Abstract
Neurotensin (NT), a gastrointestinal hormone, binds its receptor [neurotensin receptor (NTR)] to regulate the growth of normal and neoplastic intestinal cells; molecular mechanisms remain largely undefined. Glycogen synthase kinase-3 (GSK-3) regulates diverse cellular processes, including cell growth and apoptosis. Here, we show that NT induces the phosphorylation of GSK-3α/β in the human colon cancer cell line HT29, HCT116, or SW480, which possesses highaffinity NTR. The effect of NT was blocked by inhibitors of protein kinase C (PKC), but not by inhibitors of mitogen-activated protein kinase/extracellular signalregulated kinase (MEK1) or phosphatidylinositol-3 kinase, suggesting a predominant role for PKC in GSK-3β phosphorylation by NT. Pretreatment with Gö6976 (which inhibits PKCα and PKCβ1) or downregulation of endogenous PKCα or PKCβ1 blocked NT-mediated GSK-3β (but not GSK-3α) phosphorylation. Moreover, a selective PKCβ inhibitor, LY379196, reduced NT-mediated GSK-3β (but not GSK-3α) phosphorylation, suggesting a role for PKCβ1 in the NT-mediated phosphorylation of GSK-3β and an undefined kinase in the NT-mediated phosphorylation of GSK-3α. Treatment with NT or the GSK-3 inhibitor SB216763 increased the expression of cyclin D1, a downstream effector protein of GSK-3 and a critical protein for the proliferation of various cells. Our results indicate that NT uses PKC-dependent pathways to modulate GSK-3, which may play a role in the NT regulation of intestinal cell growth.
Keywords: Neurotensin, PKC, GSK-3, intestinal cells, cyclin D1
Introduction
Regulatory hormones, which are localized to specialized endocrine cells in the small bowel, control numerous physiological functions of the gastrointestinal (GI) tract, including secretion, motility, and mucosal growth [1]. The gut peptide neurotensin (NT), a tridecapeptide localized to enteroendocrine cells of the distal small bowel [2,3], facilitates fatty acid translocation [4], affects gut motility [5], and stimulates the growth of normal gut mucosa [6,7]. In addition to its trophic effects on normal GI tissues, NT stimulates the proliferation of certain pancreatic, colonic, and prostatic cancers bearing high-affinity neurotensin receptors (NTRs) [8]. NTR and its mRNA are expressed in many human colon cancer cell lines and primary colorectal cancers [9]. Therefore, it is important to characterize NT-mediated signal transduction pathways in human colon cancer cells, which may identify potential targets for therapeutic interventions.
Previously, we have shown that NTactivates the extracellular signal-regulated kinase (ERK) pathway in human colon and pancreatic cancer cells [10,11]. In agreement with our studies, other investigators have demonstrated that NTactivates ERK in colonic adenocarcinoma cells [12], colonic epithelial cells [13], and pancreatic cancer cells [14]. NT-stimulated ERK activation is dependent on Ras and is involved in NT-induced IL-8 expression [13]. NT-mediated stimulation of IL-8 gene expression involves a nuclear factor κB-dependent pathway [13]. In addition to the activation of ERK, NT induces the activation of protein kinase C (PKC) and phosphatidylinositol-3 kinase (PI3-kinase)/Akt in some cells [15–17]. NT plays an important role in the stimulation of intestinal cell proliferation; however, the signal pathways mediated by NT are largely undefined.
PKC, particularly the PKCα and PKCβ1 isoforms, has been shown to phosphorylate glycogen synthase kinase (GSK) 3α and GSK-3β [18,19]. PKC is ubiquitously expressed and involved in diverse cellular functions, such as the potentiation of the proliferative response to G protein-coupled receptor agonists [20–22]. NT stimulates the formation of inositol trisphosphate [1,4,5], increases intracellular calcium [23,24], and activates PKC [25]. However, the functional effects of PKC-mediated NT signaling are not known.
GSK-3 is an evolutionarily conserved signaling molecule that plays an important role in diverse biologic processes [26]. More recent studies indicate a role for GSK-3 in the control of cell proliferation and survival in mammalian cells and in the identification of GSK-3 as a component of the Wnt signaling pathway, which controls development in invertebrates and vertebrates [27–29]. GSK-3 phosphorylates cyclin D1, resulting in its degradation [30,31]. Two isoforms of GSK-3, GSK-3α and GSK-3β, have been identified [32]. Both isoforms are phosphorylated at Ser-21 in GSK-3α and at Ser-9 in GSK-3β [26], resulting in the inhibition of enzymes. Although studies have focused primarily on GSK-3β, GSK-3α has similar (but not identical) biochemical properties [26,33]. Several kinases have been shown to phosphorylate these inhibitory sites in vitro [26]. Protein kinase B (PKB/Akt), a serine/threonine kinase located downstream of PI3-kinase, phosphorylates both of these sites in vitro and in vivo [34], suggesting that certain growth factors repress GSK-3 activity through the PI3-kinase-PKB/Akt signaling cascade. In addition, p90RSK, a downstream target of the MEK/ERK pathway, and certain PKC isoforms have been shown to phosphorylate and inactivate both isoforms of GSK-3 [19,35]. These findings suggest that GSK-3 represents an important convergence point that integrates signals from multiple signaling cascades.
In our current study, we show that NT stimulates GSK-3 phosphorylation in human colon cancer cells that possess the high-affinity NTR through an intracellular signaling pathway involving PKC, independent of previously identified PI3-kinase-PKB/Akt and MEK/ERK pathways. These results indicate that, depending on the stimulatory context, the activity of GSK-3 can be regulated through multiple signaling mechanisms. Moreover, the NT-stimulated induction of cell growth noted in NTR+ colon cancers may be mediated, in part, through PKC-dependent GSK-3 inhibition.
Materials and Methods
Materials
GF109203x, Ro-318220, PD98059, and Gö6976 were provided by Calbiochem (La Jolla, CA). LY319796 was a generous gift from Eli Lilly Co. (Indianapolis, IN). The GSK-3 inhibitor SB-216763 was purchased from Tocris (Ellisville, MO). Rabbit anti-phospho-GSK-3α/β (Ser-21 and Ser-9), rabbit anti-phospho-Akt, and rabbit anti-Akt antibodies were purchased from Cell Signaling (Beverly, MA). Mouse monoclonal anti-GSK-3 (clone 4G-1E) and secondary antibodies were obtained from Upstate Biotechnology (Lake Placid, NY). Phorbol-12-myristate-13 acetate (PMA), wortmannin, NT, myelin basic protein (MBP), and rabbit anti-β-actin antibody were from Sigma (Solon, OH). Mouse anti-phospho-ERK1/2, rabbit anti-ERK1, rabbit anti-PKCα, rabbit anti-PKCβ1, and rabbit anti-cyclin D1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). [γ-32P] adenosine triphosphate (ATP) was obtained from PerkinElmer Life Sciences (Boston, MA). The enhanced chemiluminescence (ECL) system for Western immunoblot analysis and protein A-Sepharose was from Amersham Biosciences (Piscataway, NJ). The concentrated protein assay dye reagent was from Bio-Rad Laboratories (Hercules, CA). Tissue culture media and reagents were from Invitrogen (Carlsbad, CA). All other reagents were of molecular biology grade and were from Sigma.
Cell Culture
The human colon cancer cell lines HT29, HCT116, and SW480 were obtained from the American Type Culture Collection (Manassas, VA). HT29 and HCT116 cells were grown in McCoy's 5A supplemented with 10% fetal bovine serum (FBS). SW480 cells were grown in RPMI 1640 supplemented 10% FBS. Before stimulation with NT, cells were grown to subconfluence in 60-mm dishes and starved in serum-free medium for 24 hours, unless otherwise indicated.
Western Blot Analysis
Total protein (100 µg) was resolved on a 10% polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. Filters were incubated for 1 hour at room temperature in a blotting solution. Phospho-GSK-3α/β, GSK-3, cyclin D1, PKCα, PKCβ1, and actin were detected with specific antibodies following blotting with a horseradish peroxidase-conjugated secondary antibody and were visualized using an ECL detection system.
In Vitro Kinase Assays
PKCα or PKCβ1 activity was determined in cell extracts, as described previously [36]. Briefly, total PKCα or PKCβ1 was determined by measuring the incorporation of 32P into MBP. Extracts from HT29 cells treated with or without NT were incubated with PKCα or PKCβ1 antibody overnight and with protein A beads for 3 hours at 4°C by gentle rocking. Immunocomplexed beads were washed twice with cell lysis buffer and twice with kinase buffer (25 mM Tris, pH 7.4; 2 mM dithiothreitol; 0.1 mM Na3VO4; 10 mM MgCl2; and 5 µCi of [γ-32P]ATP). Immunocomplexes were resuspended in 40 µl of kinase buffer supplemented with 10 µM ATP and 5 µg of MBP and incubated for 30 minutes at 30°C. Kinase reactions were terminated with a sodium dodecyl sulfate (SDS) sample buffer. Samples were size-fractionated by SDS polyacrylamide gel electrophoresis (PAGE), and 32P-labeled MBP was quantified autoradiographically after fixing and drying the gel.
Results
NT Induces the Phosphorylation of GSK-3α/β
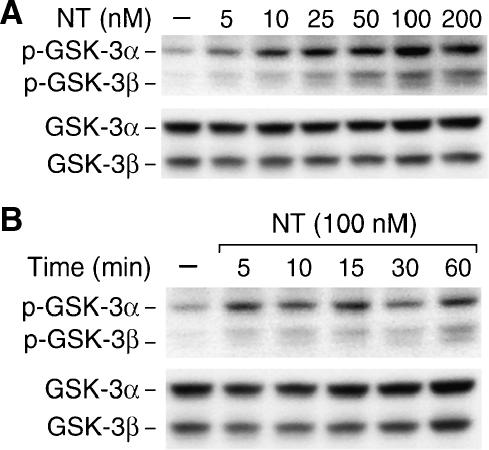
GSK-3 not only functions as a regulator of metabolism but also plays a critical role in the signal transduction of many growth factors such as IGF-1, insulin, and neuronal growth factor [26]. To assess whether GSK-3 is also regulated by NT, a GI hormone that binds its receptor to regulate the proliferation of normal and neoplastic intestinal cells [8], we treated HT29 cells, a human colon cancer cell line that possesses high-affinity NTR [9], with different concentrations of NT. Phosphorylation of GSK-3 was determined by immunoblotting with a GSK-3 phospho-specific antibody that recognizes GSK-3α phosphorylated at Ser-21 and GSK-3β phosphorylated at Ser-9 (Figure 1). NT treatment induced a dosage-dependent (Figure 1A) and time-dependent (Figure 1B) phosphorylation of GSK-3α and GSK-3β at the respective serine residues. The effect of NT on GSK-3 was noted at a dosage of 5 nM and reached a maximum at 100 nM.
Figure 1.
Treatment with NT induces GSK-3α/β phosphorylation in HT29 cells. HT29 cells were serum-starved for 24 hours, followed by treatment with different concentrations of NT (A) for 20 minutes or NT (100 nM) over a time course (B). Total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-GSK-3α/β antibody; membranes were stripped and reprobed with anti-GSK-3α/β antibody. Representative data from five separate experiments are shown.
NT Induces Signaling Pathways That May Mediate the Induction of GSK-3α/β Phosphorylation
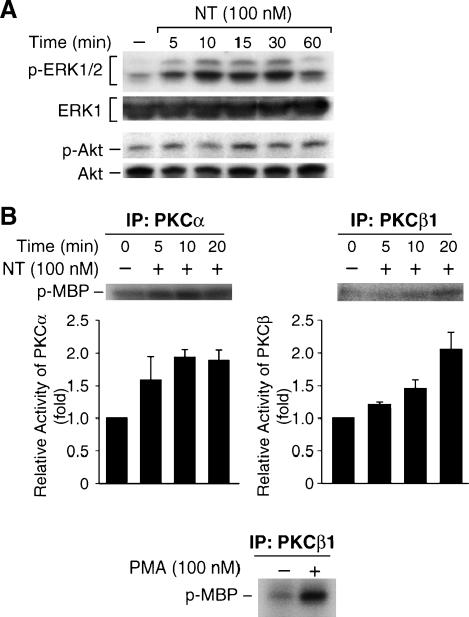
Several serine/threonine kinases, including PI3-kinase/Akt [34], p90RSK [37], a downstream target of MEK/ERK, and some PKC isoforms [18,19], have been shown to phosphorylate and inhibit GSK-3 in vitro. A number of studies suggest that ERK, PKC [12–14], and PI3-kinase/Akt pathways are involved in NT signaling in certain cells [15,16, 23,38]. In addition, NT-induced MAP kinase activation can be attenuated by the PKC inhibitor GF109203x [12]. Therefore, we measured ERK1/2 and Akt activity by Western blot analysis using anti-phospho-ERK1/2 or anti-phospho-Akt antibody, and PKCα and PKCβ1 activity by an immunocomplex kinase assay, after treating HT29 cells with NT for various times. As shown in Figure 2A, treatment with NT increased ERK1/2 and Akt phosphorylation in a time-dependent fashion in HT29 cells, suggesting the activation of ERK1/2 and Akt by NT. In vitro kinase assay showed that PKCα and PKCβ1 activity increased at 5 minutes after NT treatment. As a positive control, PMA induced PKCβ1 activity in HT29 cells (Figure 2B). Our results demonstrate the activation of ERK1/2, Akt, PKCα, and PKCβ by NT; any of these pathways may mediate NT-induced GSK-3 phosphorylation in HT29 cells.
Figure 2.
Treatment with NT increases ERK1/2, Akt, PKCα, and PKC/β activity in HT29 cells. HT29 cells were serum-starved for 24 hours, followed by treatment with NT (100 nM) for various times. (A) Total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-ERK1/2 or anti-phospho-Akt antibody; membranes were stripped and reprobed with anti-ERK1/2 or Akt antibody, respectively. (B) Total protein was immunoprecipitated (IP) with either anti-PKCα or PKCβ1 antibody, and resultant immune complexes were analyzed for PKCα or PKCβ1 activity using MBP as substrate. Signals from three separate experiments were quantitated densitometrically and expressed in relation to PKCα or PKCβ1 activity (corrected for control p-MBP). HT29 cells were treated with PMA (100 nM) for 10 minutes, and PKCβ1 activity was assayed using MBP as substrate.
NT Induces GSK-3β Phosphorylation through PKC Activation
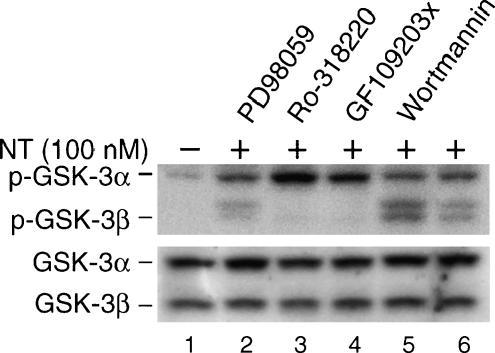
We next examined the involvement of these kinases in the NT-induced phosphorylation of GSK-3 (Figure 3). Cells were pretreated with inhibitors to MEK (PD98059), PKC (Ro-318220 and GF109203x), or PI3-kinase (wortmannin) for 30 minutes before stimulation with NT (100 nM). GSK-3 phosphorylation was analyzed by Western blot analysis (Figure 3). As expected, treatment with NT increased the phosphorylation of both GSK-3α and GSK-3β (lane 6). Interestingly, NT-mediated phosphorylation of GSK-3β, but not GSK-3α, was completely blocked by treatment with Ro318220 (lane 3) or GF109203x (lane 4). In contrast, treatment with PD98059 or wortmannin did not block either GSK-3α or GSK-3β phosphorylation by NT (lanes 2 and 5). Collectively, these results suggest a role for the PKC pathway, but not for MEK/ERK or PI3-kinase, in the NT-induced phosphorylation of GSK-3β.
Figure 3.
Inhibition of PKC, but not of MEK or PI3-kinase, blocks NT-stimulated GSK-3β (but not GSK-3α) phosphorylation. (A) HT29 cells were serum-starved for 24 hours. Cells were pretreated with or without the MEK1 inhibitor PD98059 (40 µM), PKC inhibitors Ro-318220 (2 µM) and GF109203x (2 µM), or the PI3-kinase inhibitor wortmannin (200 nM) for 30 minutes, followed by treatment with NT (100 nM) alone or in combination with the inhibitors. After 20 minutes of treatment, total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-GSK-3α/β antibody. Membranes were stripped and reprobed with anti-GSK-3α/β. Representative data from three separate experiments are shown.
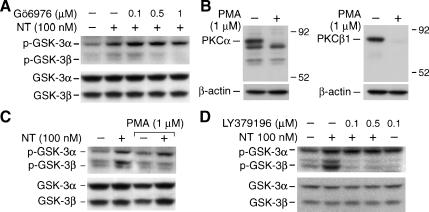
To examine the specific isoforms of PKC involved in mediating NT signaling to GSK-3, we determined whether NT-induced GSK-3 phosphorylation is blocked by the conventional PKC inhibitor Gö6976 (which inhibits PKCα and PKCβ1). HT29 cells were pretreated with Gö6976 for 30 minutes before stimulation with NT, and GSK-3 phosphorylation was visualized by Western blot analysis. Pretreatment with GÓ6976 blocked NT-mediated GSK-3β phosphorylation in a dose-dependent fashion (Figure 4A). The role of PKCα and PKCβ1 was further examined in cells treated with or without 1 µM PMA for 24 hours to downregulate these enzymes (Figure 4, B and C). Treatment with PMA (1 mM) for 24 hours significantly reduced the basal expression of PKCα and PKCb1 (Figure 4B). To address the potential role of these proteins in the induction of GSK-3 phosphorylation by NT, HT29 cells were treated with PMA (1 µM) for 24 hours, followed by the addition of NT (100 nM) for 20 minutes. GSK-3 phosphorylation was determined by Western blot analysis (Figure 4C). In agreement with changes in PKCα and PKCβ1 levels, the phosphorylation of GSK-3β, but not GSK-3α, was abolished by chronic PMA treatment, further suggesting a role for PKCα or PKCβ1 in the NT-mediated phosphorylation of GSK-3β.
Figure 4.
Inhibition of PKCβ1 blocked NT-induced GSK-3α/β phosphorylation in HT29 cells. (A) HT29 cells were serum-starved for 24 hours; cells were pretreated with Gö976 (which inhibits PKCα, and PKCβ1) for 30 minutes, followed by treatment with NT (100 nM) for 20 minutes. Total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-GSK-3α/β antibody; membranes were stripped and reprobed with anti-GSK-3α/β antibody. (B) Western analysis of PKCα and PKCβ1 expression levels in HT29 cells. Cell lysates, before and after PMA (1 µM) treatment for 24 hours, were fractionated through 10% polyacrylamide gels and transferred to PVDF membranes. Membranes were probed with antibodies to PKCα, PKCβ1, and β-actin (loading control), and the final detection was carried out by ECL. (C) HT29 cells were pretreated with PMA (1 µM) for 24 hours followed by treatment with 100 nM NT for 20 minutes. Total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-GSK-3α/β antibody; membranes were stripped and reprobed with anti-GSK-3α/β antibody. (D) HT29 cells were serum-starved for 24 hours and then pretreated with LY379196 (which inhibits PKCβ1) for 30 minutes, followed by treatment with NT (100 nM) for 20 minutes. Total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-GSK-3α/β antibody; membranes were stripped and reprobed with anti-GSK-3α/β antibody. Representative data from three separate experiments are shown.
To further examine the specific isoforms of PKC involved in mediating NT signaling to GSK-3, we used LY379196, a currently available potent and selective PKCβ inhibitor with an IC50 = 30–50 nM for PKCβ [39,40], to examine the role of a single classic PKC isozyme, PKCβ1. Pretreatment with LY379196 completely blocked NT-mediated GSK-3β (but not GSK-3α) phosphorylation (Figure 4D). These results suggest a role for PKCβ1 in the NT-mediated phosphorylation of GSK-3β and for an undefined kinase that may be responsible for the NT-induced phosphorylation of GSK-3α.
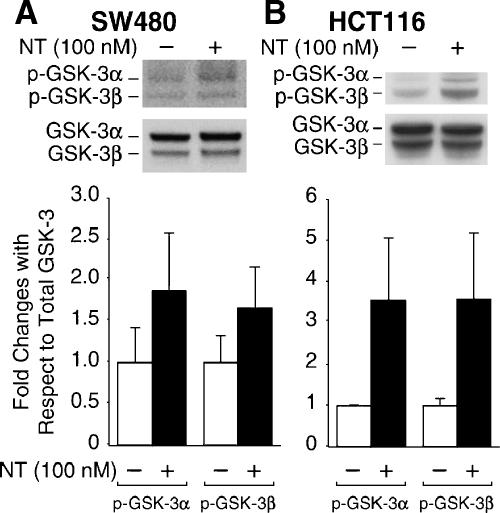
NT-Mediated GSK-3α/β Phosphorylation Occurs in Other Human Colon Cancer Cell Lines with NTR
We have shown that NT induces GSK-3 phosphorylation in the HT29 human colon cancer cell line. To determine whether this induction is limited to HT29 cells or occurs in other colon cancer cells expressing the high-affinity NTR, SW480 and HCT116 cells were treated with NT (100 nM) for 20 minutes. Total protein was extracted, and Western blot analysis was performed. As shown in Figure 5, NT induced GSK-3α/β phosphorylation in SW480 (Figure 5A) cells and HCT116 (Figure 5B). These results confirm our findings in HT29 cells and, moreover, suggest a general regulation of GSK-3α/β phosphorylation by NT in colon cancer cells that possess NTR.
Figure 5.
NT induces GSK-3α/β phosphorylation in SW480 and HCT116 cells. SW480 (A) or HCT116 (B) cells were serum-starved for 24 hours. Cells were treated with NT (100 nM) for 20 minutes. Total protein was extracted from cells and resolved by SDS-PAGE, transferred to PVDF membranes, and probed with anti-phospho-GSK-3α/β antibody; membranes were stripped and reprobed with anti-GSK-3α/β antibody. Signals from three separate experiments were quantitated densitometrically and expressed as fold changes with respect to total GSK-3α and PKCβ, respectively.
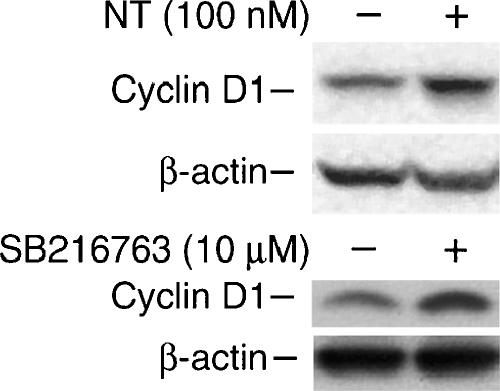
Treatment with NT or GSK-3α/β Inhibition Increases Cyclin D1 Expression in HT29 Cells
Cyclin D1 protein, which plays a critical role in intestinal epithelial cell growth and carcinogenesis [41–43], is stabilized by GSK-3β inhibition [44–46]. To investigate whether NT-mediated GSK-3 phosphorylation is related to increased cyclin D1 levels, we first determined whether NT treatment increases cyclin D1 expression in HT29 cells. As shown in Figure 6 (upper panel), cyclin D1 expression was increased at 4 hours after the addition of NT. Subsequently, we used SB216763, a potent inhibitor of GSK-3α and GSK-3β, in an ATP-competitive manner [47] to determine whether inhibition of GSK-3 leads to increased cyclin D1 expression levels in HT29 cells. As expected, treatment with SB216763 increased cyclin D1 expression in HT29 cells (Figure 6, lower panel).
Figure 6.
Inhibition of GSK-3 increases cyclin D1 expression in HT29 cells. HT29 cells were serum-starved for 24 hours, followed by treatment with NT or a specific GSK-3 inhibitor, SB216763, for 4 hours. Protein was extracted, and cyclin D1 expression was assessed by Western blot analysis. Membranes were stripped and reprobed with antiactin. Representative data from three separate experiments are shown.
Discussion
GSK-3-mediated signaling is implicated in multiple biologic processes, including embryonic development, cell differentiation, apoptosis, and insulin response, by the phosphorylation of a broad range of substrates, including several critical transcription factors (e.g., c-Myc, c-Jun, and c-Myb, and the translation factor eIF2B) [30,31]. GSK-3 phosphorylates b-catenin (at Ser-33, Ser-37, and Thr-41) and leads to its ubiquitination and proteasomal degradation, mimicking the effects of Wnt signaling activation [48]. The activation of canonical Wnt signaling stabilizes cytosolic β-catenin, resulting in its translocation to the nucleus and the stimulation of T-cell factor/lymphoid enhancer factor and target genes such as cyclin D1 [49]. Recently, we have shown that GSK-3 is involved in the regulation of TRAIL expression in human colon cancer cells [50], and inhibition of GSK-3 inhibits intestinal cell differentiation (Wang et al., unpublished data). GSK-3 is rapidly phosphorylated at Ser-21 in GSK-3α or at Ser-9 in GSK-3β, resulting in the inhibition of GSK-3 kinase activity [51,52]. Numerous stimuli lead to the inactivation of GSK-3 through S9/S21 phosphorylation, including growth factors such as epidermal growth factor and platelet-derived growth factor, which stimulate the GSK-3-inactivating kinase p90RSK through MAP kinases [37], activators of p70 ribosomal S6 kinase (p70S6K) [53], and activators of cAMP-activated protein kinase A (PKA) [54] and PKC [18,19]. Consistent with its position downstream of the PI3-kinase-PKB/Akt pathway, GSK-3 activity suppresses cell proliferation and survival [28,29]. In striking contrast, virtually nothing is known about the regulation of GSK-3 phosphorylation at serine residues by GI growth hormones that stimulate intestinal growth. In the present study, we show that the GI hormone NT induces a rapid increase in GSK-3 phosphorylation. Our results also indicate that PKC activation increases the phosphorylation of GSK-3β, implying a pathway that may be involved in physiological functions mediated by NT.
NT plays an important role in the stimulation of cell proliferation through multiple signaling pathways, including the activation of the ERK1/2 [12–14], PKC, and PI3-kinase/Akt pathways in some cells [15–17]. Although we show that NT stimulated ERK1/2, Akt, and PKC, only the inhibition of PKC blocked NT-mediated GSK-3β phosphorylation. In agreement with our results, Vilimek and Duronio [55] showed that cytokine-stimulated phosphorylation of GSK-3 is dependent on PKC, but not on PI3-kinase/Akt. These data suggest that NT, similar to cytokines, acts on GSK-3 through PKC activation and is independent of the PI3-kinase/Akt pathway.
We have shown that NT activates PKC, resulting in the phosphorylation of GSK-3. Bozou et al. [24] reported that NT only weakly stimulates PKC activity in HT29 cells. In this study, PKC activation was assessed indirectly by measuring epidermal growth factor binding to HT29 cells. We showed that NTstimulated obvious PKCα and PKCβ1 activation. The differences noted in activation may be a result of the different methods used for PKC activity assays. We assessed PKC activity directly by immunoprecipitation using anti-PKCα or anti-PKCβ1 antibody followed by in vitro kinase assay using MBP as substrate.
Our results indicate that the PKC inhibitors Gö6976 and LY379196 (which selectively inhibit either PKCα and PKCβ1, respectively) [56,57] or downregulation of PKCα and PKCβ1 (by prolonged PMA treatment) blocked NT-mediated GSK-3β (but not GSK-3α) phosphorylation. These findings suggest a role for PKCβ1 in the NT-mediated phosphorylation of GSK-3β; NT-mediated GSK-3− phosphorylation likely occurs through other kinase(s). Although it was noted that PKCα and PKCβ1 phosphorylate GSK-3β and GSK-3α [19], our results are largely consistent with a previous report by Goode et al. [18]. In this study, the effect of individual PKC isoforms on GSK-3 kinase activity in vitro was assessed. PKCα and PKCβ1 were among the PKC isoforms shown to phosphorylate GSK-3β, but not GSK-3α; however, the kinase that phosphorylates GSK-3α was not identified [10]. GSK-3α and GSK-3β are conserved with high homology within their kinase domains (98% identity), but only at 36% identity in the last 76 C-terminal residues [32]. GSK-3α and GSK-3β, although structurally similar, are not functionally identical. This became obvious in the ablation of the GSK-3β isoform in mice, which resulted in an embryonic lethal phenotype due to severe liver degeneration during midgestation [33]. The inability of GSK-3α to rescue GSK-3β-null mice indicates that a degenerative liver phenotype arises specifically from the loss of the β isoform. The phenotype of mice lacking GSK-3α has not yet been reported. It is interesting to speculate on a potentially unique role of GSK-3α in NT-mediated cellular responses. Future studies will assess the kinase(s) that contributes to NT-mediated GSK-3α phosphorylation.
Several studies have shown that NT stimulates intestinal cell growth [6–8,58]; however, the molecular mechanisms by which NT promotes cell proliferation and growth are largely undefined. In the present study, we showed that NT treatment or GSK-3 inhibition induced cyclin D1 expression. GSK-3 is a well-known regulator of cyclin D1 protein [59,60]. Phosphorylation of cyclin D1 on Thr-286 by GSK-3β results in the exclusion of cyclin D1 from the nucleus to initiate its proteasomal degradation [45]. Conversely, inactivated GSK-3β results in the suppression of cyclin D1 phosphorylation at Thr-286, thereby suppressing cyclin D1 degradation. Inhibition of GSK-3β stabilizes β-catenin expression, catalyzes its localization to the nucleus, and upregulates the downstream target gene cyclin D1 [59]. Extracellular mitogens promote cellular proliferation through receptor-mediated signaling circuitry, which ultimately converges on the cell cycle machine, and D-type cyclins function as critical sensors of these signals. During the G1 phase, cyclin D1 accumulates and assembles with either cyclin-dependent kinase in response to mitogenic growth factors. The active cyclin D1 holoenzyme promotes G1 progression by the inactivation of the growth-suppressive properties of the retinoblastoma protein and by virtue of its ability to titrate CDK inhibitors such as p27Kip1 and p21Cip1 [42] .Titration of p27Kip1 and p21Cip1, in turn, facilitates the activation of the cyclin E/CDK2 complex and its subsequent entry and progression through the DNA synthetic (S) phase of the cell cycle. G iven the critical roles of cyclin D1 in cell cycle progression and cell growth, our results provide a novel mechanism by which NT/GSK-3 may promote the proliferation and growth of colon cancer cells.
In conclusion, our results indicate that GSK-3 activity can be modulated by growth factors, such as NT, that work through the activation of PKC. Our present study underscores multiple levels of cross-talk between signaling cascades that regulate functionally critical molecules such as GSK-3. Moreover, our findings suggest that the NT-mediated proliferation of cancer cells possessing high-affinity NTR may be mediated, in part, through the modulation of GSK-3 phosphorylation by PKC signaling molecules.
Acknowledgements
The authors thank Eileen Figueroa and Karen Martin for manuscript preparation.
Abbreviations
- ECL
enhanced chemiluminescence
- FBS
fetal bovine serum
- GSK-3
glycogen synthase kinase-3
- MBP
myelin basic protein
- NT
neurotensin
- NTR
neurotensin receptor
- PI3-kinase
phosphatidylinositol-3 kinase
- PMA
phorbol-12-myristate-13 acetate
- PVDF
polyvinylidene difluoride
- PKA
protein kinase A
- PKB/Akt
protein kinase B
- PKC
protein kinase C
Footnotes
This work was supported by grants R37AG10885, RO1 DK48498, RO1 CA104748, and PO1 DK35608 from the National Institutes of Health.
References
- 1.Townsend CM, Jr, Bold RJ, Ishizuka J. Gastrointestinal hormones and cell proliferation. Surg Today. 1994;24:772–777. doi: 10.1007/BF01636304. [DOI] [PubMed] [Google Scholar]
- 2.Evers BM. Endocrine gene neurotensin: molecular mechanisms and a model of intestinal differentiation. World J Surg. 2002;26:799–805. doi: 10.1007/s00268-002-4055-3. [DOI] [PubMed] [Google Scholar]
- 3.Evers BM, Wang X, Zhou Z, Townsend CM, Jr, McNeil GP, Dobner PR. Characterization of promoter elements required for cellspecific expression of the neurotensin/neuromedin N gene in a human endocrine cell line. Mol Cell Biol. 1995;15:3870–3881. doi: 10.1128/mcb.15.7.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Parker MC, Ferris CF, Leeman SE. Neurotensin stimulates [3H]oleic acid translocation across rat small intestine. Am J Physiol. 1986;251:G823–G829. doi: 10.1152/ajpgi.1986.251.6.G823. [DOI] [PubMed] [Google Scholar]
- 5.Thor K, Rosell S. Neurotensin increases colonic motility. Gastroenterology. 1986;90:27–31. doi: 10.1016/0016-5085(86)90070-3. [DOI] [PubMed] [Google Scholar]
- 6.Evers BM, Izukura M, Chung DH, Parekh D, Yoshinaga K, Greeley GH, Jr, Uchida T, Townsend CM, Jr, Thompson JC. Neurotensin stimulates growth of colonic mucosa in young and aged rats. Gastroenterology. 1992;103:86–91. doi: 10.1016/0016-5085(92)91099-p. [DOI] [PubMed] [Google Scholar]
- 7.Chung DH, Evers BM, Shimoda I, Townsend CM, Jr, Rajaraman S, Thompson JC. Effect of neurotensin on gut mucosal growth in rats with jejunal and ileal Thiry-Vella fistulas. Gastroenterology. 1992;103:1254–1259. doi: 10.1016/0016-5085(92)91512-3. [DOI] [PubMed] [Google Scholar]
- 8.Thomas RP, Hellmich MR, Townsend CM, Jr, Evers BM. Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues. Endocr Rev. 2003;24:571–599. doi: 10.1210/er.2002-0028. [DOI] [PubMed] [Google Scholar]
- 9.Maoret JJ, Pospai D, Rouyer-Fessard C, Couvineau A, Laboisse C, Voisin T, Laburthe M. Neurotensin receptor and its mRNA are expressed in many human colon cancer cell lines but not in normal colonic epithelium: binding studies and RT-PCR experiments. Biochem Biophys Res Commun. 1994;203:465–471. doi: 10.1006/bbrc.1994.2205. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers RA, Zhang Y, Hellmich MR, Evers BM. Neurotensinmediated activation of MAPK pathways and AP-1 binding in the human pancreatic cancer cell line, MIA PaCa-2. Biochem Biophys Res Commun. 2000;269:704–708. doi: 10.1006/bbrc.2000.2335. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers RA, II, Bonnor RM, Wang X, Hellmich MR, Evers BM. Signal transduction mechanisms in neurotensin-mediated cellular regulation. Surgery. 1998;124:239–246. (discussion, 246-237) [PubMed] [Google Scholar]
- 12.Poinot-Chazel C, Portier M, Bouaboula M, Vita N, Pecceu F, Gully D, Monroe JG, Maffrand JP, LeFur G, Casellas P. Activation of mitogen-activated protein kinase couples neurotensin receptor stimulation to induction of the primary response gene Krox-24. Biochem J. 1996;320(Pt 1):145–151. doi: 10.1042/bj3200145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem. 2001;276:44464–44471. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 14.Ryder NM, Guha S, Hines OJ, Reber HA, Rozengurt E. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: neurotensin responsiveness and mitogenic stimulation. J Cell Physiol. 2001;186:53–64. doi: 10.1002/1097-4652(200101)186:1<53::AID-JCP1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Warhurst G, Fogg KE, Higgs NB, Tonge A, Grundy J. Ca(2+)-mobilising agonists potentiate forskolin- and VIP-stimulated cAMP production in human colonic cell line, HT29-cl.19A: role of [Ca2+]i and protein kinase C. Cell Calcium. 1994;15:162–174. doi: 10.1016/0143-4160(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 16.Hassan S, Dobner PR, Carraway RE. Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells. Regul Pept. 2004;120:155–166. doi: 10.1016/j.regpep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Guha S, Rey O, Rozengurt E. Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2002;62:1632–1640. [PubMed] [Google Scholar]
- 18.Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267:16878–16882. [PubMed] [Google Scholar]
- 19.Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase 3: EDG receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann J. Protein kinase C isozymes as potential targets for anticancer therapy. Curr Cancer Drug Targets. 2004;4:125–146. doi: 10.2174/1568009043481579. [DOI] [PubMed] [Google Scholar]
- 21.Umar S, Sellin JH, Morris AP. Murinecolonic mucosa hyperproliferation: II. PKC-beta activation and cPKC-mediated cellular CFTR overexpression. Am J Physiol Gastrointest Liver Physiol. 2000;278:G765–G774. doi: 10.1152/ajpgi.2000.278.5.G765. [DOI] [PubMed] [Google Scholar]
- 22.Parhamifar L, Jeppsson B, Sjolander A. Activation of cPLA2 is required for leukotriene D4-induced proliferation in colon cancer cells. Carcinogenesis. 2005;26:1988–1998. doi: 10.1093/carcin/bgi159. [DOI] [PubMed] [Google Scholar]
- 23.Amar S, Kitabgi P, Vincent JP. Activation of phosphatidylinositol turnover by neurotensin receptors in the human colonic adenocarcinoma cell line HT29. FEBS Lett. 1986;201:31–36. doi: 10.1016/0014-5793(86)80565-8. [DOI] [PubMed] [Google Scholar]
- 24.Bozou JC, Rochet N, Magnaldo I, Vincent JP, Kitabgi P. Neurotensin stimulates inositol trisphosphate-mediated calcium mobilization but not protein kinase C activation in HT29 cells. Involvement of a G-protein. Biochem J. 1989;264:871–878. doi: 10.1042/bj2640871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin stimulates protein kinase C-dependent mitogenic signaling in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2003;63:2379–2387. [PubMed] [Google Scholar]
- 26.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korswagen HC, Coudreuse DY, Betist MC, van de Water S, Zivkovic D, Clevers HC. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 2002;16:1291–1302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 29.Cui H, Meng Y, Bulleit RF. Inhibition of glycogen synthase kinase 3beta activity regulates proliferation of cultured cerebellar granule cells. Brain Res Dev Brain Res. 1998;111:177–188. doi: 10.1016/s0165-3806(98)00136-9. [DOI] [PubMed] [Google Scholar]
- 30.Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase3: properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 31.Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 32.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 34.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 35.De Mesquita DD, Zhan Q, Crossley L, Badwey JA. p90-RSK and Akt may promote rapid phosphorylation/inactivation of glycogen synthase kinase 3 in chemoattractant-stimulated neutrophils. FEBS Lett. 2001;502:84–88. doi: 10.1016/s0014-5793(01)02669-2. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Wang X, Evers BM. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 37.Brady MJ, Bourbonais FJ, Saltiel AR. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 1998;273:14063–14066. doi: 10.1074/jbc.273.23.14063. [DOI] [PubMed] [Google Scholar]
- 38.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates interleukin-8 expression through modulation of IkappaBalpha phosphorylation and p65 transcriptional activity: involvement of protein kinase Calpha. Mol Pharmacol. 2005;67:2025–2031. doi: 10.1124/mol.104.010801. [DOI] [PubMed] [Google Scholar]
- 39.Lin WW, Chen BC. Distinct PKC isoforms mediate the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW264. macrophages. Br J Pharmacol. 1998;125:1601–1609. doi: 10.1038/sj.bjp.0702219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen HC, Huang WC, Ali A, Woodgett JR, Lin WW. Negative regulation of phosphatidylinositol 3-kinase and Akt signalling pathway by PKC. Cell Signal. 2003;15:37–45. doi: 10.1016/s0898-6568(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 41.Rouet-Benzineb P, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, Bado A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J Biol Chem. 2004;279:16495–16502. doi: 10.1074/jbc.M312999200. [DOI] [PubMed] [Google Scholar]
- 42.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21:857–864. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 44.Hamelers IH, van Schaik RF, Sipkema J, Sussenbach JS, Steenbergh PH. Insulin-like growth factor I triggers nuclear accumulation of cyclin D1 in MCF-7S breast cancer cells. J Biol Chem. 2002;277:47645–47652. doi: 10.1074/jbc.M208727200. [DOI] [PubMed] [Google Scholar]
- 45.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 47.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 48.Sadot E, Conacci-Sorrell M, Zhurinsky J, Shnizer D, Lando Z, Zharhary D, Kam Z, Ben-Ze'ev A, Geiger B. Regulation of S33/S37 phosphorylated beta-catenin in normal and transformed cells. J Cell Sci. 2002;115:2771–2780. doi: 10.1242/jcs.115.13.2771. [DOI] [PubMed] [Google Scholar]
- 49.Wong NA, Pignatelli M. Beta-catenin—a linchpin in colorectal carcinogenesis? Am J Pathol. 2002;160:389–401. doi: 10.1016/s0002-9440(10)64856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Wang X, Hernandez A, Hellmich MR, Gatalica Z, Evers BM. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. J Biol Chem. 2002;277:36602–36610. doi: 10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- 51.Cross DA, Alessi DR, Vandenheede JR, McDowell HE, Hundal HS, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krause U, Bertrand L, Maisin L, Rosa M, Hue L. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur J Biochem. 2002;269:3742–3750. doi: 10.1046/j.1432-1033.2002.03069.x. [DOI] [PubMed] [Google Scholar]
- 54.Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vilimek D, Duronio V. Cytokine-stimulated phosphorylation of GSK-3 is primarily dependent upon PKCs, not PKB. Biochem Cell Biol. 2006;84:20–29. doi: 10.1139/o05-154. [DOI] [PubMed] [Google Scholar]
- 56.Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH, III, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A, et al. (S)-13-[(Dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiaza-cyclohexadecene-1,3(2H)-dione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem. 1996;39:2664–2671. doi: 10.1021/jm950588y. [DOI] [PubMed] [Google Scholar]
- 57.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 58.Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer. 1999;80:448–454. doi: 10.1002/(sici)1097-0215(19990129)80:3<448::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 59.Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, Seldin DC. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res. 2005;65:5792–5801. doi: 10.1158/0008-5472.CAN-05-1021. [DOI] [PubMed] [Google Scholar]
- 60.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]