expression is absent in PPC-1 cells through the hypermethylation of ETB promoter region EDNRB [21], and that ET-1 survival signaling works through the ETA receptor using the phosphatidyinositol 3-kinase (PI3-K) pathway [22,23].
Figure 1.

(A–C) ET-1 + ABT-627 + taxanes combination treatment resulted in significantly fewer viable cells than treatment with taxanes alone. PPC-1 cells transfected with an ETA-overexpressing vector were pretreated with vehicle, ET-1, ET-1 + ABT-627 ETA antagonist, or ET-1 + A192621 ETB antagonist, followed by treatment with paclitaxel or docetaxel. Crystal violet analysis was performed after 3 weeks to determine cell survival after treatments: (A) docetaxel, (B) docetaxel + ET-1, and (C) docetaxel + ABT-627 + ET-1. Results are reported as the mean of the number of colonies ± SE from three independent experiments. (D) ET-1 increased the number of colonies in docetaxel-treated and paclitaxel-treated PPC-1 cells. Blocking the ETA receptor with ABT-627 significantly reduced cell survival. Results are reported as the mean of the number of colonies ± SE from three independent experiments (P < .05).
Docetaxel treatment produced similar effects. Crystal violet assessment of untreated and ET-1-treated groups yielded too many colonies to be counted; the number of viable cells was significantly decreased by the administration of 10-8 M docetaxel alone. Cells pretreated with the ABT-627 ETA antagonist exhibited a significant decrease in viability after docetaxel treatment when compared to docetaxeltreated cells preincubated with ET-1 alone (P = .0338), illustrating an additive effect of ETA blockade with docetaxel (Figure 1D). Representative images of paclitaxel-treated cell plates are shown above their respective treatment conditions (Figure 1, A–C).
The efficacy of ABT-627 in both the presence and the absence of ET-1 pretreatment suggests the presence of an endogenous ET-1 ligand that promotes survival through the ETA receptor—an observation consistent with our previously published findings that prostate cancer cell lines, including PPC-1, secrete significant levels of mature ET-1 [12]. Additionally, treatment of PPC-1-CMV control cells yielded results similar to those of PPC-1-ETA (data not shown), suggesting that PPC-1 cells expressed enough endogenous ETA receptors to yield a protective response.
Combination Therapy Results in More Apoptosis
To determine if the decrease in cell viability occurred through an apoptotic mechanism, we used an ELISA-based assay to measure DNA fragmentation in PPC-1-ETA cells that had undergone various treatments. Treatment with ABT-627 significantly increased the number of apoptotic cells (P = .044) (Figure 2). As monotherapy, administration of 10-7 and 10-8 M paclitaxel and of 10-7 and 10-8 M docetaxel also significantly increased cell death in a dose-dependent fashion (P < .0001, .0001, .0001, and .045, respectively). Coadministration of 10-6 M ABT-627, however, significantly increased the amount of cell death following 10-7 M paclitaxel (P = .0094) and 10-8 M docetaxel (P = .02), respectively, over treatment with the corresponding taxane monotherapy. However, this significance of ABT-627 combination therapy was not present following lower-dose paclitaxel or higher-dose docetaxel, suggesting a specific therapeutic window in which combination therapy is most effective. Overall, these in vitro results demonstrate an additive effect of ABT-627 and cytotoxic taxane therapy on apoptosis. These data suggest that, for in vivo studies, docetaxel can be used at a dose lower than that of paclitaxel in combination with ABT-627 to effectively reduce tumor volume.
Figure 2.
Combination treatment with ABT-627 + paclitaxel or docetaxel resulted in significantly more cell deaths than treatment with taxane alone. Prostate cancer cells were pretreated with serum-free medium, ET-1, or ABT-627 ETA antagonist, followed by treatment with vehicle, 10 nM paclitaxel, 100 nM paclitaxel, 10 nM docetaxel, or 100 nM docetaxel. Apoptosis was quantified with a spectrophotometric ELISA-based assay. Results are reported as the mean ± SE from three independent experiments (P < .05).
Combination Therapy Reduces Xenograft Growth Rates More Than Does Monotherapy
The efficacy of low-dose docetaxel monotherapy versus ABT-627 + docetaxel combination therapy was assessed in vivo using a xenograft mouse model. Athymic mice bearing established PPC-1-ETA xenograft tumors were treated with vehicle, ABT-627 alone, docetaxel alone, or ABT-627 + docetaxel. Figure 3 illustrates the tumor volumes of these mice over the treatment period. Mice treated with vehicle had the same tumor burdens as those treated with either ABT-627 or docetaxel monotherapy. However, mice given the combination therapy had significantly smaller tumor volumes than mice in the other treatment groups. Note that, at the end of the experiment, all mice treated with vehicle or ABT-627 remained alive; in contrast, 6 of 9 mice treated with docetaxel and 5 of 10 mice given the combination therapy survived. This was most likely secondary to the toxic effects of drug accumulation following seven doses of docetaxel.
Figure 3.
Mice treated with combination ABT-627 + docetaxel had significantly smaller tumor burdens and growth rates than mice treated with vehicle, ABT-627, or docetaxel alone. ETA-overexpressing PPC-1 cells were injected as xenograft tumors in athymic mice. Mice with established tumors were treated with vehicle, docetaxel, ABT-627 ETA antagonist, or a combination of docetaxel + ABT-627. Tumors were measured every 2 days. Results are reported as mean tumor size ± SE.
A tumor growth mathematical model was used to more accurately assess tumor growth patterns in xenograft mice (Table 1). Using an exponential growth model, only the combination treatment group displayed a tumor growth rate distinguishable from those of the other groups. The combination treatment group had a tumor doubling time of 8.7 days (12.1% coefficient of variation), whereas the other groups all had a tumor doubling time of 5.8 days (25.8% coefficient of variation). The use of a separate growth rate for the combination treatment group improved the description of the data (P = .0045, χ2 = 8, df = 1).
Table 1.
ETA Receptor Antagonist and Docetaxel Treatment in Mice Bearing PPC-1 Xenograft Tumors.
| Treatment | Baseline Tumor (mm3) | Final Tumor Volume (mm3) | Doubling Time (mm3/day) |
| Vehicle | 63.73 | 690.5 ± 165.0 | 5.93 |
| ABT-627 | 54.58 | 775.5 ± 164.2 | 5.36 |
| Docetaxel | 55.57 | 657.4 ± 158.3 | 5.41 |
| ABT-627 + docetaxel | 55.1 | 321.7 ± 76.1* | 8.29* |
Statistically different from all other treatment groups (P < .05).
Combination Therapy Significantly Decreases Tumor Cell Proliferation, But Not Vascularity
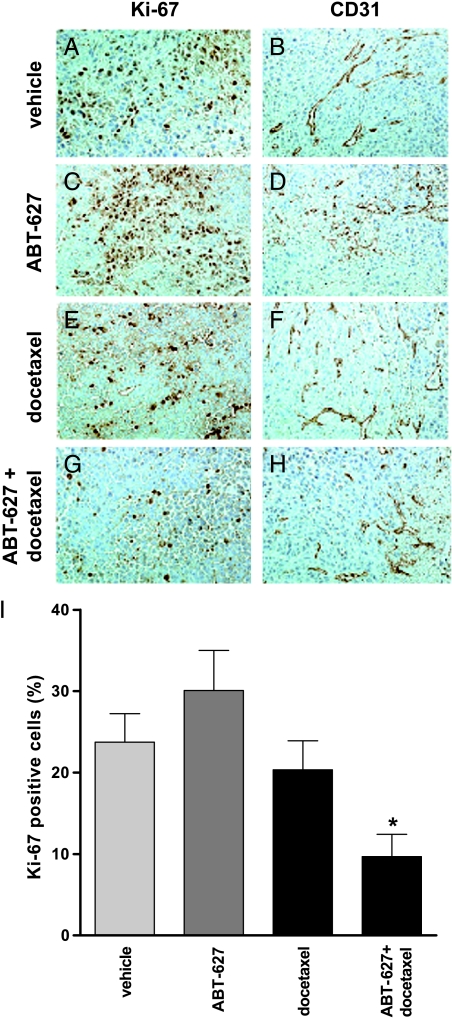
Xenograft tumors were immunostained with Ki-67 and CD31 antibodies to illustrate cell proliferation and tumor vascularity, respectively. Positive cells were counted and averaged between three individuals. Figure 4 illustrates that tumors of mice treated with ABT-627 + docetaxel combination therapy had significantly fewer proliferative cells than tumors of mice treated with either vehicle, ABT-627 alone, or docetaxel alone (P = .0225, .0031, and .0457, respectively). Representative images of Ki-67-stained tumors are illustrated in Figure 4, A, C, E, and G.
Figure 4.
Mice treated with combination therapy had significantly fewer proliferative cells than those given monotherapy or vehicle alone. Tumor vascularity was not affected. ETA-overexpressing PPC-1 xenograft tumors were grown in athymic mice treated with vehicle, docetaxel, ABT-627 ETA, or a combination of docetaxel + ABT-627. After 21 days of treatment, tumors were removed and analyzed by immunohistochemistry for Ki-67 (for the evaluation of cell proliferation) and CD31 (for tumor vasculature analysis) expression. (A and B) Vehicle, (C and D) ABT-627, (E and F) docetaxel, (G and H) ABT-627 + docetaxel, and (I) Ki-67+ cells were quantified in each tumor section. Combination therapy reduced tumor cell proliferation relative to vehicle-treated or monotherapy-treated mice (P < .05). In contrast, CD31 staining remained unchanged with treatment.
In contrast, CD31 staining illustrated no significant difference between treatment groups (data not shown). Representative images of xenograft tumors stained with CD31 demonstrate no significant difference in tumor vascularity following monotherapy or combination therapy. Serum was also harvested from xenograft mice and analyzed for VEGF levels by ELISA. Similar to CD31 data, ELISA revealed no significant difference in VEGF levels between mice in any of the treatment groups (data not shown). Analysis of ETA expression in the tumors demonstrated that receptor levels remained high in all treatment groups, suggesting that selection for subpopulations of PPC-1 cells with altered ETA expression did not contribute to the change in tumor volume with combination therapy (data not shown). ETB receptor expression was not detectable in prostate tumor cells, whereas endothelial cells of the tumor vasculature were used as internal controls and were positive for ETB expression in all treatment groups.
Combination Treatment Does Not Significantly Change Tissue Docetaxel Concentration
Given ET's vasoactive properties, LC/MS analysis was performed to determine whether tissue concentrations of docetaxel increased with coadministration of ABT-627. The average tumor concentration of docetaxel in mice treated with docetaxel monotherapy was 110.7 ± 53.26 nM. Mice treated with combination therapy had a slightly decreased tumor docetaxel concentration of 16.33 ± 13.33 nM, but this difference was not significant (P = .0843).
Discussion
The normal prostate expresses both ET receptors [24]. Luminal epithelial cells secrete ET-1 [25], which is normally found in greatest concentration within the ejaculate of both intact and vasectomized men [26]. Although its physiological role in the prostate has yet to be determined, ET involvement in neoplastic transformation is becoming evident. ET-1 activation of ETA receptors localized on prostate stromal cells has antiapoptotic effects. Earlier studies from our laboratory have shown that ETA/ET-1 receptor-ligand binding correlates with a time-dependent and dosedependent activation of PI3-K, leading to phosphorylation of AKT and the subsequent inactivation of the proapoptotic protein, Bad [23]. In addition, prostate tumor cell exposure to ET-1 decreases the overall expression of Bad, as well as of the other proapoptotic proteins Bak and Bax. In contrast, activation of the ETB receptors found within epithelial luminal cells counteracts ETA by inducing both apoptotic and ET-1 clearance pathways. Studies have shown that reexpression of ETB may increase apoptosis through an increased expression of the same proapoptotic proteins Bad, Bak, and Bax [23].
ET receptor expression is altered in prostate cancer. Although ETA receptor expression increases with disease aggressiveness [27], ETB receptor levels decrease. The decrease in ETB results, in part, from the hypermethylation of the EDNRB promoter region [21]. Hypermethylation correlates with the grade and the stage of prostate cancer [28,29]. EDNRB promoter methylation leads to a decrease in ETB-induced ET-1 clearance. Advanced prostate cancer also corresponds with a decreased activity of the ET-1-cleaving enzyme, neutral endopeptidase 24.11 (NEP) [30]. Given these observations, it is not surprising that patients with ARPC have greater serum ET-1 levels than those with localized disease or than those with no disease [12]. This suggests a prostate cancer model in which the decreased expression of NEP and ETB results in increased ET-1 levels and uninhibited ETA activation, culminating in the promotion of carcinogenic pathways.
In addition to its effects on apoptosis, the ETaxis has also been implicated in mitogenesis through the activation of the protein kinase C, PI3-K, and MAP kinase signaling pathways [31]. Within a prostate cancer model, ET-1 has been shown to induce the proliferation of prostate cancer cells, especially when used synergistically with polypeptide growth factors such as epidermal growth factor [32]. This mitogenic effect also occurs in osteoblasts—the bone-forming cells responsible for painful bone lesions characteristic of advanced prostate cancer. With approximately 104 ETA receptors per cell, osteoblasts have a surprisingly high density of receptors [33]. In vitro studies have shown that ET-1 both induces osteoblastic bone proliferation and inhibits osteoclastic bone resorption; in a murine model, the administration of a selective ETA receptor antagonist significantly attenuates this effect [34,35]. In addition to the inhibitory potency of ET-1 in osteoclast bone resorption, there is a concentrationdependent reduction in osteoclast motility at nanomolar levels of ligand [36]. ET-1 produced by marrow endothelial cells, as well as prostate tumor cells residing in the bone, will have important roles in the local regulation of osteoclast function. In ovarian cancer models, ET-1 induces VEGF and other angiogenic factors as well, leading to tumor neovascularization [37]; however, our present study did not detect a significant effect of ETA blockade on tumor vascularity or serum VEGF.
Our data showed that the combination of ETA antagonism and cytotoxic taxane therapy is significantly more efficacious than treatment with either modality alone. In vitro, both paclitaxel and docetaxel, when combined with ABT-627, had additive effects on both decreasing cell viability and increasing apoptosis in PPC-1 cells. In vivo, ABT-627 and docetaxel combination therapy significantly reduced the rate of growth of xenograft tumors more than did monotherapy. Furthermore, Ki-67 staining of xenograft tumors illustrated that combination therapy attenuated the number of proliferating cells significantly more than treatment with either docetaxel or ABT-627 alone.
Additive effects can be explained mechanistically; although both drugs activate the proapoptotic proteins of the Bcl-2 family, each has distinct nonoverlapping anticarcinogenic mechanisms. The taxanes primarily inhibit microtubular depolymerization, whereas ABT-627 blocks ETA-associated proliferative and antiapoptotic pathways [22,23]. Taxanes also work by decreasing Bcl-2 binding to Bax—the same proapoptotic protein inhibited by ET-1 [38].
Given ET's vasoactive properties, it would be reasonable to assume that the increased effectiveness of combination therapy would be due to ABT-627-mediated vascular changes. However, serum VEGF analysis and CD31 vascular endothelium labeling of xenograft tumor demonstrated no differences between mice in the different treatment groups. Additionally, xenograft tumors illustrated no difference in docetaxel concentrations following ETA blockade. Thus, it appears that ABT-627-mediated enhanced cytotoxicity was not due to changes in blood flow or vascularity. Prior studies have shown ET-1 to increase blood flow in a breast cancer model through the activation of the ETB receptor [39]; our results suggest that ETA blockade does not have similar effects in a prostate cancer model. However, in this study, we examined concentrations at a single time point 12 hours after the last of seven docetaxel doses. A pharmacokinetic study to further examine docetaxel distribution in tumors at serial time points with and without ABT-627 would provide a more complete picture of the role of ETA blockade in reducing tumor growth with combination therapy.
Prostate cancer is characterized by low rates of proliferation, making it resistant to most forms of cytotoxic chemotherapy. Prostate cancer chemotherapy is not curative; in a recent phase III clinical study, the Southwest Oncology Group reported that treatment of ARPC with docetaxel + estramustine increased disease survival by only 3 months in patients who received only mitoxantrone with prednisone [40]. As such, the demand for new treatments remains high. With implicated roles in tumor cell survival, apoptosis, mitogenesis, neovascularization, and osteogenesis, the ETaxis is well suited for therapeutic manipulation. Initially designed as an antihypertensive, the ETA antagonist ABT-627 has a half-life of 25 hours, making it ideally suited for long-term once-daily dosing. Adverse effects are mild and correspond with the drug's vasoactive properties, including headache, rhinitis, and peripheral edema [4].
As monotherapy, ABT-627 has already been well-studied clinically. Following encouraging phase I and II studies [16,41,42], recent phase III clinical trials have focused on the efficacy of ABT-627 in combating ARPC. These studies assessed time to disease progression, prostate-specific antigen progression, bone metabolism markers, and quality of life in prostate cancer patients given ABT-627 vs placebo. Although the first completed set of these trials demonstrated that ABT-627 both significantly delayed disease progression and attenuated the increase in bone and tumor markers in the evaluable population, the drug did not significantly delay disease progression in the intent-to-treat population. Although the lack of a significant effect for ABT-627 monotherapy in this first phase III trial was disappointing, a therapeutic effect from treatment clearly exists.
In light of these clinical results, our study supports the hypothesis that ABT-627 may have greater clinical utility when used in combination with taxane treatment. Ongoing phase I clinical studies are already examining ABT-627 + docetaxel combination therapy in patients with advanced disease. Although the results of these trials have yet to be published, it will be of great interest to see if clinical studies confirm our results. Although our data support the concept that ABT-627 has additive effects on treating advanced prostate cancer with the cytotoxic taxanes, further studies are necessary to optimize the combination to evoke the most effective therapeutic response. Only then will we be able to determine the full therapeutic potential of ET manipulation in the treatment of advanced prostate cancer.
Conclusion
The ET axis is involved in several tumorigenic pathways in prostate cancer. Both in vitro and in vivo, blockade of the ETA receptor with ABT-627 demonstrated significant additive effects when used in combination with the cytotoxic taxanes paclitaxel and docetaxel. Given the efficacy, low toxicity profile, bioavailability, and half-life of ABT-627, additional studies should be performed to further delineate the therapeutic role of this promising agent.
Acknowledgements
The authors would like to thank Marie Acquafondata for her expertise with immunohistochemical analyses and the Department of Urology scientific writer Moira Hitchens for her expert assistance in the writing of this manuscript.
Abbreviations
- ABT-627
atrasentan
- ARPC
androgen-refractory prostate cancer
- ELISA
enzyme-linked immunosorbent assay
- ET-1
endothelin-1
- ETA
endothelin A
- ETB
endothelin B
- HPLC
high-performance liquid chromatography
- PI3-K
phosphatidyinositol 3-kinase
- VEGF
vascular endothelial growth factor
Footnotes
We acknowledge the Mellam Family Foundation and Abbott Laboratories for providing us with funding resources.
References
- 1.Khan MA, Carducci MA, Partin AW. The evolving role of docetaxel in the management of androgen independent prostate cancer. J Urol. 2003;170:1709–1716. doi: 10.1097/01.ju.0000088787.95124.4b. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Longterm biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 3.Chodak GW, Keane T, Klotz L. Critical evaluation of hormonal therapy for carcinoma of the prostate. Urology. 2002;60:201–208. doi: 10.1016/s0090-4295(02)01677-1. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JB. Endothelin receptor antagonists. World J Urol. 2005;23:19–27. doi: 10.1007/s00345-004-0478-9. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann E, Bogemann M, Bierer S, Eltze E, Hertle L, Wulfing C. The endothelin axis in urologic tumors: mechanisms of tumor biology and therapeutic implications. Expert Rev Anticancer Ther. 2006;6:73–81. doi: 10.1586/14737140.6.1.73. [DOI] [PubMed] [Google Scholar]
- 6.Bagnato A, Tecce R, Di Castro V, Catt KJ. Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res. 1997;57:1306–1311. [PubMed] [Google Scholar]
- 7.Baley PA, Resink TJ, Eppenberger U, Hahn AW. Endothelin messenger RNA and receptors are differentially expressed in cultured human breast epithelial and stromal cells. J Clin Invest. 1990;85:1320–1323. doi: 10.1172/JCI114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AJ, Belinsky S, Franklin W, Beard S. Molecular and physiologic evidence for 5′CpG island methylation of the endothelin B receptor gene in lung cancer. Chest. 2002;121:27S–28S. doi: 10.1378/chest.121.3_suppl.27s-a. [DOI] [PubMed] [Google Scholar]
- 9.Economos K, MacDonald PC, Casey ML. Endothelin-1 gene expression and biosynthesis in human endometrial HEC-1A cancer cells. Cancer Res. 1992;52:554–557. [PubMed] [Google Scholar]
- 10.Egidy G, Juillerat-Jeanneret L, Jeannin JF, Korth P, Bosman FT, Pinet F. Modulation of human colon tumor-stromal interactions by the endothelin system. Am J Pathol. 2000;157:1863–1874. doi: 10.1016/S0002-9440(10)64825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahav R, Heffner G, Patterson PH. An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci USA. 1999;96:11496–11500. doi: 10.1073/pnas.96.20.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, Simons JW. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 13.Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes—hypothetical roles in CNS pathophysiology. Brain Res Brain Res Rev. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Venuti A, Salani D, Manni V, Poggiali F, Bagnato A. Expression of endothelin 1 and endothelin A receptor in HPV-associated cervical carcinoma: new potential targets for anticancer therapy. FASEB J. 2000;14:2277–2283. doi: 10.1096/fj.00-0024com. [DOI] [PubMed] [Google Scholar]
- 15.Verhaar MC, Grahn AY, Van Weerdt AW, Honing ML, Morrison PJ, Yang YP, Padley RJ, Rabelink TJ. Pharmacokinetics and pharmacodynamic effects of ABT-627, an oral ETA selective endothelin antagonist, in humans. Br J Clin Pharmacol. 2000;49:562–573. doi: 10.1046/j.1365-2125.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 17.Godara G, Cannon GW, Cannon GM, Jr, Bies RR, Nelson JB, Pflug BR. Role of endothelin axis in progression to aggressive phenotype of prostate adenocarcinoma. Prostate. 2005;65:27–34. doi: 10.1002/pros.20252. [DOI] [PubMed] [Google Scholar]
- 18.Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, Natali PG, Bagnato A. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res. 2003;63:2447–2453. [PubMed] [Google Scholar]
- 19.Strychor S, Eiseman JL, Parise RA, Egorin MJ, Joseph E, Zamboni WC. Plasma, tumor, and tissue disposition of docetaxel in SCID mice bearing SKOV-3 human ovarian xenografts. Proc AACR. 2005;2005:4165. doi: 10.1007/s00280-007-0620-7. [DOI] [PubMed] [Google Scholar]
- 20.Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ. Sensitive liquid chromatography-mass spectrometry assay for quantitation of docetaxel and paclitaxel in human plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2003;783:231–236. doi: 10.1016/s1570-0232(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 21.Nelson JB, Lee WH, Nguyen SH, Jarrard DF, Brooks JD, Magnuson SR, Opgenorth TJ, Nelson WG, Bova GS. Methylation of the 5′ CpG island of the endothelin B receptor gene is common in human prostate cancer. Cancer Res. 1997;57:35–37. [PubMed] [Google Scholar]
- 22.Pflug BR, Zheng H, Udan MS, D'Antonio JM, Marshall FF, Brooks JD, Nelson JB. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.02.007. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Nelson JB, Udan MS, Guruli G, Pflug BR. Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia. 2005;7:631–637. doi: 10.1593/neo.04787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi S, Tang R, Wang B, Opgenorth T, Stein E, Shapiro E, Lepor H. Localization of endothelin receptors in the human prostate. J Urol. 1994;151:763–766. doi: 10.1016/s0022-5347(17)35083-8. [DOI] [PubMed] [Google Scholar]
- 25.Langenstroer P, Tang R, Shapiro E, Divish B, Opgenorth T, Lepor H. Endothelin-1 in the human prostate: tissue levels, source of production and isometric tension studies. J Urol. 1993;150:495–499. doi: 10.1016/s0022-5347(17)35534-9. [DOI] [PubMed] [Google Scholar]
- 26.Casey ML, Byrd W, MacDonald PC. Massive amounts of immunoreactive endothelin in human seminal fluid. J Clin Endocrinol Metab. 1992;74:223–225. doi: 10.1210/jcem.74.1.1727824. [DOI] [PubMed] [Google Scholar]
- 27.Gohji K, Kitazawa S, Tamada H. Expression of endothelin receptor A associated with prostate cancer progression. J Urol. 2001;165:1033–1036. [PubMed] [Google Scholar]
- 28.Woodson K, Hanson J, Tangrea J. A survey of gene-specific methylation in human prostate cancer among black and white men. Cancer Lett. 2004;205:181–188. doi: 10.1016/j.canlet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs WB, Nelson WG. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 30.Papandreou CN, Usmani B, Geng Y, Bogenrieder T, Freeman R, Wilk S, Finstad CL, Reuter VE, Powell CT, Scheinberg D, et al. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat Med. 1998;4:50–57. doi: 10.1038/nm0198-050. [DOI] [PubMed] [Google Scholar]
- 31.Pirtskhalaishvili G, Nelson JB. The endothelin receptor. A novel target for anticancer therapy. Am J Cancer. 2002;1:81–91. [Google Scholar]
- 32.Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, Simons JW. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- 33.Takuwa Y, Ohue Y, Takuwa N, Yamashita K. Endothelin-1 activates phospholipase C and mobilizes Ca2+ from extra-and intracellular pools in osteoblastic cells. Am J Physiol. 1989;257:E797–E803. doi: 10.1152/ajpendo.1989.257.6.E797. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JB, Nabulsi AA, Vogelzang NJ, Breul J, Zonnenberg BA, Daliani DD, Schulman CC, Carducci MA. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J Urol. 2003;169:1143–1149. doi: 10.1097/01.ju.0000042162.08938.27. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon DB, Chung LW, Inoue N. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53:1063–1069. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 36.Alam AS, Gallagher A, Shankar V, Ghatei MA, Datta HK, Huang CL, Moonga BS, Chambers TJ, Bloom SR, Zaidi M. Endothelin inhibits osteoclastic bone resorption by a direct effect on cell motility: implications for the vascular control of bone resorption. Endocrinology. 1992;130:3617–3624. doi: 10.1210/endo.130.6.1597159. [DOI] [PubMed] [Google Scholar]
- 37.Salani D, Di Castro V, Nicotra M. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000;157:1537–1547. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- 39.Rai A, Gulati A. Evidence for the involvement of ET(B) receptors in ET-1-induced changes in blood flow to the rat breast tumor. Cancer Chemother Pharmacol. 2003;51:21–28. doi: 10.1007/s00280-002-0534-3. [DOI] [PubMed] [Google Scholar]
- 40.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 41.Zonnenberg BA, Groenewegen G, Janus TJ, Leahy TW, Humerickhouse RA, Isaacson JD, Carr RA, Voest E. Phase I dose-escalation study of the safety and pharmacokinetics of atrasentan: an endothelin receptor antagonist for refractory prostate cancer. Clin Cancer Res. 2003;9:2965–2972. [PubMed] [Google Scholar]
- 42.Carducci MA, Nelson JB, Bowling MK, Rogers T, Eisenberger MA, Sinibaldi V, Donehower R, Leahy TL, Carr RA, Isaacson JD, et al. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol. 2002;20:2121–2180. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]