Abstract
The assembly of focal adhesions and actin stress fibers by cells plated on fibronectin depends on adhesion-mediated signals involving both integrins and cell-surface heparan sulfate proteoglycans. These two cell-surface receptors interact with different domains of fibronectin. To attempt to identify the heparan sulfate proteoglycans involved, we used fibronectin-null (FN−/−) mouse fibroblasts to eliminate the contribution of endogenous fibronectin during the analysis. FN−/− fibroblasts plated on the cell-binding domain of fibronectin or on antibodies directed against mouse β1 integrin chains attach but fail to spread and do not form focal adhesions or actin stress fibers. When such cells are treated with antibodies directed against the ectodomain of mouse syndecan-4, they spread fully and assemble focal adhesions and actin stress fibers indistinguishable from those seen in cells plated on intact fibronectin. These results identify syndecan-4 as a heparan sulfate proteoglycan involved in the assembly process. The antibody-stimulated assembly of focal adhesions and actin stress fibers in cells plated on the cell-binding domain of fibronectin can be blocked with C3 exotransferase, an inhibitor of the small GTP-binding protein Rho. Treatment of cells with lysophosphatidic acid, which activates Rho, results in full spreading and assembly of focal adhesions and actin stress fibers in fibroblasts plated on the cell-binding domain of fibronectin. We conclude that syndecan-4 and integrins can act cooperatively in generating signals for cell spreading and for the assembly of focal adhesions and actin stress fibers. We conclude further that these joint signals are regulated in a Rho-dependent manner.
Adhesion of cells to their surrounding extracellular matrix (ECM) in vivo regulates their morphology, proliferation, migration, survival, and differentiation (1–5). In vitro, the interactions of cells with ECM molecules such as fibronectin result in cell attachment, spreading, and the assembly of focal adhesions and actin stress fibers (6). Focal adhesions are macromolecular complexes made up of transmembrane adhesion receptors and intracellular proteins with structural and signaling functions (6, 7). Two independent adhesion receptor-mediated signals are required for the assembly of these macromolecular complexes when cells are plated on fibronectin. One signal is mediated through integrins and involves the RGD-containing cell-binding domain of fibronectin (7–9). The second signal is mediated through cell-surface heparan sulfate proteoglycans (HSPGs) and involves heparin-binding domains of fibronectin (10). The formation of complete focal adhesions and stress fibers in the context of integrins has been shown to require integrin clustering, integrin occupancy, tyrosine phosphorylation, and cytoskeletal integrity (7, 8, 11, 12). Integrin-signaling pathways involve the small GTP-binding protein Rho (5, 9, 13–15).
Isolated proteolytic fragments of fibronectin containing either the cell-binding domain or the heparin-binding domain are not sufficient as substrates for cells to assemble focal adhesions and stress fibers even though these individual domains support cell attachment and some spreading (10). However, focal adhesions and stress fiber assembly can occur in cells plated on the cell-binding domain of fibronectin if the cultures are supplemented with the heparin-binding domain incorporated either in the substrate or added in solution (10). Because syndecan-4 is a HSPG of focal adhesions (16, 17), we hypothesized that this proteoglycan may provide a second signal leading to the assembly of focal adhesions and stress fibers. Here we report that FN−/− mouse fibroblasts plated on the cell-binding domain of fibronectin form focal adhesions and actin stress fibers if they are treated with antibodies directed against the ectodomain of mouse syndecan-4. This syndecan-4-mediated effect requires the GTP-binding protein Rho. We conclude that syndecan-4 and integrins act cooperatively in generating the signals necessary for cell spreading, and the assembly of focal adhesions and actin stress fibers when cells are plated on fibronectin.
MATERIALS AND METHODS
Generation of FN−/− Embryonic Stem (ES) Cells.
The Fn1 m1Hyn null mutation (18) was obtained on a 129Sv/Jae background by breeding a chimeric founder with 129Sv/Jae females. Heterozygous Fn1 m1Hyn animals were bred to obtain time-mated females (day 0.5 = noon on the day of vaginal plug identification). To delay implantation, 2.5 days postcoitum (dpc), females were ovariectomized and injected subcutaneously with 0.15 ml of a 10-mg/ml solution of Depo-Provera (Sigma M1629) in physiological saline. Six and a half dpc, blastocysts were flushed from uterine horns by using ES cell medium (as described in ref. 18), supplemented with 1,000 units/ml lymphocyte inhibitory factor, and cultured on a feeder layer of embryonic fibroblasts in gelatin-coated four-well plates (Nunc 176740). Ten and a half dpc, the expanded inner cell masses from cultured blastocysts were picked by using a drawn-out Pasteur pipette and trypsinized for 10 min at 37°C in 3 μl of trypsin (0.25%)/EDTA. Single-cell suspensions were replated on feeder layers in four-well plates. Fifteen and a half dpc, clones were trypsinized and transferred to a 12-well plate (passage 0). Clones were expanded to passage 3 and aliquots were stored at −135°C. DNA from passage 3 clones was analyzed by PCR as previously described (18). From approximately 20 original blastocysts, two independent null ES cell clones were generated.
Derivation of FN−/− Fibroblasts.
FN−/− mouse fibroblasts were derived from the FN−/− ES cells. The ES cells were differentiated into a mixed population by culture in 1.0% dimethyl sulfoxide. The cultures were enriched for cells with a fibroblastic morphology by several passages with trypsinization. Cells were then immortalized by infection with a recombinant retrovirus that transduced simian virus 40 large T antigen (19) and cloned by limiting dilution. Clones were analyzed by immunofluorescence and flow cytometry (20) and selected on the basis of ability to deposit exogenous fibronectin into fibrillar extracellular matrix and strong surface expression of α5 and β1 subunits of an integrin fibronectin receptor. These cells did not incorporate radiolabeled amino acids into secreted fibronectin (data not shown). There was no detectable staining for extracellular or intracellular fibronectin unless exogenous fibronectin was added (see Fig. 2).
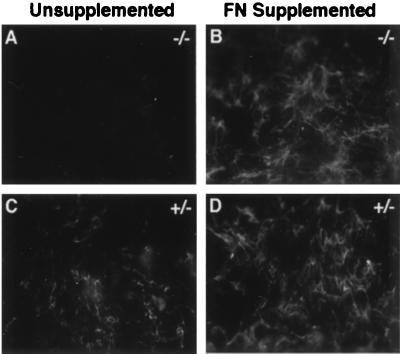
Figure 2.
Fibroblasts differentiated from ES cells heterozygous (+/−) or homozygous (−/−) for the fibronectin mutation were cultured on glass coverslips for 2 days in DMEM containing 10% fetal calf serum without (A and C) or with 10 mg/ml human plasma fibronectin (B and D). The cells were washed, fixed in 4% paraformaldehyde, and stained with rabbit anti-human fibronectin that crossreacted with mouse and bovine fibronectins (20).
Cell Culture.
For the experiments reported here, FN−/− fibroblasts (clone 5F) were maintained at subconfluent densities in DMEM supplemented with 10% fetal bovine serum (FBS), streptomycin (250 μg/ml), and penicillin (250 units/ml) (Life Technologies, Gaithersburg, MD) in a humidified atmosphere of 10% CO2 at 37°C. The cells were rinsed with DMEM without FBS, trypsinized, and plated on coverslips that had been coated for 2 hr at room temperature with 50 μl of a solution of fibronectin (200 μg/ml; Collaborative Biomedical Products, Bedford, MA), the 120-kDa α-chymotryptic cell-binding domain of fibronectin (200 μg/ml; Life Technologies), polylysine (100 μg/ml; Sigma), or a rat monoclonal antibody directed against the mouse β1 integrin chain (200 μg/ml; 9EG7; PharMingen). FN−/− fibroblasts were allowed to attach to the substrate for 2 hr and maintained for an additional 2 or 4 hr, during which they were supplemented with 50 μl of either affinity-purified antibodies directed against the ectodomain of mouse syndecan-4 (0.175 μg/ml), the 40-kDa α-chymotryptic heparin-binding domain of fibronectin (0.2 ng/ml; Chemicon), C3 exotransferase (15 μg/ml; List Biological Laboratories, Campbell, CA), or lysophosphatidic acid (500 ng/ml; Sigma). Cells were fixed in 4% paraformaldehyde and examined for focal adhesions and stress fibers.
Antibody Production.
Rabbit polyclonal antibodies were directed against a unique amino acid sequence of the ectodomain of mouse syndecan-4. The immunogen was a synthetic peptide whose sequence was deduced from a mouse syndecan-4 cDNA clone. The sequence (I90 to G122) deduced from our clone differed from the published sequence (21) at position 117, where we have V117 instead of A117. For the immunization protocol, 3.5 mg of peptide was mixed with 0.75 mg of methylated BSA in 1.0 ml of PBS and mixed with the RIBI R-730 adjuvant before immunization (22). We refer to this antibody as MS-4-E. Antibodies (immune and preimmune) were affinity purified on a protein-G column according to the manufacturer’s (Pierce) recommendations. The specificity of the anti-mouse syndecan-4 antibodies was evaluated by Western blot analysis after SDS/PAGE of 1.8 mg each of purified mouse syndecan-4 ectodomain (amino acids 24–144) and full-length chicken syndecan-4 core protein (23) expressed as maltose-binding fusion proteins in bacteria. After electrophoresis, the proteins were transferred to an Immobilon-P membrane (Millipore), blocked with 5% nonfat dry milk in PBS/0.2% Tween 20, and incubated with a 1/300 dilution of purified rabbit IgG or anti-mouse syndecan-4 antibodies (MS-4-E) or a 1/3,000 dilution of purified antibodies directed against the ectodomain of chicken syndecan-4 core protein (CS-4-E; ref. 23). The bound antibodies were detected with a horseradish-conjugated secondary antibody and the enhanced chemiluminescence reagents (Renaissance, NEN) according to the manufacturer’s protocol.
Flow Cytometry.
Surface expression of syndecan-4 on FN−/− fibroblasts was analyzed by flow cytometry (FACScan; Becton Dickinson) as described (24). Briefly, 2 × 105 cells, released from fibronectin-coated culture dishes in ice-cold PBS, were incubated for 60 min at 4°C with a 1/10 dilution of the affinity-purified anti-mouse syndecan-4 antibody (MS-4-E) or a 1/100 dilution of a rat monoclonal antibody directed against the mouse β1 integrin chain (9EG7) in the presence of propidium iodide. After washing with PBS containing 5% fetal calf serum, the cells were stained with a 1/200 dilution of fluorescein isothiocyanate-conjugated goat anti-rabbit or goat anti-rat antibodies (Santa Cruz Biotechnology). Fluorescence intensity was measured within 1 hr. Damaged cells that had incorporated propidium iodide were excluded from the fluorescence measurements.
Immunocytochemistry.
After fixation, the cells were stained for vinculin or actin. The vinculin monoclonal antibody (hVin-1, Sigma) was used at a 1/400 dilution. To visualize the primary antibody staining, the slides were incubated with a 1/100 dilution of fluorescein isothiocyanate-conjugated anti-mouse antibodies (Jackson ImmunoResearch) for 45 min at 37°C. TRITC-phalloidin (Molecular Probes) was used for actin staining according to the manufacturer’s instructions. Immunocytochemical analysis was carried out with a Leica confocal microscope. Of the emission channels used during double-labeling experiments, excitation levels and gain were set to eliminate bleed-through from one channel to the other as outlined by the manufacturer. This lack of bleed-through was verified experimentally. Nonspecific staining was determined by use of secondary antibody alone.
RESULTS
Characterization of ES Cells from FN−/− Embryos.
Numerous attempts to obtain FN−/− ES cells from previously isolated heterozygous cells (18) by using selection in high concentrations of G418 (25) failed to yield any homozygous lines. Accordingly, we generated FN−/− ES cells by deriving new ES cell lines from blastocysts arising from intercrosses of FN-null heterozygotes on a 129Sv/Jae background by established methods (26). Resulting ES cell lines were genotyped by PCR (Fig. 1) and two null lines were obtained from 20 embryo cultures.
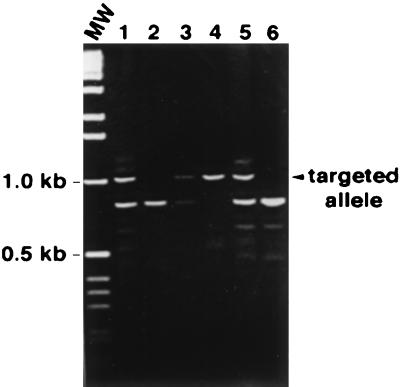
Figure 1.
Representative PCR analysis of DNA from ES cells generated from blastocysts of a FN/129 het × het mating. Shown are the 900-bp product from the wild-type allele and the 1,060-bp product from the targeted allele. Lanes: MW, 1-kb DNA ladder; 1, 3, and 5, heterozygous ES cell clones; 2 and 6, wild-type ES cell clones; and 4, the homozygous null ES cell clone used for the derivation of FN null fibroblasts.
Characterization of FN−/− Fibroblasts.
FN+/− fibroblasts deposited a fibronectin matrix with or without the addition to the culture medium of 10 μg/ml fibronectin, whereas FN−/− cells deposited fibronectin into a fibrillar matrix only if fibronectin was added to the medium (Fig. 2). Transcription of the syndecan-4 core protein gene by FN−/− cells was demonstrated by reverse transcription–PCR analysis of 1 μg of total RNA prepared from 107 FN −/− or 3T3 fibroblasts cultured on fibronectin. PCR reactions were performed with primers specific for syndecan-4 (forward: NT +76 to +100 and reverse: NT +443 to +464) resulting in the amplification of a 388-bp DNA fragment. For control of specificity, H2O or RNA without reverse transcription into cDNA was added to the PCR reactions instead of cDNA from FN−/− or 3T3 fibroblasts (Fig. 3A). The FN−/− cells express the core protein of syndecan-4 on their cell surfaces as indicated from the flow cytometric analysis. A shift in fluorescence intensity is evident with the anti-mouse syndecan-4 antibody and the anti-β1 integrin chain antibody but not with the secondary antibodies (Fig. 3B). The specificity of the anti-mouse syndecan-4 antibodies is indicated from tests in which bacterially expressed mouse syndecan-4 ectodomain fusion protein is recognized by the anti-mouse syndecan-4 antibody (MS-4-E) but not by an anti-chicken syndecan-4 antibody (CS-4-E), and the bacterially expressed chicken syndecan-4 core protein fusion protein is recognized by the anti-chicken syndecan-4 antibody but not by the anti-mouse syndecan-4 antibody (Fig. 3C).
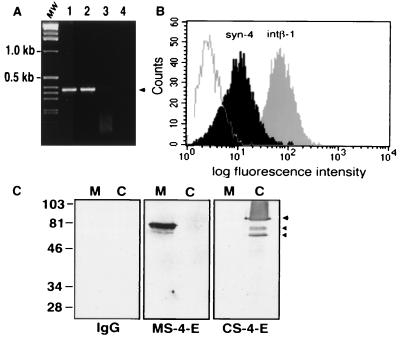
Figure 3.
(A) PCR analysis of FN−/− and 3T3 fibroblasts. The 388-bp band (arrowhead) indicates the presence of mRNA for syndecan-4 in 3T3 (lane 1) and FN−/− (lane 2) fibroblasts. No band is visible in PCR reactions where the cDNA is replaced by nonreverse-transcribed RNA from FN−/− cells (lane 3) or H2O (lane 4). MW, 1-kb DNA ladder. (B) Surface expression of syndecan-4 and β1 integrins analyzed by flow cytometry after staining with affinity-purified rabbit antibody against mouse syndecan-4 or with a rat monoclonal antibody against mouse β1 integrin. (C) Bacterially expressed mouse syndecan-4 ectodomain (M) or chicken full-length syndecan-4 core protein (C) was analyzed by SDS/PAGE and Western blotting with preimmune (IgG), anti-mouse syndecan-4 antibodies (MS-4-E), or anti-chicken syndecan-4 antibodies (CS-4-E). The lower bands (arrowheads) in lane C of the right panel are degradation products of the full-length fusion protein (arrow).
Assembly of Focal Adhesions and Actin Stress Fibers in FN−/− Cells Plated on the Cell-Binding Domain of Fibronectin.
FN−/− fibroblasts attach when plated on fibronectin, and 98% of the attached cells are fully spread (Table 1) and form focal adhesions and establish an elaborate actin cytoskeleton (Fig. 4A). In contrast, when cells are plated on the 120-kDa cell-binding domain of fibronectin, 94% of the cells maintain a round morphology without organized focal adhesions and stress fibers (Fig. 4B). The 6% of the cells that are spread form focal adhesions and a cytoskeleton, although both types of structures are less well developed than those seen in cells plated on full-length fibronectin. When cells plated on the 120-kDa cell-binding domain of fibronectin are supplemented with the 40-kDa heparin-binding domain in solution, 47% of the attached cells spread and develop focal adhesions and an actin cytoskeleton (Fig. 4C). The extent of spreading with this treatment is not as pronounced as that seen when cells are plated on intact fibronectin. These results are in agreement with those reported by Woods et al. (10). A very striking change in morphology is seen when FN−/− fibroblasts plated on the 120-kDa cell-binding domain of fibronectin are supplemented with medium containing antibodies directed against the ectodomain of mouse syndecan-4. In this case, 90% of the attached cells spread and form focal adhesions and an elaborate actin cytoskeleton (Fig. 4D). These cells spread to the same extent as those plated on full-length fibronectin. No such changes are evident when the cells are treated with the same concentration of preimmune antibodies instead of the anti-syndecan-4 antibodies (Fig. 4E). Similarly, when antibodies directed against mouse β1 integrin chains are used as substrate instead of the cell-binding domain of fibronectin, 85% of the attached FN−/− cells are fully spread and assemble focal adhesions and actin stress fibers in the presence of anti-mouse syndecan-4 antibodies (Fig. 4F). In the presence of the same concentration of preimmune antibodies, 94% of the cells remain round without an organized cytoskeleton (Fig. 4G). In contrast, when FN−/− fibroblasts are plated on polylysine, they spread somewhat (Fig. 4 H and I), but 95% of the cells do not assemble focal adhesions or an actin cytoskeleton in the presence of either anti-syndecan-4 antibodies (Fig. 4H) or IgG (Fig. 4I). The staining pattern of vinculin is diffuse and the actin has a cortical distribution in these cells.
Table 1.
Summary of morphology of FN−/− fibroblasts cultured under various conditons
| Substrate | Supplement | % of attached cells that assemble focal adhesions and actin stress fibers | Corresponding figures |
|---|---|---|---|
| FN | None | 98 | 4A |
| 120 kDA | None | 6 | 4B |
| 40 kDa | 47 | 4C | |
| Anti-syndecan-4 antibodies | 90 | 4D | |
| IgG | 5 | 4E | |
| Anti-β1 mAb | None | 4 | |
| Anti-syndecan-4 antibodies | 85 | 4F | |
| IgG | 6 | 4G | |
| Polylysine | None | 5 | |
| Anti-syndecan-4 antibodies | 6 | 4H | |
| IgG | 5 | 4I | |
| 120 kDa | None | 6 | 5A |
| Anti-syndecan-4 antibodies | 89 | 5B | |
| Anti-syndecan-4 antibodies + C3 | 8 | 5C | |
| LPA | 95 | 5D |
A minimum of 50 cells were counted for each experiment. FN, fibronectin; 120 kDa, 120 kDa α-chymotryptic cell-binding domain of fibronectin; anti-β1 mAb, rat monoclonal antibody directed against mouse β1 integrin chain; 40 kDa, 40 kDa α-chymotryptic heparin-binding domain of fibronectin; anti-syndecan-4 antibodies, the antibodies are directed against the ectodomain of mouse syndecan-4 core protein; C3, C3 exotransferase; LPA, lysophosphatidic acid.
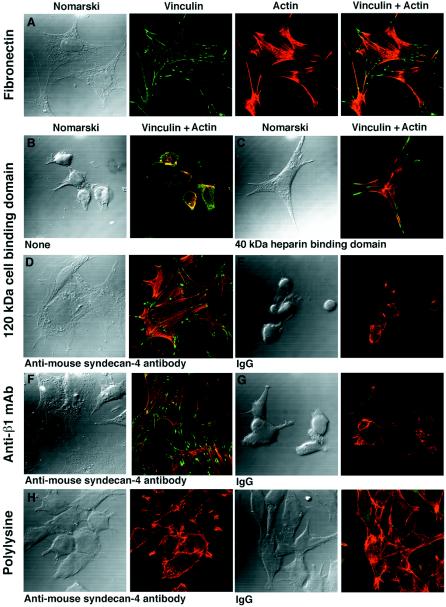
Figure 4.
Analysis of the spreading and assembly of focal adhesions and actin stress fibers by FN−/− fibroblasts. The degree of spreading is seen from the Nomarski micrographs. Focal adhesions are visualized with the vinculin staining and actin stress fibers by means of tetramethyl rhodamine isothiocyanate–phalloidin staining. (A) FN−/− fibroblasts plated on fibronectin. The cells are fully spread. They have assembled focal adhesions and actin stress fibers. (B–E) FN−/− fibroblasts plated on the 120-kDa cell-binding domain of fibronectin. Cell spreading and assembly of focal adhesions and actin stress fibers are present in cells supplemented with the 40-kDa heparin-binding domain of fibronectin (C) or supplemented with antibodies directed against the ectodomain of mouse syndecan-4 (D) but absent in unsupplemented cells (B) or in cells supplemented with IgG (E). (F and G) FN−/− fibroblasts plated on rat monoclonal antibody directed against mouse β1 integrin chains. Cell spreading and assembly of focal adhesions and actin stress fibers are present in cells supplemented with antibodies directed against the ectodomain of mouse syndecan-4 (F) but not in those supplemented with IgG (G). (H and I) FN−/− fibroblasts plated on polylysine. No assembly of focal adhesions and actin stress fibers is evident in cells supplemented either with antibodies directed against the ectodomain of mouse syndecan-4 (H) or with IgG (I).
Thus, the anti-syndecan-4 antibodies can substitute for the heparin-binding domain of fibronectin for full spreading and assembly of focal adhesions and actin stress fibers in cells plated on either the cell-binding domain of fibronectin or on anti-mouse β1 integrin chain antibodies but not in cells plated on polylysine. We conclude that occupancy of both syndecan-4 and integrins is necessary for full cell spreading and assembly of focal adhesions and actin stress fibers.
The Cytoskeletal Reorganization Is Regulated by Rho.
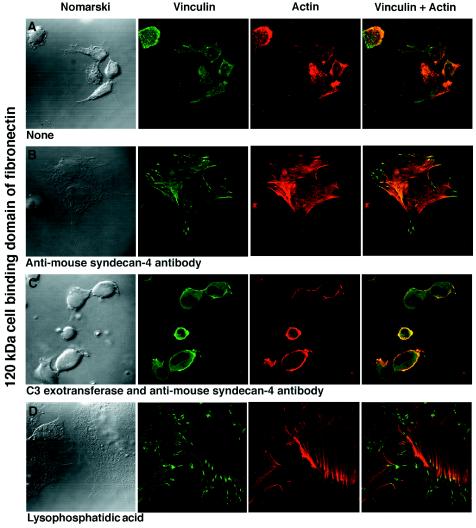
The small GTP-binding protein Rho is a key regulator of focal adhesion and stress fiber formation for signaling events through integrins (15). To test whether the activity of Rho is also required for the effects of anti-syndecan-4 antibodies, FN−/− fibroblasts were allowed to attach to the 120-kDa cell-binding domain of fibronectin for 2 hr. During the next 2 hr, half of the cells remained unsupplemented and half were supplemented with Clostridium botulinum C3 exotransferase, which ADP ribosylates and inactivates Rho. During the last 2 hr, half of each group was supplemented with the affinity-purified anti-mouse syndecan-4 antibodies. In the group that did not receive C3 exotransferase and was not supplemented with the anti-syndecan-4 antibody, 94% of the attached cells remained round (Table 1) and did not assemble focal adhesions or actin stress fibers (Fig. 5A). On supplementation with the anti-mouse syndecan-4 antibody during the last 2 hr, 89% of the cells were fully spread with well-defined focal adhesions and actin stress fibers (Fig. 5B). In contrast, the addition of the anti-mouse syndecan-4 antibody to cells that had been treated with C3 exotransferase failed to lead to cell spreading and assembly of focal adhesions and actin stress fibers in 92% of the cells examined (Fig. 5C). Only 8% of the cells that adhered to the 120-kDa cell-binding domain of fibronectin had a spread morphology (Table 1). In the group of cells that were treated with C3 exotransferase but no anti-syndecan-4 antibody, 6% of the adherent cells had a spread morphology (data not shown). Thus, the C3 exotransferase treatment blocks the assembly of focal adhesions and stress fibers that can be induced by anti-syndecan-4 antibodies in cells plated on the cell-binding domain of fibronectin. Finally, the addition of lysophosphatidic acid (LPA), which signals through Rho, to cells plated on the 120-kDa cell-binding domain of fibronectin leads to full spreading and extensive focal adhesion and actin stress fiber assembly (Fig. 5D) in 95% of the attached cells.
Figure 5.
Assembly of focal adhesions and actin stress fibers is absent in unsupplemented FN−/− fibroblasts plated on the 120-kDa cell-binding domain of fibronectin (A). The cell spreading and assembly of focal adhesions and actin stress fibers as a result of supplementation with antibodies directed against the ectodomain of mouse syndecan-4 (B) is inhibited by the C3 exotransferase treatment that inhibits Rho (C). Supplementation with LPA leads to full spreading and assembly of focal adhesions and actin stress fibers in cells plated on the 120-kDa cell-binding domain of fibronectin alone (D).
Therefore, focal adhesions and stress fibers in cells adherent to the cell-binding domain of fibronectin can be induced either by LPA, known to activate Rho, or by antibodies to syndecan-4, in a Rho-dependent fashion. The simplest implication of these results is that Rho-mediated regulation of focal adhesion and stress fiber assembly in cells plated on fibronectin results from cooperative signaling through integrins and syndecan-4.
DISCUSSION
Cell-adhesion-mediated events result in the activation of a number of signaling pathways that lead to the formation of focal adhesions and actin stress fibers in cells plated on fibronectin. These pathways control the activation of protein tyrosine kinases and members of the small GTP-binding protein Rho (5, 9, 13–15). These adhesion-dependent events have been studied extensively in the context of integrins. However, it has been evident for some time that integrin-mediated signals are not sufficient for the assembly of focal adhesions and actin stress fibers. Cell-surface HSPG-mediated signals are also required (10). In those previous studies, the potential complications of contributions from endogenous fibronectin were eliminated by treating the cells with cycloheximide to inhibit protein synthesis. In the present study, we used fibroblasts null for fibronectin to eliminate any endogenously synthesized fibronectin that might interfere with the analysis. This genetic approach was used to minimize any complications that the cycloheximide inhibition of protein synthesis might have on proteins other than fibronectin. Using the FN−/− fibroblasts, we identify syndecan-4 as a HSPG that can act cooperatively with integrins in the assembly of focal adhesions and actin stress fibers in cells plated on fibronectin. FN−/− cells plated on the cell-binding domain of fibronectin or on anti-mouse β1 integrin chain antibodies fail to spread and assemble focal adhesions and actin stress fibers. However, FN−/− cells plated on the cell-binding domain or on the anti-β integrin chain antibodies that are supplemented with antibodies directed against the ectodomain of mouse syndecan-4 spread extensively and assemble focal adhesions and actin stress fibers indistinguishable from those observed in cells plated on intact fibronectin. The anti-syndecan-4 antibodies alone have no effect, because cells plated on polylysine neither spread nor assemble focal adhesions and actin stress fibers on the addition of the antibodies. These results indicate that occupancy of integrins alone or of syndecan-4 alone is not sufficient for full spreading and assembly of focal adhesions and actin stress fibers. Rather, occupancy of both integrin and syndecan-4 is required for a complete cellular adhesion response.
The anti-syndecan-4 antibody-stimulated assembly of focal adhesions in the FN−/− cells plated on the cell-binding domain can be abolished by inhibiting Rho with C3 exotransferase. Furthermore, the activation of Rho with LPA leads to full spreading of cells plated on the cell-binding domain alone. Such cells show striking assembly of focal adhesions and actin stress fibers. Together, these results indicate a cooperativity between syndecan-4 and integrins in the generation of a complete cellular adhesion response, and they suggest that the activation of Rho necessary for the assembly of focal adhesions and stress fibers depends on both integrin- and syndecan-4-mediated cell-adhesion signals.
A great deal of evidence has accumulated toward an understanding of the principal mechanisms underlying the connections between integrins and the components of the focal adhesion macromolecular complex (5, 11, 12). The connection involving syndecan-4 is not yet well understood. However, it has recently been shown that protein kinase C (PKC) localizes to focal adhesions and that activated PKC-α interacts with the variable region of the cytoplasmic domain of syndecan-4. Regulation of kinase activity requires multimerization of the cytoplasmic domain (27, 28) and involves the cooperative activity of phosphatidylinositol 4,5-biphosphate (29). Activation of PKC can also overcome the requirement for heparin-binding domains of fibronectin for the assembly of focal adhesions (30), and it regulates the recruitment of syndecan-4 into focal adhesions (17). Recently, we have identified a cytoplasmic protein, syndesmos, which interacts specifically with the cytoplasmic domain of syndecan-4 (unpublished work). When overexpressed in NIH 3T3 cells, syndesmos mediates cell spreading and actin cytoskeleton organization. Thus, syndesmos may be involved in linking syndecan-4 to the focal adhesion complex. Additional more generalized signaling may result from PDZ-containing proteins such as syntenin (31) and CASK/LIN-2 (32, 33), which interact with the cytoplasmic domains of all four syndecan family members through the highly conserved C-terminal EFYA sequence.
Acknowledgments
The authors are grateful to Drs. Takao Sakai and Qinghong Zhang for help with derivation and characterization of FN−/− fibroblasts and Dr. Yimin Ge for help with the confocal microscopy. We thank Laird Bloom for constructive criticism of the manuscript. This work was supported in part by grants from the Child Health and Human Development Institute (HD-22016) and from the Cutaneous Biology Research Center through the MGH/Shiseido Company agreement to P.F.G.; the National Heart, Lung and Blood Institute (HL21644) to D.F.M.; the Howard Hughes Medical Institute and the National Heart, Lung and Blood Institute (PO1-HL41484) to R.O.H. F.E. was supported by a Research Fellowship from the Deutsche Forschungsgemeinschaft and S.D.R. by a fellowship from the American Cancer Society (PF-3945). R.O.H. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- HSPG
heparan sulfate proteoglycan
- ES
embryonic stem
- FN−/−
fibronectin null
- LPA
lysophosphatidic acid
References
- 1.Adams J C, Watt F M. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenas J, Muschler J, Bissell M J. Dev Biol. 1996;180:433–444. doi: 10.1006/dbio.1996.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 4.Giancotti F G. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 5.Howe A, Aplin A E, Alahari S K, Juliano R L. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Chrzanowska-Wodnicka M. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 7.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 8.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 10.Woods A, Couchman J R, Johansson S, Hook M. EMBO J. 1986;5:665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto S, Akiyama S K, Yamada K M. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons J T. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 14.Tapon N, Hall A. Curr Topics Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 15.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods A, Couchman J R. Mol Biol Cell. 1994;5:183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baciu P C, Goetinck P F. Mol Biol Cell. 1995;6:1503–1513. doi: 10.1091/mbc.6.11.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George E L, Georges-Labouesse E N, Patel-King R S, Rayburn H, Hynes R O. Development (Cambridge, UK) 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 19.Fässler R, Pfaff M, Murphy J, Noegel A A, Johansson S, Timpl R, Albrecht R. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai T, Zhang Q, Fässler R, Mosher D F. J Cell Biol. 1998;141:527–538. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuzuki S, Kojima T, Katsumi A, Yamazaki T, Sugiura I, Saito H. J Biochem. 1997;122:17–24. doi: 10.1093/oxfordjournals.jbchem.a021724. [DOI] [PubMed] [Google Scholar]
- 22.Benoit R, Bohlen P, Ling N, Briskin A, Esch F, Brazeau P, Ying S-Y, Guillemin R. Proc Natl Acad Sci USA. 1982;79:917–921. doi: 10.1073/pnas.79.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baciu P C, Acaster C, Goetinck P F. J Biol Chem. 1994;269:696–703. [PubMed] [Google Scholar]
- 24.Echtermeyer F, Schöber S, Pöschl E, von der Mark H, von der Mark K. J Biol Chem. 1998;271:2071–2075. doi: 10.1074/jbc.271.4.2071. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen R M, Conner D A, Chao S, Geisterfer-Lowrance A A T, Seidman J G. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 27.Oh E S, Woods A, Couchman J R. J Biol Chem. 1997;272:11805–11811. doi: 10.1074/jbc.272.18.11805. [DOI] [PubMed] [Google Scholar]
- 28.Oh E S, Woods A, Couchman J R. J Biol Chem. 1997;272:8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- 29.Oh E S, Woods A, Lim S T, Theibert A W, Couchman J R. J Biol Chem. 1998;273:10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- 30.Woods A, Couchman J R. J Cell Sci. 1992;101:277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]
- 31.Grootjans J J, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen A R, Wood D F, Marfatia S M, Walther Z, Chisti A H, Anderson J M. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsueh Y-P, Yang F-C, Kharazia V, Naisbitt S, Cohen A R, Weinberg R J, Sheng M. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]