Abstract
Reproduction in C. elegans relies on continuously proliferating germ cells which, during germline development, exit mitosis, undergo meiosis and differentiate into gametes. Supplementing the diet of C. elegans with dihommogamma-linolenic acid (20:3n-6, DGLA), a long chain omega-6 polyunsaturated fatty acid, results in sterile worms that lack germ cells. The effect is remarkably specific for DGLA, as eicosapentaenoic acid (20:5n-3, EPA) and other long-chain polyunsaturated fatty acids with similar physical properties have little or no effect on fertility. Germ cells undergoing mitosis during larval stages are especially sensitive to DGLA, but exposure to DGLA during adulthood also reduces germ cells and brood size, in part by inducing inappropriate apoptosis of meiotic germ cells. Mutant strains with defects in fatty acid desaturation and elongation display altered susceptibility to DGLA, indicating that the sterility effect of the dietary lipid depends on the amount of DGLA present in membranes as well as on the capacity to convert DGLA to other fatty acids. We propose that DGLA produces a signal that interacts with one or more pathways regulating germ cell survival. Our DGLA findings are the first report of a role for a specific fatty acid affecting the development and maintenance of germ cells in C. elegans.
Keywords: Polyunsaturated fatty acid, Arachidonic acid, Dihommogamma-linolenic acid, Germline, Apoptosis
Introduction
Polyunsaturated fatty acids (PUFAs) are essential in the mammalian diet for proper development of the nervous system, efficient immune response, and proper skin function (de Urquiza et al., 2000; Horrobin, 2000; Hwang, 2000; Sampath and Ntambi, 2004; Uauy et al., 2001). PUFAs are important membrane components that influence membrane integrity and fluidity and are implicated in numerous signaling pathways that affect cell growth, differentiation, and programmed cell death (Brash, 2001; Tang et al., 2002). In addition, upon stimulation, PUFAs are selectively released from cell membranes by phospholipases and are readily converted to biologically active eicosanoids by cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes (Piomelli, 1993). Important aspects of mammalian reproduction, such as implantation and parturition are influenced by eicosanoids and other lipids (Hertelendy and Zakar, 2004; Hertelendy et al., 1995; Ye et al., 2005). Despite extensive research, the mechanisms by which unsaturated fatty acids and their derivatives affect cell processes are poorly understood.
The most commonly occurring PUFAs fall into two families, n-3 or n-6 (omega-3 or omega-6), named so because of the position of the first double bond relative to the methyl end of the molecules. Studies in humans and animal models provide evidence that high dietary intake of n-6 fatty acids, common in Western diets, stimulate the development of mammary and colon cancer (reviewed in (Bartsch et al., 1999)) and, due to increased inflammation and platelet aggregation, may contribute to coronary artery disease (James et al., 2000). In contrast, ingestion of n-3 PUFAs, found mainly in fish and marine mammals, provides many health benefits, including the prevention of coronary heart disease, the lowering of plasma lipid levels, and the slowing of autoimmune disorders (Sampath and Ntambi, 2004; Simopoulos, 1999).
The nematode C. elegans is a good model for studying the physiological functions of unsaturated fatty acids. Unlike mammals, nematodes possess all the required fatty acid biosynthetic enzymes and have no dietary requirements for essential fatty acids (Wallis et al., 2002)(Fig. 1). Moreover, a series of mutants has been isolated that are unable to synthesize specific PUFAs due to mutations in genes encoding fatty acid desaturases and elongases (Watts and Browse, 2002). Further manipulation of nematode membrane fatty acids can be achieved by dietary supplementation with fatty acids (Watts et al., 2003). In mammals, C20 PUFAs play important roles in cellular signaling due their conversion into eicosanoids, their ability to activate nuclear hormone receptor transcription factors, and their ability to affect membrane composition and function (Bazan, 2003; Benatti et al., 2004; Ma et al., 2004). C. elegans fat-3 mutants lack Δ-6 desaturation activity and therefore fail to produce the precursors for the elongation of C18 PUFAs to C20 PUFAs. This strain is viable in spite of the dramatic changes in fatty acid composition. However, the fat-3 mutant exhibits a number of phenotypes, including sluggish movement, reduced numbers of synaptic vesicles at neuromuscular junctions, reduced sensory abilities, and a low brood size (Kahn-Kirby et al., 2004; Lesa et al., 2003; Watts et al., 2003).
Fig. 1.

Schematic diagram of C. elegans fatty acid synthesis. C. elegans possesses all of the desaturase and elongase enzymes to produce a range of C18 and C20 PUFAs from saturated fatty acid precursors. Fatty acids are abbreviated as in 20:5n-3, which has 20 carbons and five methylene-interrupted double bonds, the first occurring at the n-3 position. See text for details.
Wild-type C. elegans hermaphrodites typically produce 250-300 offspring during their reproductive lifetime. During larval development a complex program of mitosis, meiosis, and differentiation produces the somatic germline tissues, germ cells, and differentiating gametes (Schedl, 1997). The hermaphrodite gonad consists of two reflexed arms that share a common uterus and vulva at the midbody of the animal. Germ cells at the distal end of the gonad maintain a stem cell-like population that depends on a proliferation signal from the somatic distal tip cell (Seydoux and Schedl, 2001). As proliferating cells gradually progress proximally in the gonad they cease to proliferate and enter meiotic prophase. The meiosis versus cell proliferation decision is controlled by the conserved GLP-1/NOTCH signaling pathway (Austin and Kimble, 1987). The first germ cells that enter meiotic prophase in each gonad arm differentiate into approximately 160 sperm, after which a switch in sexual fate occurs and germ cells differentiate into oocytes. This developmental switch depends on RNA binding proteins that bind to the 3’ untranslated region of the fem-3 mRNA that controls the sperm-oocyte switch (Ahringer and Kimble, 1991; Kraemer et al., 1999).
In this study, we find that dietary supplementation and subsequent incorporation of dihommogamma-linolenic acid (DGLA, 20:3n-6) leads to sterility in C. elegans. Levels of DGLA as low as 12% of total fatty acids cause the degeneration of germ cells during larval development, and induce excess programmed cell death in germ cells of adult animals. In contrast, ingestion of high doses of eicosapentaenoic acid (EPA, 20:5n-3) and other long chain polyunsaturated fatty acids has little effect on germline development or fertility. Moreover, we demonstrate that nematode mutants with defective biosynthesis, modification and transport of C20 PUFAs show altered sensitivity to the dietary DGLA.
Materials and methods
Strains
Caenorhabditis elegans N2 variety Bristol was the wild-type parent of all mutant strains. The following mutations were used: fat-3(wa22), fat-1(wa9), fat-4(wa14), fat-1;fat-4 (wa9;wa14), elo-1(wa7) (Watts and Browse, 2002), OH172 [(lin-15(n765); otEx105[lim-7∷ GFP; lin-15(+)] (Hall et al., 1999), nrf-5 (sa513), ndg-4(lb108), nrf-6(sa525), nrf-1(sa524) (Choy and Thomas, 1999), ced-4(n1162) (Yuan and Horvitz, 1992), rme-1(b1045) (Grant et al., 2001), and rme-2(b1008) (Grant and Hirsh, 1999). Strains were maintained on NGM agar using standard techniques (Wood, 1988). All experiments were performed at 20°C. The OH172 strain expressing lim-7∷ GFP was constructed in the lin-15 background. The GFP line is highly penetrant, but because the non-multivulva/multivulva (Muv) does not correlate well with the presence or absence of the extrachromosomal array, the line was maintained by picking GFP-positive animals. Many GFP-positive animals showed a Muv phenotype due to the lin-15 background.
Fatty acid supplementation and sterility assays
NGM plates were prepared with the addition of 0.075-0.25 mM fatty acid sodium salt (NuChek Prep) in the presence of 0.1% tergitol (NP-40). Because of the instability of polyunsaturated fatty acids we used freshly opened vials of fatty acids and stored the nematode growth plates in the dark. E. coli strain OP50 grew on the plates for 2-3 days at room temperature before the addition of embryos or L1 larvae. Sterility was scored by examining animals under a stereo dissecting microscope for the presence or absence of embryos in the uterus. Unless otherwise noted, 100 animals were scored for each fatty acid treatment. After scoring for sterility, the entire population from the plate (200-300 animals) was washed off the plate and prepared for gas chromatography analysis of fatty acid composition.
Lipid analysis
For analysis of fatty acid composition of whole worms, 200-500 adults were washed off of one or two 6 cm plates with water. Glass tubes containing the washed worms were placed on ice and the adult worms were allowed to settle. Most of the water was removed with a glass pipette and fatty acid methyl esters were prepared by adding 1 ml of 2.5% methanolic H2SO4. The mixture was incubated for 1 h at 80°C and extracted with hexane for gas chromatography analysis. For the isolation and separation of lipids, worms were grown for 4 days starting from isolated early embryos on 50 NGM plates with 0.1% tergitol (NP-40) with or without 0.11 mM dihommogamma linolenate. Lipids were extracted from frozen worm pellets with (10:10:1) chloroform/methanol/formic acid at -20° for at least 12 h (Bligh and Dyer, 1959). The extract was washed with 0.2 MH3PO4, 1 M KCl (Hajra, 1974). Lipids were recovered in the chloroform phase, dried under N2, and redissolved in chloroform containing 0.01% BHT. Individual lipids were purified from the extracts by one-dimensional thin layer chromatography. Neutral lipids were separated on silica gel HL plates (Analtech) using 80:20:3 hexane/diethyl ether/acetic acid solvent mixture. Polar lipids were separated using a 65:43:3:2.6 chloroform/methanol/water/acetic acid solvent mixture. Separated lipids were visualized using I2 vapor, compared to authentic standards and scraped immediately for fatty acid methyl ester derivatization. The internal standard (15:0) was added prior to esterification. Fatty acid methyl esters were prepared with 2.5% methanolic H2SO4 for analysis by gas chromatography (Watts and Browse, 2002).
Fluorescence microscopy
SYTO 12 staining of apoptotic corpses was performed according to (Gumienny et al., 1999). Diamindinophenylindole (DAPI) staining was performed using the method described in (Kadyk et al., 1997). DAPI stained samples were mounted in Vectashield and examined by fluorescence microscopy and Nomarski optics.
Results
Dietary DGLA causes sterility in C. elegans
During our studies of fatty acid supplementation of fat-3 and wild-type nematodes (Watts and Browse, 2002), we discovered that dietary supplementation with dihommogamma-linolenic acid (DGLA, 20:3n-6) caused sterility. The doses of DGLA that are sufficient to cause sterility did not affect worm growth or produce any other detectable phenotypes. Dose-response curves demonstrated that the percentage of a wild-type nematode population that was infertile increased in proportion to increased concentrations of supplemented DGLA. To assess the actual exposure of the animals to supplemented PUFAs, we measured the fatty acid composition of the E. coli food source as well as in the nematodes after 4 days growth on the supplemented plates. We found that DGLA was incorporated into E. coli lipids and closely reflected the degree of accumulation in the nematode lipids as well. Furthermore, worms growing on PUFA supplemented plates without E. coli did not accumulate PUFAs (data not shown). Therefore, uptake of PUFAs in worms appears to depend on PUFAs incorporated into their bacterial food source.
In a series of five experiments, the degree of sterility varied between experiments with respect to the concentration of DGLA in plates (data not shown). However when we compared the extent of sterility with the amount of DGLA that was incorporated into lipids of the worms, we noticed a high degree of concordance between the experiments (Fig. 2A). The total DGLA measured included DGLA synthesized by the worm as well as the assimilated dietary DGLA. We calculated the EC50, the effective concentration of DGLA as a percent of fatty acids in nematode lipids in which 50% of the population becomes sterile. In the five combined experiments the EC50 is 11.6% ± 1.2% DGLA.
Fig. 2.

Dose response curves of wild type (WT) and fatty acid desaturase and elongase mutants grown on plates containing various concentrations of DGLA. (A) Data were obtained from fertility and fatty acid analysis from five, independent dose-response experiments. The DGLA content of worm lipids represents DGLA that synthesized endogenously plus DGLA that is assimilated from the diet. The EC50 calculated from these experiments is 11.6% DGLA (±1.2%). (B) Comparison of wild type and PUFA synthesis mutants and their sensitivity to DGLA. The graph shows the percentage of each population (n = 100-150 worms) that was sterile vs. the concentration of DGLA in supplemented plates. Sterility was scored under a stereo dissecting microscope for the presence of embryos in the uterus. (C) The same experiment as in panel B, comparing the percent sterile animals vs. the DGLA content of nematode lipids (endogenously synthesize DGLA plus assimilated dietary DGLA). The EC50 calculated from these data is 12.9% DGLA (±1.1).
C. elegans possesses two distinct desaturase activities that further modify DGLA, the Δ5 desaturase, which produces AA from DGLA, and the omega-3 desaturase, which converts DGLA to 20:4n-3 (Fig. 1). Mutants in these desaturase activities accumulate higher levels of DGLA in their phospholipids than wild type (Watts and Browse, 2002). Mutant strains with deficient omega-3 desaturase (fat-1), as well as mutants deficient in Δ5 desaturase (fat-4) are more sensitive to DGLA than wild type, and mutants lacking both the omega-3 and Δ5 desaturase activities (fat-1;fat-4) are extremely sensitive to DGLA (Fig. 2B). On the other hand, strains that are deficient in the production of DGLA, including fat-3, which lack Δ6 desaturase activity, and elo-1, which fail to elongate GLA (18:3 n-6) to DGLA, are more resistant to DGLA (Fig. 2B). This demonstrates that the ability of DGLA to induce sterility depends on the amount of DGLA present in the membranes of various worm strains, as well as the capacity to convert DGLA to other fatty acids. When nematode sterility is graphed with respect to the DGLA composition of the nematodes, we find that the dose response curves of the different mutants converge (Fig. 2C). Again, the calculated EC50 of the various genotypes converge between 11 and 14%, with an average EC50 of 12.9% ± 1.1% DGLA. The exception to the 11-14% rule is the fat-1;fat-4 double mutant, which is deficient in both the omega-3 and the Δ5 desaturases. On unsupplemented plates this strain accumulates approximately 22% DGLA, yet shows only low sterility (3%). The fat-1;fat-4 worms, however, are hypersensitive to DGLA (Fig. 2B). They become sterile when exposed to less than 0.05 mM dietary DGLA, a concentration that has no effect on wild-type animals or on the fat-1 and fat-4 single mutants. It is not clear why the germ line is protected from excess endogenously produced DGLA in the fat-1;fat-4 double mutant.
Effects of various dietary PUFAs on fertility
When we examined worms after supplementation with other PUFAs, we found that most PUFAs did not affect the fertility of the nematodes. The only fatty acid to cause sterility similar to DGLA was its immediate 18-carbon precursor gamma-linolenic acid (18:3n-6, GLA). Fatty acid composition analysis of the worms fed GLA revealed that greater than half of the dietary GLA was elongated to DGLA in the nematodes, resulting in high accumulation of DGLA. Similar to DGLA-supplemented worms, the GLA-supplemented worms that accumulated greater than 15% DGLA were 100% sterile. In order to determine whether the GLA itself or the elongated DGLA was responsible for the infertility, we made use of the elo-1 strain that is severely compromised in its ability to elongate GLA (Watts and Browse, 2002). The elo-1 strain supplemented with 0.2 mM GLA accumulated up to 44% GLA in its lipids, and only 2% of this fatty acid was elongated to DGLA, resulting in a concentration of 1% DGLA in total lipid extracts in these animals. We found that the GLA-supplemented elo-1 nematodes were 100% fertile. In contrast, 100% of wild-type worms in the same experiment were sterile, and fatty acid analysis revealed that they accumulated 13.5% GLA and 22.8% DGLA. These results demonstrate that DGLA, whether supplemented directly, or elongated from its precursor GLA, induces sterility, but that GLA itself has little effect on the worms.
Feeding high doses of linoleic acid (18:2n-6, LA), linolenic acid (18:3n-3, ALA), and eicosapentaenoic acid (20:5n-3, EPA) had no effect on the C. elegans fertility. Nematodes grown on plates containing 0.2 mM of the fatty acids were 100% fertile and we found the following accumulation in nematode lipids: LA, 35%; ALA, 41%; and EPA, 48%. Culturing worms on very high concentrations (>25 mM) of any of the C18 or C20 PUFAs, such that the supplemented fatty acid accounted for greater than 50% of total lipids caused an overall slowing of growth or larval arrest. Worms that reached adulthood often display a short, fat body shape (Dumpy) and a protruding vulva. These phenotypes appear to be a general fatty acid toxicity affect, in contrast to the sterility, which is observed at concentrations of DGLA that do not cause other growth defects in the worms.
In three independent experiments we observed that between 17% and 73% of a population of nematodes became sterile when growing on plates containing arachidonic acid (20:4n-6, AA). GC analysis revealed that these worms contained 30-40% arachidonic acid in their lipids. Several subsequent dose response experiments revealed that, unlike DGLA, AA must accumulate to over 20% of worm lipids before any sterile animals are observed. An average of 46% sterile animals were observed in animals that accumulated 35-45% AA in their lipids. The highest degree of sterility achieved by AA was 73%, which corresponded to 52% AA accumulation in nematode lipids. These animals also displayed slow growth, sluggish movement, and a Dumpy body shape, traits typical of general fatty acid toxicity that are induced by a variety of dietary PUFAs. Thus, while dietary AA induces some sterility, it does so at higher concentrations than DGLA and the affected populations are never 100% sterile.
Lipid analysis reveals that all lipids assimilate dietary DGLA
We presumed that the dietary DGLA would be incorporated into all types of lipids in the nematode. However, in order to investigate if any differential accumulation of DGLA occurred, we performed an extensive lipid and fatty acid analysis of young adult C. elegans grown in the presence or absence of 0.11 mM DGLA. In this experiment, 95% of the supplemented animals were sterile and 100% of the unsupplemented animals were fertile. Our analysis shows that the amount of storage lipids (TAGs) relative to total lipids increased in lipid-supplemented worms (Table 1). The DGLA supplementation did not increase the levels of free fatty acids in the worms. The free fatty acids accounted for β2% of total lipids in both fed worms and unfed controls (data not shown). It is noteworthy that when wild-type worms consume unsupplemented E. coli that are devoid of polyunsaturated fatty acids, the C20 PUFAs that they synthesize endogenously are almost all excluded from triacylglyceride (TAG) fraction, and instead are incorporated into phosphatidylethanolamine (PE), phosphatidylcholine (PC), and phosphatidylserine (PS). When dietary DGLA is present, we found excess DGLA in all lipids of the worms, including TAGs and all of the major membrane phospholipids (Table 1). In addition to the increase in DGLA in all lipids, we found a corresponding increase in 20:4n-3, which is produced from DGLA by omega-3 desaturation. The major saturated fatty acids, palmitic acid (16:0) and stearic acid (18:0), increased in supplemented worms, while the monounsaturated fatty acids, oleic acid (18:1n-9) and vaccenic acid(18:1n-7) declined, as did the C20 n-3 PUFA EPA. Thus, DGLA supplementation resulted in altered fatty acid composition of all of the major nematode lipids.
Table 1.
Lipid analysis of adult C. elegans grown unsupplemented (NoFA) or on 0.11 mM DGLA plates
| TAGs |
Phospholipids |
PE |
PC |
PI |
PS |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NoFA | DGLA | NoFA | DGLA | NoFA | DGLA | NoFA | DGLA | NoFA | DGLA | NoFA | DGLA | |
| % total lipids | 61 | 74 | 34 | 23 | ||||||||
| % phospholipids | 53 | 46 | 32 | 38 | 9 | 8 | 5 | 7 | ||||
| 16:0 | 7 | 9 | 6 | 10 | 10 | 11 | 5 | 12 | 6 | 7 | 7 | 6 |
| C17iso | 3 | 2 | 2 | 2 | 4 | 2 | 2 | 2 | 2 | 2 | 3 | 2 |
| 16:1 | 3 | 5 | 0 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 1 |
| 17Δ | 16 | 7 | 6 | 2 | 6 | 2 | 8 | 2 | 2 | 0 | 3 | 1 |
| 18:0 | 4 | 8 | 15 | 21 | 15 | 27 | 5 | 12 | 38 | 50 | 25 | 29 |
| 18:1n-9 | 8 | 7 | 4 | 1 | 3 | 1 | 4 | 2 | 3 | 2 | 3 | 1 |
| 18:1n-7 | 21 | 26 | 30 | 22 | 28 | 22 | 26 | 22 | 16 | 10 | 26 | 21 |
| 18:2 | 7 | 3 | 3 | 1 | 3 | 1 | 4 | 1 | 1 | 1 | 2 | 0 |
| 19Δ | 11 | 4 | 0 | 1 | 5 | 1 | 8 | 2 | 5 | 1 | 2 | 4 |
| 20:3n-6 | 1 | 11 | 4 | 14 | 2 | 10 | 4 | 13 | 2 | 5 | 4 | 13 |
| 20:4n-6 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
| 20:4n-3 | 1 | 3 | 4 | 11 | 2 | 6 | 4 | 11 | 2 | 3 | 3 | 7 |
| 20:5 | 1 | 2 | 9 | 7 | 5 | 3 | 13 | 7 | 12 | 11 | 8 | 6 |
| % total C20 PUFAs | 5 | 17 | 18 | 33 | 10 | 19 | 23 | 34 | 17 | 20 | 17 | 28 |
Abbreviations: TAG, triacylglycerides; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PI, phosphatidylinositol; PS, phosphatidylserine; 17Δ , 9,10-methylene hexadecanoic acid; 19Δ , 11,12-methylene octadecanoic acid.
Absence of germ cells and gametes in nematodes exposed to dietary DGLA
C. elegans hermaphrodites are born with two primordial germ cells which begin to divide in late L1 larval stage and continue to proliferate throughout development, producing around 1000 germ cell nuclei per gonad by adult stage (Seydoux and Schedl, 2001) (Fig. 3A). The adult gonad consists of two reflexed arms containing a syncitium of germline nuclei sharing a common cytoplasm. By convention, a germline nucleus and its surrounding cytoplasm is called a germ cell. We used the DNA-binding dye DAPI to visualize and count germ cells in sterile adults that had developed in the presence of DGLA. Wild-type adults that were raised on DGLA were deficient in germ cells. The degree of germ cell deficit correlated with the amount of DGLA associated with worm lipids. In one experiment worms were exposed throughout their life cycle to a high concentration of DGLA which resulted in 31.3% incorporation of DGLA into worm lipids. In 95% of these animals, we did not identify recognizable oocytes, sperm, or non-gamete germ cells (n = 37) (Fig. 3B). The remaining two animals each contained 1-5 germ cells per gonad, some of which appeared to have more condensed DNA (Fig. 3C). We observed a similar lack of germ cells in males raised on DGLA (data not shown), indicating that this effect is not sex specific. DAPI staining of worms raised on lower concentrations of DGLA, under conditions that did not cause 100% sterility, revealed reduced numbers of germ cells, spermatocytes, and sperm (Fig. 3D); differentiated oocytes were never observed in sterile worms. DAPI staining of the fertile worms grown on the same plates revealed a more severe phenotype in the anterior gonad than in the posterior gonad (Fig. 3E). In this treatment, 40% of the supplemented worms showed a difference in the number of germ cells in each of the two gonadal arms. A vast majority of these (88%) showed a more severe phenotype in the anterior, with fewer non-gamete germ cells and differentiated gametes visible in the anterior gonad.
Fig. 3.

Germ cells are absent or greatly reduced in DGLA supplemented nematodes. Nuclei were visualized by DAPI staining of fixed nematodes. In all panels, anterior is to the left. (A) Wild type on control plates showing many germ cell nuclei, differentiating gametes and embryos developing in the uterus. (B) Sterile wild type grown on 0.15 mM DGLA showing a lack of germ cells, gametes, and embryos. (C) High magnification of germ cells in a sterile wild type grown on 0.15 mM DGLA. (D) High magnification of germ cells and sperm in a sterile wild type grown on 0.1 mM DGLA. (E) Fertile wild type grown on 0.1 mM DGLA showing an apparently normal posterior gonad, one developing embryo in the uterus, and an anterior gonad devoid of germ cells. Scale bars in panels A, B, and E: 100 μm. Scale bars in panels C and D: 100 μm.
Gonad migration defects are caused by DGLA
By microscopic observation with Nomarski optics, we found that the somatic uterus and vulva morphology appear normal in DGLA-fed worms that lack germ cells. To determine whether the proliferation or maintenance of other somatic gonad cells was also affected by DGLA, we used the OH172 strain, which expresses GFP in the somatic gonadal sheath cells under control of the lim-7 promoter (Hall et al., 1999). Observation of OH172 animals that were raised on DGLA revealed that the gonadal sheath cells were present in supplemented worms (Fig. 4). This indicates that DGLA does not interfere with the proliferation and maintenance of these somatic cells. However, we observed that the gonad is shriveled due to lack of germ cells. In addition, the GFP expression revealed gonad migration defects. We found that DGLA-fed worms often developed small gonads that lacked a U-shaped bend, and occasionally aberrant migration occurred that resulted in a Z-shaped bend (Fig. 4). In addition, the anterior gonad was also more likely to be affected than the posterior gonad.
Fig. 4.

Altered gonad migration in DGLA supplemented nematodes. (A) Typical GFP expression in gonadal sheath cells in OH172 raised on unsupplemented plates, the gonads show the predicted U-shaped bend. (B) Abnormal gonad migration in OH172 raised on 0.15 mM DGLA. The anterior gonad has no bend, while the posterior gonad displays improper gonadal migration (Z-shaped bend). The table reports the percentage of the supplemented population (n = 49) showing normal gonad shape, no bend, or Z shaped migration (Mig) in the anterior and the posterior gonads.
Larval stages exposed to DGLA are susceptible to permanent loss of germ cells
To define the onset and cause of the germ cell deficiency, we transferred larvae onto, and off of, fatty acid plates during various stages of development and scored fertility and numbers of germ cells when worms reached maturity (Fig. 5). Sterility was scored by observing mature animals under a steromicroscope for the absence of embryos in the uterus. A subset of sterile animals was fixed and stained with DAPI in order to visualize the germ cells. Early embryos were isolated by treatment of gravid adults with hypochlorite solution and were plated at time 0 onto NGM plates containing 0.1% tergitol only (control), or 0.1% tergitol and 0.12 mM DGLA. By 24 h, the embryos had hatched and the majority of the L1 larvae had molted into the L2 stage. At hatching, the L1 animals contain a gonadal primordium consisting of four cells, two precursors of the somatic gonad, named Z1 and Z4, and two precursors of germ-line tissue, Z2 and Z3 (Hirsh et al., 1976). At late L1 stage Z2 and Z3 begin mitotic divisions. The dividing germ cells closest to the distal end of the germ line maintain a mitotic stem cell population throughout the larval and adult life of the worm. During L3 stage, cells in the most proximal end of the developing gonad leave mitosis and enter meiosis.
Fig. 5.
L2/L3 larval stages are critically sensitive to DGLA. Early embryos were isolated from gravid adults by hypochlorite treatment. Embryos were plated onto control plates containing 0.1% tergitol or DGLA plates containing 0.1% tergitol and 0.12 mM DGLA and subsequently transferred between these two media as indicated. By 24 h after plating, the embryos had hatched and worms had reached L2 larval stage. After 48 h, worms had reach the L3 or early L4 stage, at 72 h, worms were young adults, and at 96 h, worms that were fertile were gravid adults. Sterility was scored at 96 h under a stereo dissecting microscope for the presence of embryos in the uterus (n = 100 worms for each treatment).
In this experiment, worms growing on DGLA plates for their entire lifetime were 100% sterile (Fig. 5, treatment 1), while worms growing on control plates were fully fertile (treatment 2). We found that eggs hatching in the presence of DGLA that are exposed to dietary DGLA during L1 larval stage and were subsequently shifted to unsupplemented plates at 24 h (when most eggs had reached the early L2 stage) developed into mostly fertile adults (treatment 3), while worms undergoing the opposite treatment, hatching on control plates but shifted to DGLA plates at 24 h were 100% sterile (treatment 4). Animals that spend their first 48 h on DGLA always developed into sterile adults (treatment 5), while only 33% of worms that develop for 48 h on control plates and then are shifted to DGLA plates become sterile (treatment 6).
In treatment 7, eggs were hatched on normal plates, then shifted to DGLA plates at L2 stage for 24 h, then shifted back to regular plates to complete development. We scored sterility and fatty acid composition and found that 100% of animals were sterile, even though the fatty acid composition of adults had returned to normal (total lipids of adult worms contained only 4.0% DGLA). The reverse experiment (treatment 8) resulted in 38% sterile animals. Finally, in treatment 9, worms were exposed to DGLA from 48 h to 72 h, during late larval development. In this treatment, 38% of animals became sterile adults.
The period in which worms are most sensitive to DGLA was the second 24-h period, when worms were developing through the L2 and L3 stages. Exposure to DGLA during this period always resulted in 100% sterile animals. During L2 and L3 larval stages germ cells are proliferating mitotically. Shifting later in development resulted in fewer sterile nematodes, although the DGLA is still potent at later stages (treatments 8 and 9), since approximately one third of the animals do become sterile. Furthermore, some germ cells do degenerate even when young adults are shifted to DGLA (see below). These studies indicate that dietary fatty acids induce a permanent degeneration of mitotic germ cells, not merely a temporary cessation in cell division. In all of the treatments resulting in 100% sterility, DAPI staining revealed that only 0-5 germ cells per gonad arm remained after DGLA exposure, indicating that mitotic germ cells are eliminated during larval exposure to DGLA.
DGLA induces inappropriate programmed cell death in the germ line
We observed that worms that developed on unsupplemented media and were subsequently transferred to DGLA as young adults and L4 larvae produced significantly fewer offspring than those that remained on control plates. An average of 30.3 (±23.3) progeny were produced by L4s transferred to 0.15 mM DGLA plates compared to 196.3 (±12.9) progeny produced by L4s transferred to control plates. Furthermore, DAPI staining revealed that 4-day-old adults that were not exposed to DGLA had mostly depleted their sperm stores, but accumulated an average of 30 oocytes and more than 1000 non-gamete germ cells, while worms that were exposed to DGLA as adults still possessed an average of 113 sperm but showed only 2 oocytes and an average of 116 non-gamete germ cells. Before being transferred to DGLA plates, the germ cells in L4 larvae have proliferated to approximately 500 germ cells per gonad arm. Therefore, even if the DGLA resulted in the degeneration of the mitotically dividing cells, we would still expect a higher number of progeny derived from the germ cells that already progressed into meiosis. Since we observe reduced numbers of germ cells, oocytes, and progeny, we examined whether meiotic germ cells in adults are also affected by DGLA.
Under normal growth conditions, approximately 50% of meiotic female germ cells in adult hermaphrodites are fated to undergo programmed cell death. As in mammalian tissue, cells are eliminated to maintain tissue homeostasis (Gumienny et al., 1999). We examined whether DGLA may be causing excess cell death in adult worms, which would result in fewer progeny and germ cells. We stained worms with SYTO 12 to identify apoptotic corpses. The combined results from four independent experiments revealed 75% more corpses in adults that had been placed on 0.2 mM fatty acid plate for 20 h (Table 2A and Fig. 6). The extra corpses were often located more distally in the germ line than the wild-type corpses, indicating the inappropriate induction of apoptosis in these germ cells. We also occasionally observed germ cell deaths in L3 and L4 larvae that were placed onto DGLA. Programmed cell death of germ cells is not normally observed in these larval stages (Gumienny et al., 1999).
Table 2.
SYTO 12 staining of adults grown unsupplemented or exposed to 0.2 mM DGLA for 20 h
| Genotype | DGLA treatment | n | No. of SYTO 12 positive cells in the posterior gonad (±SEM) |
|---|---|---|---|
| (A) | |||
| N2 | No | 51 | 4.0 (±0.2) |
| N2 | Yes | 40 | 8.3 (±0.5) |
| ced-4 | No | 29 | 0 |
| ced-4 | Yes | 42 | 0 |
| (B) | |||
| N2 | No | 53 | 3.5 (±0.4) |
| elo-1 | No | 26 | 3.8 (±0.5) |
| fat-3 | No | 62 | 3.4 (±0.3) |
Fig. 6.

Adult exposure to DGLA causes increased apoptosis of germ cells. Young adults were placed on 0.2 mM DGLA or control plates for 20 h and apoptotic corpses were stained with SYTO 12.
To confirm that the SYTO 12-positive cells arose from programmed cell death, we transferred adult ced-4 mutants onto fatty acid plates. The ced-4 mutants lack the caspase activator activity required for programmed cell death in C. elegans (Gumienny et al., 1999; Yuan and Horvitz, 1992). SYTO 12-positive cells were not observed, indicating that the formation of corpses that are stained with SYTO12 do arise from apoptosis. However, we observe that ced-4 animals that develop on supplemented plates accumulate DGLA to similar levels as wild-type controls and lack germ cells as adults. Similarly, ced-4 animals placed onto DGLA plates as L4s display a similar reduction of germ cells to wild type on DGLA plates. Therefore, in addition to inducing excess programmed cell death of meiotic nuclei in the germ line, the ingested DGLA likely acts in an alternative death pathway that may affect both mitotic and meiotic germ cells.
We then asked whether endogenously produced DGLA is required for proper programmed cell death in the germ line. Mutants in fat-3 and elo-1 are severely deficient in their production of DGLA (Watts and Browse, 2002). However, SYTO 12 staining revealed that the mutants undergo programmed cell death of germ cells at the same frequencies as wild type (Table 2B). Therefore, endogenously synthesized DGLA is not required for programmed cell death in the germ line.
A subset of NRF proteins are required for DGLA-induced sterility
To investigate possible mechanisms of transport of dietary fatty acids from the intestine to the developing germ line, we examined several strains carrying mutations in genes encoding proteins that play a role in lipid transport to the gonad. Newly fertilized C. elegans embryos contain large amounts of yolk, which consists of lipids and lipid binding proteins called vitellogenins. Yolk is synthesized in the intestine and is taken up by developing oocytes by endocytosis (Grant and Hirsh, 1999). The bulk of cholesterol transport from the intestine to developing oocytes relies on the endocytotic pathway including the RME-2 yolk receptor (Matyash et al., 2001). To test whether the endocytotic mechanism is necessary for DGLA to induce sterility, strains carrying mutations in rme-1, which encodes an EH protein that functions in endocytic recycling, and rme-2, which encodes the yolk receptor, were exposed to DGLA. These mutants are both defective in oocyte uptake of yolk. We found that these mutants were just as susceptible to DGLA induced sterility as wild-type animals (Fig. 7A). Therefore, the yolk receptor and the endocytotic machinery are not necessary for the DGLA-induced demise of germ cells. These results suggest that the DGLA that is transported to the germ line during larval development does not depend on the expression of the yolk receptor, indicating that another system of lipid transport is operating during larval development.
Fig. 7.
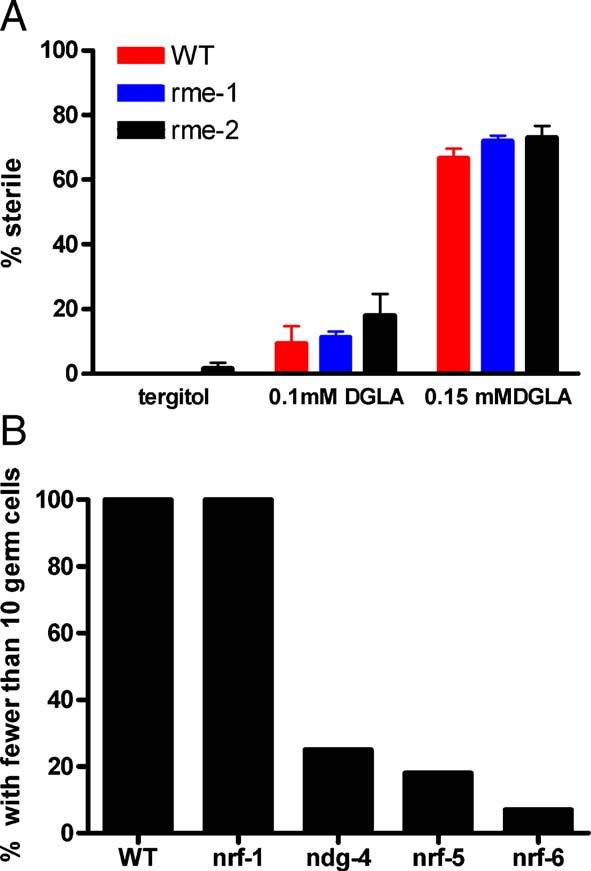
(A) Strains carrying mutations in rme-1 and rme-2 show similar susceptibility to DGLA as wild type. Sterility was scored under a stereo dissecting microscope for the presence of embryos in the uterus (n = 50 for each treatment). (B) Strains carrying mutations in ndg-4, nrf-5, and nrf-6 are resistant to DGLA. After 4 days growth on fatty acid plates containing 0.12 mM DGLA, adult worms were scored for the presence of germ cells by DAPI staining. At least 15 animals were DAPI stained and scored for each genotype.
Presumably lipids are transferred to the gonad during larval development to provide energy for mitosis, meiosis, and transcriptional and translational activities of germ cell nuclei. We tested another class of mutants that produce pale eggs with incompletely penetrant embryonic lethality. Pale eggs, which may have decreased yolk and lipid stores, are a secondary phenotype of a subclass of the nrf mutants that were isolated in screens for resistance the muscle contraction response to fluoxitine (Prozac) (Choy and Thomas, 1999). One of the genes had been previously identified as ndg-4, which was isolated during a screen for the resistance to lethality from the lipoxygenase inhibitor nordihydroguaiaretic acid (NDG) (Shreffler et al., 1995). C. elegans does not have an identified lipoxygenase homologue and the target of NDG in C. elegans has not been identified. Another gene, nrf-5, has been shown to encode a secreted lipid binding protein similar to mammalian cholesterol-ester binding proteins (Choy et al., in press). Since the eggs produced by the nrf-5, nrf-6, and ndg-4 mutants may be deficient in lipid stores, we reasoned that these gene products might be needed to transport dietary lipids into the reproductive tract. When grown in the presence of tergitol and DGLA, DAPI staining revealed much greater numbers of non-gamete germ cells and oocytes in the three mutant strains than in wild type. This finding indicated that the DGLA was having a lesser effect on nrf-5, nrf-6, and ndg-4 than on wild type or nrf-1 (a nrf mutant that does not exhibit pale eggs or embryonic lethality) (Fig. 7B). Over half of the treated nrf-5, nrf-6, and ndg-4 animals showed normal germ cell numbers, while 100% of the wild type and nrf-1 animals had gonads devoid of germ cells. Furthermore, GC analysis of wild type, nrf-6 and ndg-4 strains revealed that they accumulated similar amounts of DGLA in their lipids (16% for wild type, 17% for ndg-4, and 26% for nrf-6), demonstrating that the resistance to DGLA was not due to failure to assimilate the dietary fatty acid.
Discussion
These studies reveal a specific effect of the polyunsaturated fatty acid DGLA in maintenance of germ cells in C. elegans. Feeding of this n-6 PUFA during larval development results in the loss of mitotic germ cells and irreversible sterility. Dietary DGLA also causes excess apoptosis of adult meiotic germ cells. Feeding the n-3 C20 PUFA, EPA, as well as the C18 PUFAs LA and ALA, has no effect on larval germ cell maintenance and does not induce excess apoptosis in the adult germ line. Using the elo-1 mutant, we demonstrated that the sterility caused by dietary GLA is actually due to the production of DGLA, since elo-1 mutants that do not elongate DGLA did not become sterile when grown in the presence of GLA. Another C20 n-6 PUFA, AA also shows some detrimental effects on the germ line, but only at much higher concentrations than DGLA.
The n-3 fatty acid EPA differs from DGLA only by the presence of two additional double bonds and the physical properties of these molecules are similar. Therefore, it seems unlikely that the effects of DGLA are due to altered membrane fluidity of cellular membranes. Nor are they likely to be due to the generation of reactive intermediates due to generalized oxidation of excess membrane PUFAs, since all of the highly-unsaturated C20 PUFAs would be predicted to produce similar products. Rather, we suggest that the excess DGLA interferes with signaling events in the germ line. The specificity of the DGLA invokes the possibility of the existence of a specific receptor for DGLA. Dietary fatty acids and eicosanoids have been implicated in the control of expression in mammals, often by specific binding to various nuclear receptors such as PPARα,PPARγ, LXR, and HNF-4α (Pegorier et al., 2004).
Alternatively, a specific oxidized product of DGLA could trigger the events that lead to infertility. The mechanisms involved in this phenomenon may be related to the fatty acid signaling involved in apoptosis of proliferating tumor cells. It is well established by animal feeding studies and cell culture studies that essential fatty acids can be cytotoxic to proliferating cells, such as tumors (Jiang et al., 1998). While the metabolism of fatty acid by cyclooxygenase or lipoxygenase enzymes is often implicated in this process (Tang et al., 2002), unesterified AA has also been demonstrated to signal apoptosis in mammals (Cao et al., 2000). We and others (Lesa et al., 2003) have been unable to identify homologs of cyclooxygenase or lipoxygenase enzymes in the C. elegans genome, nor are prostaglandin receptors evident. However, C. elegans contains 80 cytochrome P450 monooxygenases that could potentially produce epoxy, hydroxy, and lipoxin products from DGLA (Menzel et al., 2001).
Dietary supplementation of polyunsaturated fatty acids can induce major changes in membrane fatty acid composition. PUFAs will accumulate in total lipid fractions such that up to 50% of fatty acids in total lipids consists of the dietary PUFA. Interestingly, even though many of the supplemented fatty acids are substrates for desaturase enzymes that would normally convert them to more unsaturated species, we found that when relatively high concentrations of fatty acids (N0.1 mM) were used, the supplemented fatty acids accumulated in nematode lipids. This indicates that further desaturation of these fatty acids is limited. The repression of the yeast desaturase OLE1 by dietary PUFAs has been found to be due to reduced transcription as well as changes in stability of the OLE1 mRNA (Gonzalez and Martin, 1996). Nevertheless, as shown in Fig. 2B, C. elegans mutants that eliminate omega-3 and Δ5 desaturase activity are more susceptible to DGLA than wild type.
Germline proliferation in C. elegans begins in the late L1 larval stage and continues throughout adulthood (Seydoux and Schedl, 2001). We show that ingestion of DGLA during larval development results in sterile adults that lack germ cells. This could be due to an inhibition of cell proliferation or to the degeneration of germ cells. While our studies do not rule out that DGLA inhibits proliferation, they do reveal several lines of evidence that DGLA causes the degeneration of germ cells. First, animals treated with DGLA often contain no germ cells. Mitotic germ cell proliferation depends on GLP-1/LIN-12/LAG-2 signaling, nevertheless the primordial germ cells in lag-2 or glp-1 loss of function mutants undergo one or two rounds of cell division, enter meiosis early and differentiate into sperm (Austin and Kimble, 1987; Lambie and Kimble, 1991). Second, DGLA exposure during the L2 and L3 larval stages leads to a lack of germ cells in adults. Cell division does not recommence after removal from DGLA, indicating that the germ cells are permanently affected at this stage. This L2/L3 period of rapid germ cell proliferation is partially dependent on the somatic gonadal sheath lineages (Killian and Hubbard, 2005; McCarter et al., 1997). We cannot rule out that DGLA may act in the somatic gonadal cells and the effect on the germ cells is indirect. Third, we show that DGLA induces inappropriate apoptosis of germ cells in adults. This is not the only cause of germ cell degeneration since ced-4 animals, which do not undergo germ cell apoptosis, also lose germ cells during larval and adult exposure to DGLA. EPA does not cause excess apoptosis. Interestingly, ectopic expression of the C. elegans omega-3 desaturase fat-1 in primary rat neuron cell cultures resulted in a significant inhibition of growth factor withdrawal-induced apoptotic cell death (Ge et al., 2002). Similarly, our data reveal that the fat-1 mutants lacking the omega-3 desaturase activity are more susceptible to DGLA-induced germ cell destruction. Indeed, in a range of mutant strains, the extent of germ cell death and sterility is highly correlated to the level of DGLA in worm lipids (Fig. 2C). It is possible that DGLA acts in a pathway that operates a checkpoint mechanism that acts to eliminate defective germ cells. Our studies show that the fat-3 and elo-1 mutants have normal frequency of programmed cell death in the germ line, even though they are deficient in the synthesis of endogenous DGLA. Therefore, DGLA is not required for programmed cell death, although excess dietary DGLA clearly promotes increased apoptosis of germ cells.
In animals supplemented with relatively low concentrations of DGLA that do not produce 100% sterility, we observe more severe effects in the anterior gonad than in the posterior. The more severe anterior gonad defects perhaps reflects the changing fatty acid composition of the fats that are transported to the gonad as food is digested during its procession from the pharynx through the digestive tract. The resistance of nrf-5, nrf-6, and ndg-4 mutants to dietary DGLA suggests that these molecules may play a role in the transport of DGLA from the intestine to the gonad. The ndg-4 and nrf-6 genes encode members of a novel gene family of multipass transmembrane proteins that are expressed in the intestine (Choy and Thomas, 1999), suggesting that they may form membrane channels. The nrf-5 gene encodes a secreted lipid binding protein similar to mammalian cholesterol-ester binding proteins (Choy et al., in press). A strain carrying a mutation in nrf-1 is also resistant to the muscle contraction effects of fluoxitine, but does not have pale eggs and is not resistant to DGLA, demonstrating that it is not the resistance to fluoxitine per se that renders the animals resistant to DGLA. We suggest that nrf-5, nrf-6, and NDG-4 proteins are needed to mobilize, transport and/or incorporate small, lipophilic molecules (such as fluoxitine, nordihydro-guairetic acid, and fatty acids) from the intestine into surrounding worm tissues.
The inappropriate cell death caused by DGLA during larval stages is similar to the demise of the germ line reported for nos-3 mutants and a nos-1;nos-2RNAi strain (Kraemer et al., 1999; Subramaniam and Seydoux, 1999). NOS proteins are RNA binding proteins that regulate translation and are required for proper primordial germ cell development during embryogenesis, as well as for maintenance of germ cell viability during larval development. Similar to our observations, animals with reduced NOS-1 and NOS-2 lack germ cells, resulting from germ cell death during the L3 larval stage. Increased apoptosis occurs in these animals, but death also occurs in ced-4 mutants, suggesting that the lack of NOS activity induces an alternative death pathway (Subramaniam and Seydoux, 1999). In addition, three germline RNA helicases that are components of germline P granules play important roles in the maintenance of germ cells and in oogenesis. The combined loss of GLH-1 and GLH-4 results in underproliferated gonads devoid of oocytes (Kuznicki et al., 2000), and the loss of CGH-1 leads to excess apoptosis of meiotic germ cells and sterility (Navarro et al., 2001). Furthermore, the progeny of maternal effect sterile (mes) mutants are sterile because they lack gametes and their germ cells undergo degeneration (Capowski et al., 1991; Garvin et al., 1998). MES-3 and MES-6 are members of the polycomb family of transcriptional repressors that form a complex with MES-2 and MES-6 (Paulsen et al., 1995; Xu et al., 2001). This complex regulates regression of the X chromosome in the germ line. Further experiments will be necessary to determine whether DGLA is affecting these RNA- and chromatin-interacting proteins or the downstream genes that they regulate.
Previous evidence for the involvement of lipid signaling in the germ line of C. elegans has focused on the role of sterols. C. elegans grown in the absence of cholesterol show reduced brood size, reduced numbers of germ cells, and smaller gonads (Merris et al., 2003; Shim et al., 2002). Furthermore, replacing cholesterol with the 4αmethyl sterol lophenol results in extensive necrotic cell death, gross tissue degradation and twisted, ruptured gonads (Merris et al., 2003). Gonad migration defects have been described in daf-12 and daf-9 mutants (Antebi et al., 1998; Su et al., 2000). The DAF-12 nuclear hormone receptor and the cytochrome P450 encoded by daf-9 are both required to modulate insulin signals in order to regulate adult life span, dauer formation, and metabolism as well as for the proper migration of the gonad distal tip cells. Because cholesterol deprivation phenocopies many daf-9 defects, it has been proposed that DAF-9 and DAF-12 are involved in the synthesis and reception of a steroid-like hormone (Gerisch and Antebi, 2004; Gerisch et al., 2001).
Polyunsaturated fatty acids have not previously been shown to play a role in germline development in C. elegans. Here we have provided evidence for a specific function of the n-6 fatty acid, DGLA, in maintenance and degeneration of germ cells. Our findings have broad implications that relate, for example, to the undesirable n-6:n-3 fatty acid compositions observed in certain human disease conditions. We believe that the genetic tools available in C. elegans, such as suppressor analysis and whole genome based gene expression studies, promise to provide clues to the mechanism of the DGLA-induced demise of germ cells.
Acknowledgments
We thank Eric Phillips for technical assistance, Jim Thomas for strains and discussions, Michael Miller, Lenore Barkan, Jim Wallis and two anonymous reviewers for suggestions and critical reading of the manuscript, and the Laboratory of Nematology at Wageningen University for Life Sciences, The Netherlands for temporary space for JLW. Some of the strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Center for Research Resources of NIH. Funding was provided by NIH R01 GM62521, NIH R01 DK 074114, and the Agricultural Research Center, Washington State University.
References
- Ahringer J, Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3’ untranslated region. Nature. 1991;349:346–348. doi: 10.1038/349346a0. [DOI] [PubMed] [Google Scholar]
- Antebi A, Culotti JG, Hedgecock EM. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development. 1998;125:1191–1205. doi: 10.1242/dev.125.7.1191. [DOI] [PubMed] [Google Scholar]
- Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004;23:281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brash AR. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capowski EE, Martin P, Garvin C, Strome S. Identification of grandschildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics. 1991;129:1061–1072. doi: 10.1093/genetics/129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy RK, Thomas JH. Fluoxetine-resistant mutants in C. elegans define a novel family of transmembrane proteins. Mol. Cell. 1999;4:143–152. doi: 10.1016/s1097-2765(00)80362-7. [DOI] [PubMed] [Google Scholar]
- Choy RKM, Kemner JM, Thomas JH. Fluoxitine-resistance genes in Caenorhabditis elegans function in the intestine and may act in drug transport. Genetics. :172. doi: 10.1534/genetics.103.024869. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Garvin C, Holdeman R, Strome S. The phenotype of mes-2, mes-3, mes-4 and mes-6, maternal-effect genes required for survival of the germline in Caenorhabditis elegans, is sensitive to chromosome dosage. Genetics. 1998;148:167–185. doi: 10.1093/genetics/148.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Wang X, Chen Z, Landman N, Lo EH, Kang JX. Gene transfer of the Caenorhabditis elegans n-3 fatty acid desaturase inhibits neuronal apoptosis. J. Neurochem. 2002;82:1360–1366. doi: 10.1046/j.1471-4159.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev. Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez CI, Martin CE. Fatty acid-responsive control of mRNA stability. Unsaturated fatty acid-induced degradation of the Saccharomyces OLE1 transcript. J. Biol. Chem. 1996;271:25801–25809. doi: 10.1074/jbc.271.42.25801. [DOI] [PubMed] [Google Scholar]
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat. Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Hajra AK. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974;9:502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Zakar T. Regulation of myometrial smooth muscle functions. Curr. Pharm. Des. 2004;10:2499–2517. doi: 10.2174/1381612043383926. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Molnar M, Rigo J., Jr. Proposed signaling role of arachidonic acid in human myometrium. Mol. Cell. Endocrinol. 1995;110:113–118. doi: 10.1016/0303-7207(95)03523-a. [DOI] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am. J. Clin. Nutr. 2000;71:367S–372S. doi: 10.1093/ajcn/71.1.367s. [DOI] [PubMed] [Google Scholar]
- Hwang D. Fatty acids and immune responses—A new perspective in searching for clues to mechanism. Annu. Rev. Nutr. 2000;20:431–456. doi: 10.1146/annurev.nutr.20.1.431. [DOI] [PubMed] [Google Scholar]
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Bryce RP, Horrobin DF. Essential fatty acids: molecular and cellular basis of their anti-cancer action and clinical implications. Crit. Rev. Oncol./Hematol. 1998;27:179–209. doi: 10.1016/s1040-8428(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Kadyk LC, Lambie EJ, Kimble J. glp-3 is required for mitosis and meiosis in the Caenorhabditis elegans germ line. Genetics. 1997;145:111–121. doi: 10.1093/genetics/145.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJ. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev. Biol. 2005;279:322–335. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M. NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr. Biol. 1999;9:1009–1018. doi: 10.1016/s0960-9822(99)80449-7. [DOI] [PubMed] [Google Scholar]
- Kuznicki KA, Smith PA, Leung-Chiu WM, Estevez AO, Scott HC, Bennett KL. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development. 2000;127:2907–2916. doi: 10.1242/dev.127.13.2907. [DOI] [PubMed] [Google Scholar]
- Lambie EJ, Kimble J. Two homologous regulatory genes, lin-12 and glp-1, have overlapping functions. Development. 1991;112:231–240. doi: 10.1242/dev.112.1.231. [DOI] [PubMed] [Google Scholar]
- Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 2003;116:4965–4975. doi: 10.1242/jcs.00918. [DOI] [PubMed] [Google Scholar]
- Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J. Nutr. Biochem. 2004;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Matyash V, Geier C, Henske A, Mukherjee S, Hirsh D, Thiele C, Grant B, Maxfield FR, Kurzchalia TV. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol. Biol. Cell. 2001;12:1725–1736. doi: 10.1091/mbc.12.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- Menzel R, Bogaert T, Achazi R. A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Arch. Biochem. Biophys. 2001;395:158–168. doi: 10.1006/abbi.2001.2568. [DOI] [PubMed] [Google Scholar]
- Merris M, Wadsworth WG, Khamrai U, Bittman R, Chitwood DJ, Lenard J. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4alpha-methyl sterols. J. Lipid Res. 2003;44:172–181. doi: 10.1194/jlr.m200323-jlr200. [DOI] [PubMed] [Google Scholar]
- Navarro RE, Shim EY, Kohara Y, Singson A, Blackwell TK. cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development. 2001;128:3221–3232. doi: 10.1242/dev.128.17.3221. [DOI] [PubMed] [Google Scholar]
- Paulsen JE, Capowski EE, Strome S. Phenotypic and molecular analysis of mes-3, a maternal-effect gene required for proliferation and viability of the germ line in C. elegans. Genetics. 1995;141:1383–1398. doi: 10.1093/genetics/141.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J. Nutr. 2004;134:2444S–2449S. doi: 10.1093/jn/134.9.2444S. [DOI] [PubMed] [Google Scholar]
- Piomelli D. Arachidonic acid in cell signaling. Curr. Opin. Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr. Rev. 2004;62:333–339. doi: 10.1111/j.1753-4887.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Schedl T. Developmental genetics of the germ line. In: Riddle D, Blumenthal T, Meyer B, Priess JR, editors. C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 1997. pp. 241–270. [PubMed] [Google Scholar]
- Seydoux G, Schedl T. The germline in C. elegans: origins, proliferation, and silencing. Int. Rev. Cytol. 2001;203:139–185. doi: 10.1016/s0074-7696(01)03006-6. [DOI] [PubMed] [Google Scholar]
- Shim YH, Chun JH, Lee EY, Paik YK. Role of cholesterol in germline development of Caenorhabditis elegans. Mol. Reprod. Dev. 2002;61:358–366. doi: 10.1002/mrd.10099. [DOI] [PubMed] [Google Scholar]
- Shreffler W, Magardino T, Shekdar K, Wolinsky E. The unc-8 and sup-40 genes regulate ion channel function in Caenorhabditis elegans motorneurons. Genetics. 1995;139:1261–1272. doi: 10.1093/genetics/139.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- Su M, Merz DC, Killeen MT, Zhou Y, Zheng H, Kramer JM, Hedgecock EM, Culotti JG. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development. 2000;127:585–594. doi: 10.1242/dev.127.3.585. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development. 1999;126:4861–4871. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- Tang DG, La E, Kern J, Kehrer JP. Fatty acid oxidation and signaling in apoptosis. Biol. Chem. 2002;383:425–442. doi: 10.1515/BC.2002.046. [DOI] [PubMed] [Google Scholar]
- Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–895. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem. Sci. 2002;27:467. doi: 10.1016/s0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Phillips E, Griffing KR, Browse J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegansfat-3 mutants. Genetics. 2003;163:581–589. doi: 10.1093/genetics/163.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 1988. [Google Scholar]
- Xu L, Fong Y, Strome S. The Caenorhabditis elegans maternal-effect sterile proteins, MES-2, MES-3, and MES-6, are associated in a complex in embryos. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5061–5066. doi: 10.1073/pnas.081016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Horvitz HR. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]



