Abstract
Angiostatin, a proteolytic fragment of plasminogen, is a potent antagonist of angiogenesis and an inhibitor of endothelial cell migration and proliferation. To determine whether the mechanism by which angiostatin inhibits endothelial cell migration and/or proliferation involves binding to cell surface plasminogen receptors, we isolated the binding proteins for plasminogen and angiostatin from human umbilical vein endothelial cells. Binding studies demonstrated that plasminogen and angiostatin bound in a concentration-dependent, saturable manner. Plasminogen binding was unaffected by a 100-fold molar excess of angiostatin, indicating the presence of a distinct angiostatin binding site. This finding was confirmed by ligand blot analysis of isolated human umbilical vein endothelial cell plasma membrane fractions, which demonstrated that plasminogen bound to a 44-kDa protein, whereas angiostatin bound to a 55-kDa species. Amino-terminal sequencing coupled with peptide mass fingerprinting and immunologic analyses identified the plasminogen binding protein as annexin II and the angiostatin binding protein as the α/β-subunits of ATP synthase. The presence of this protein on the cell surface was confirmed by flow cytometry and immunofluorescence analysis. Angiostatin also bound to the recombinant α-subunit of human ATP synthase, and this binding was not inhibited by a 2,500-fold molar excess of plasminogen. Angiostatin’s antiproliferative effect on endothelial cells was inhibited by as much as 90% in the presence of anti-α-subunit ATP synthase antibody. Binding of angiostatin to the α/β-subunits of ATP synthase on the cell surface may mediate its antiangiogenic effects and the down-regulation of endothelial cell proliferation and migration.
Tumor growth requires the continuous and persistent generation of blood vessels. If this angiogenesis is prevented, tumor growth is dramatically impaired and the tumor size is restricted. Endogenous angiogenic inhibitors therefore are likely to play an important role in tumor development. Angiostatin, a proteolytic fragment of plasminogen, is a potent inhibitor of angiogenesis and the growth of tumor cell metastases (1). Angiostatin can be generated in vitro by limited proteolysis of plasminogen (2), resulting in a 38-kDa plasminogen fragment containing kringles 1–3. Although the enzymatic mechanism by which angiostatin is generated in vivo is unknown, recent studies have demonstrated that the cleavage of plasminogen to yield angiostatin can be catalyzed by a serine proteinase (3), a macrophage metalloelastase (4), and matrix metalloproteinase 3 (MMP-3 or stomelysin 1) (5). Generation of angiostatin from reduction of plasmin also has been shown in vitro with human prostate carcinoma cells (6), Chinese hamster ovary cells (7), and human fibrosarcoma cells (7). Additional studies demonstrated suppression of primary tumor growth in mice injected with purified angiostatin, with evidence of increased tumor-specific apoptosis (8). The antiproliferative effect of angiostatin also may result from inhibition of cell cycle progression (9). However, little is known about the molecular mechanism(s) by which angiostatin functions to regulate endothelial cell behavior.
Cellular receptors for plasminogen, including annexin II and actin, are found on human umbilical vein endothelial cells (HUVEC) and are believed to function in the regulation of endothelial cell activities, including angiogenesis (10, 11). Receptors for plasminogen also are expressed in high numbers on tumor cells where they have been identified as critical for tumor invasion. Proteins normally found in the cytoplasm, such as α-enolase (12) and ATP synthase (13), also occur on the cell surface and function to bind plasminogen or aid in lymphocyte-mediated cytotoxicity, respectively. The β-subunit of mitochondrial ATP synthase is present on the surface of several tumor cell lines and may function to transport H+ across the plasma membrane, resulting in cytolysis. This finding is supported by studies demonstrating addition of ATP synthase to cultures of tumor cell lines induces membrane depolarization, changes in permeability, and eventual lysis of a variety of transformed cells (14–20). The presence of ATP synthase on tumor cells may help explain lymphocyte-mediated destruction of tumors.
In the current study we examined the interaction of plasminogen and angiostatin with HUVEC. Angiostatin did not compete for plasminogen binding to the endothelial cells, suggesting the presence of distinct binding sites for each protein on the cell surface. Further studies identified the angiostatin binding site on HUVEC as the α/β-subunits of ATP synthase (α/β-ATP synthase). Binding to α/β-ATP synthase was confirmed by using peptide mass fingerprinting, flow cytometry, immunohistochemical staining, Western blotting, competitive cellular binding, and proliferation assays. These studies present evidence for the identification of the α/β-ATP synthase on the endothelial cell surface and imply a potential regulatory role for plasma membrane ATP synthase in endothelial cell proliferation and migration.
MATERIALS AND METHODS
Protein Purification.
Plasminogen was purified from human plasma by affinity chromatography and separated into isoforms 1 and 2 as described (21, 22). Based on kinetic and electrophoretic analysis, all plasminogen preparations were plasmin-free. The concentration of plasminogen was determined spectrophotometrically at a wavelength of 280 nm by using an A1cm1% value of 1.67 and a molecular mass of 92 kDa for Glu1-plasminogen (23). Human plasminogen kringles 1–3 (angiostatin) were purified as described (2). The concentration of angiostatin was determined spectrophotometrically at a wavelength of 280 nm by using an A1cm1% value of 0.8 and a molecular mass of 38 kDa (2). Protein endotoxin levels were <50.0 pg endotoxin/ml as assessed by Pyrotell Limulus amebocyte lysate clotting times (Associates of Cape Cod).
Cell Culture.
Primary HUVEC were grown as described (24) in 150-mm Petri dishes and retained for up to six passages. Human dermal microvascular endothelial cells were obtained from Clonetics (San Diego), grown according to specifications, and retained for up to six passages. A549 (human lung carcinoma) cells were obtained from the American Type Culture Collection and grown according to specifications. For all experiments, cells were detached by incubation with PBS containing 2 mM EDTA, pH 7.4.
Antibody Purification.
Antibody to His-tagged recombinant α-subunit ATP synthase was generated in rabbits by intranodal injection (Covance Laboratories, Vienna, VA). Production bleeds were centrifuged, and the serum obtained was ammonium sulfate precipitated. The precipitate was resuspended in PBS/0.5 M NaCl, pH 7.5, and passed over protein A-Sepharose (Sigma), plasminogen-Sepharose, and α-subunit ATP synthase-Sepharose columns (CNBr coupling, Amersham Pharmacia). Each column was eluted with 20 mM glycine, pH 2.5. Neutralized IgG fractions were tested by immunodiffusion, ELISA, and Western blotting. Antibody to the anti-α-subunit of ATP synthase showed no crossreactivity with plasminogen or other proteins by Western blot analysis.
Polyclonal antibody obtained from A. E. Senior (Rochester Medical Center, Rochester, NY) directed against the α-subunit of ATP synthase from Escherichia coli was characterized by ELISA and Western blot analysis and showed no crossreactivity with other proteins in the F1 portion or E. coli cell membranes (25, 26).
Binding Assays.
Ligands were radioiodinated by using Iodobeads(Pierce), repurified on l-lysine-Sepharose, eluted with 100 mM ɛ-aminocaproic acid, and dialyzed in PBS, pH 7.0 before use in binding assays. HUVEC were plated at a density of 5,000 or 10,000 cells/well and incubated with increasing concentrations of 125I-labeled ligand in media containing 1% BSA for 1 h at 4°C in 96-well plates. Wells were washed, and remaining bound radioactivity was quantified by using an LKB 1272 γ-radiation counter. Nonspecific binding was measured in the presence of excess unlabeled ligand.
Membrane Purification.
Plasma membrane extracts from N-hydroxysuccinimide-biotin-labeled HUVEC were prepared by 300-psi Parr bomb nitrogen cavitation and ultracentrifugation (27). Membrane extracts were incubated with plasminogen-Sepharose or angiostatin-Sepharose columns in an inhibitor mixture buffer (27). Each Sepharose column was eluted with 50 mM Tris/100 mM ɛ-aminocaproic acid, pH 7.5, 50 mM Tris/1 M NaCl, pH 7.5, 50 mM Tris/7% dimethyl sulfoxide, and 20 mM glycine, pH 2.5 to account for all types of binding. The glycine eluates were dialyzed, lyophilized, electrophoresed on 5–15% gradient SDS/PAGE (28), and electroblotted onto Immobilon membrane (29) before experiments to identify plasminogen and angiostatin binding proteins.
Mass Spectrometer Analysis.
Plasma membrane proteins were separated on SDS/PAGE gels, and the bands of interest were excised from the gels and digested in situ with trypsin. A portion (1/20) of each sample was analyzed by matrix-assisted laser desorption ionization-MS, and the obtained mass spectrometric peptide maps were used to identify the protein in the owl Protein database release 29.6 (30, 31).
Flow Cytometry.
HUVEC and A549 cells were resuspended in ice-cold staining buffer (Hanks’ balanced salt solution/1% BSA/0.1% sodium azide) and incubated on ice for 30 min with either rabbit polyclonal antiserum raised against α-subunit ATP synthase derived from E. coli or preimmune rabbit serum. Cells were washed with ice-cold staining buffer and pelleted in a microfuge at 4°C. This wash was repeated twice, and the cells were resuspended in ice-cold staining buffer before incubation on ice for 30 min in the dark with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate. After the final wash (as above), the cells were pelleted and fixed in 10% neutral buffered formalin at a density of 1 × 106 cells/ml. Control experiments were performed by using antibody directed against the α-subunit of ATP synthase, which was preincubated with a 5-fold molar excess of recombinant α-subunit ATP synthase protein. The mean relative fluorescence after excitation at a wavelength of 488 nm was determined for each sample on a FACScan flow cytometer (Becton-Dickenson) and analyzed with cellquest software (Becton-Dickenson).
Immunofluorescence Microscopy.
HUVEC and human dermal microvascular endothelial cells were plated at 5 × 105 cells/ml on glass coverslips and allowed to adhere overnight. Cells were incubated at 4°C for 1 h in PBS, pH 7.0 containing 1% BSA with either rabbit polyclonal antiserum raised against the α-subunit of ATP synthase derived from E. coli, preimmune rabbit serum, preimmune IgG, or anti-rabbit IgG. Cells were washed and incubated at 4°C for 1 h in the dark with goat anti-rabbit IgG conjugated to indocarbocyanine (Cy3) before washing and fixing in 4% paraformaldehyde. Immunofluorescence microscopy was performed by using an Olympus BX-60 microscope (Olympus, Lake Success, NY).
Cloning of the α-Subunit of ATP Synthase.
Poly(A)+ mRNA was isolated from HUVEC by using Oligotex resin (Qiagen). RNA was reverse-transcribed into single-stranded cDNA by using AMV Reverse Transcriptase (Boehringer Mannheim). The α-subunit of ATP synthase was PCR-amplified by using Expand High Fidelity PCR system (Boehringer Mannheim). The 1.7-kb PCR product was purified from a 0.8% Tris–acetate/EDTA agarose gel by using a QIAEX II gel extraction kit. Restriction enzyme digests of the PCR fragment and vector pLE1 were carried out at 37°C for 1 h. Both digests were passed over Qiaquick purification columns, then ligated overnight at 16°C by using T4 DNA ligase. Competent E. coli DH5α (GIBCO/BRL) were transformed with the ligation mixture, plated on 2× Bacto-yeast tryptone (YT) agarose plates, and grown overnight at 37°C. Colonies were screened for the insert by restriction enzyme digestion and DNA sequencing.
Purification of the α-Subunit of ATP Synthase.
Competent E. coli BL21DE3 were transformed with the pLE1 vector containing the α-subunit, plated on 2× Bacto-yeast tryptone (YT) agarose, and grown overnight at 37°C. Twenty milliliters of 2× YT containing 50 μg/ml kanamycin was inoculated with one colony and grown overnight at 37°C (200 rpm). A 1-liter culture (2× YT, 50 μg/ml kanamycin) was inoculated with 20 ml of the noninduced overnight culture and grown at 37°C to an OD of 0.6 at a wavelength of 600 nm. Isopropyl thio-β-d-galactosidase was added to a final concentration of 1 mM and grown for an additional 3 h. Cells were harvested by centrifugation at 8,000 rpm for 10 min and stored overnight at −20°C. Lysates were prepared under denaturing conditions and batch-purified by using Qiagen Ni-NTA agarose. Resulting protein was dialyzed against PBS, pH 7.0 for use in all experiments.
Proliferation Assay.
HUVEC were plated at a density of 5,000 cells/well in media depleted of FCS overnight to allow the cells to become quiescent. Fresh media containing FCS were added to the wells along with angiostatin at a final concentration of 0.5, 0.75, and 1.0 μM. In some experiments antibody directed against the α-subunit of ATP synthase derived from E. coli also was added at a dilution of 1:10. MTS/PMS [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt/phenazine methosulfate] solution was added after 24 h, and the absorbance of formazan was quantitated on a Thermomax plate reader at a wavelength of 490 nm according to the manufacturer’s specifications (Promega). The absorbance values used to calculate the percent proliferation of the cells ranged from 0.81 for untreated, 0.60 for treated, and 0.47 for baseline quiescent cells.
RESULTS AND DISCUSSION
Binding of Plasminogen and Angiostatin to Endothelial Cells.
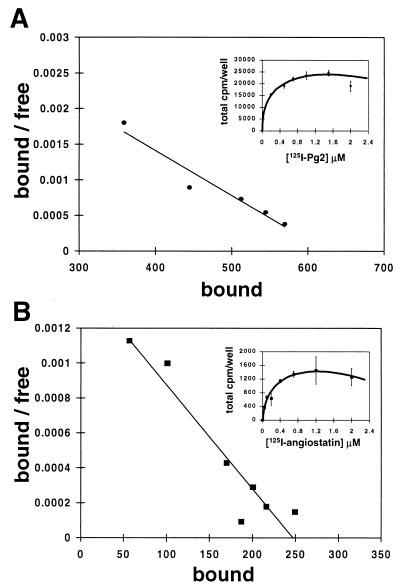
To determine whether angiostatin blocks angiogenesis by competitive interaction with endothelial cell plasminogen receptors, we analyzed the effects of angiostatin on the binding of plasminogen to endothelial cells. In control experiments, plasminogen bound to HUVEC in a concentration-dependent saturable manner with an apparent dissociation constant (Kd) of 158 nM and ≈870,000 sites per cell (Fig. 1A), comparable to values previously reported (11). Angiostatin also bound to HUVEC in a concentration-dependent saturable manner with a similar affinity (Kd of 245 nM), with ≈38,000 sites per cell (Fig. 1B). Binding studies using 125I-labeled plasminogen and a 100-fold molar excess of unlabeled angiostatin demonstrated no inhibition of plasminogen binding (Fig. 2). Similar studies were performed by using 125I-labeled angiostatin. Excess unlabeled plasminogen had little or no effect on angiostatin binding (Fig. 2). In contrast to plasminogen, binding of 125I-labeled angiostatin to HUVEC in the presence of 100 mM ɛ-aminocaproic acid was only slightly inhibited, suggesting that binding of angiostatin to endothelial cells is not a lysine binding site-dependent process (data not shown). Together these data suggest the presence of a distinct angiostatin binding site on HUVEC.
Figure 1.
Direct binding assay and Scatchard analysis of plasminogen and angiostatin with endothelial cells. HUVEC were plated at a density of 10,000 cells/well and incubated with increasing concentrations of 125I-labeled-plasminogen or -angiostatin as described in Materials and Methods. (A) 125I-labeled plasminogen binding was concentration dependent and saturable with an apparent dissociation constant (Kd) of 158 nM and 870,000 sites/cell. (B) Binding to HUVEC with 125I-labeled angiostatin was concentration dependent and saturable with a Kd of 245 nM and 38,000 sites/cell. Error bars represent SD.
Figure 2.
Competition binding assay between plasminogen and angiostatin. HUVEC were plated at a density of 10,000 cells/well and incubated with 1.0 μM 125I-labeled plasminogen in the presence of 100-fold molar excess of unlabeled angiostatin for 1 h at 4°C. Cells were washed, and the remaining radioactivity was quantified by γ-counting. (A) Total binding of 1.0 μM 125I-labeled plasminogen was designated as 100%. (B) Plasminogen binding is inhibited by ≈80% in the presence of a 25-fold molar excess of unlabeled plasminogen. (C) Plasminogen binding was not inhibited in the presence of a 100-fold molar excess of unlabeled angiostatin, suggesting distinct binding sites for each on the cells. Similar experiments by using 125I-labeled angiostatin (D) showed no inhibition of binding in the presence of a 2-fold molar excess unlabeled plasminogen (E). Error bars represent SD.
Purification of the Angiostatin Binding Site from Endothelial Cells.
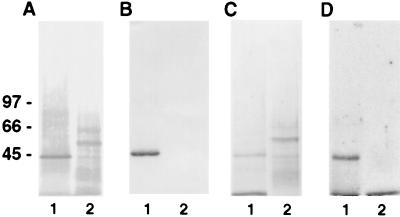
The cell surface proteins involved in binding of plasminogen or angiostatin to HUVEC were identified by subjecting N-hydroxysuccinimide-biotin-labeled HUVEC plasma membranes to affinity chromatography on plasminogen-Sepharose or angiostatin-Sepharose. Two distinct bands were identified on Western blot analysis using streptavidin-alkaline phosphatase conjugate (Fig. 3A) or by Coomassie brilliant blue stain (Fig. 3C). A companion blot, probed with an antibody to the known plasminogen receptor, annexin II, demonstrated immunologic crossreactivity with the 44-kDa membrane protein isolated from the plasminogen-Sepharose column (Fig. 3B, lane 1), but not with the 55-kDa protein isolated from the angiostatin-Sepharose column (Fig. 3B, lane 2). Ligand blot analysis of the affinity-purified plasma membranes using 125I-labeled plasminogen (Fig. 3D, lanes 1 and 2) demonstrated binding of plasminogen only to the 44-kDa protein and not to the 55-kDa species, providing additional evidence that HUVEC contain an angiostatin binding site distinct from the known plasminogen binding protein, annexin II.
Figure 3.
Affinity purification of plasminogen and angiostatin binding sites. Plasma membranes were prepared as described in Materials and Methods. SDS/PAGE containing membrane proteins then were analyzed by Western blotting. Membranes were incubated in 10 mM Tris⋅HCl, 0.15 M NaCl, 0.05% Nonidet P-40, pH 7.5 containing (A) streptavidin-alkaline phosphatase conjugate antibody or (B) anti-annexin II antibody and developed by using 5-bromo-4-chloroindol-3-yl-phosphate nitro blue tetrazolium. Membrane stained with Coomassie brilliant blue (C), showing affinity-purified membrane proteins. Membrane incubated with 125I-labeled plasminogen (D), showing binding to the plasminogen-purified membrane and not the angiostatin. Lane 1 represents protein eluted from the plasminogen-Sepharose column. Lane 2 represents protein eluted from the angiostatin-Sepharose column. The relative molecular masses of α-ATP synthase and β-ATP synthase are ≈55 and ≈50 kDa, respectively.
Peptide Mass Fingerprinting of the Angiostatin Binding Site.
To identify the unique angiostatin binding site component, the affinity-purified binding proteins were analyzed by amino-terminal sequencing, mass spectrometer analysis, and peptide mass fingerprinting. Both the 44- and 55-kDa proteins were analyzed by reduced SDS/PAGE and digested with trypsin in situ (32). The resulting peptides were extracted and the mass of approximately 30 peptides was determined by using a Bruker Reflex matrix-assisted laser desorption ionization-time of flight mass spectrometer, providing a unique signature by which to identify the protein by peptide mass searches (30, 31). The 55-kDa angiostatin binding membrane protein was identified as the α/β-subunits of ATP synthase (Table 1), whereas the plasminogen binding protein was confirmed as annexin II. Although expression of the β-subunit of ATP synthase has been reported on the surface of several tumor cell lines (13), we provide evidence for surface expression of the α/β-subunits of ATP synthase on HUVEC.
Table 1.
Bruker Reflex Matrix-assisted laser desorption ionization-time of flight mass spectrometer analysis of 55-kDa peptides
| Sequence | Peptide mass, monoisotopic, Da
|
|
|---|---|---|
| Measured | Calculated | |
| QMSLLLR | 859.48 | 859.495 |
| AVDSLVPIGR | 1025.58 | 1025.587 |
| VGLKAPGIIPR | 1119.68 | 1119.713 |
| TIAMDGTEGLVR | 1261.40 | 1261.634 |
| ISVREPMQTGIK | 1357.70 | 1357.739 |
| IMNVIGEPIDER | 1384.68 | 1384.702 |
| AHGGYSVFAGVGER | 1405.66 | 1405.674 |
| FTQAGSEVSALLGR | 1434.73 | 1434.747 |
| TSIAIDTIINQKR | 1471.81 | 1471.836 |
| EAYPGDVPYLHSR | 1552.71 | 1552.731 |
| VALVYGQMNEPPGAR | 1600.79 | 1600.803 |
| TGAIVDVPVGEELLGR | 1623.87 | 1623.883 |
| LVLEVAQHLGESTVR | 1649.88 | 1649.910 |
| IMDPNIVGSEHYDVAR | 1814.85 | 1814.862 |
| VLDSGAPIKIPVGPETLGR | 1918.08 | 1918.089 |
| AIAELGIYPAVDPLDSTSR | 1986.99 | 1987.026 |
| IMNVIGEPIDERGPIKTK | 2009.10 | 2009.098 |
| IPSAVGYQPTLATDMGTMQER | 2265.06 | 2265.077 |
| EVAAFAQFGSDLDAATQQLLSR | 2337.15 | 2337.160 |
The proteins were separated on an SDS/PAGE gel, the band of interest was excised from the gel, and then digested in situ with trypsin. Then 1/20 of the sample was analyzed by matrix-assisted laser desorption ionization-MS. The obtained mass spectrometric peptide map was used to identify the α/β-subunits of ATP synthase in the owl Protein database release 29.6 (30, 31). Analysis of protein sequences of α and β ATP synthase from the Institute of Biology and Chemistry of Proteins showed ≈23% homology and ≈57% similarity.
Binding of the α-Subunit ATP Synthase Antibody to the Surface of HUVEC by Flow Cytometry and Immunofluorescence Microscopy.
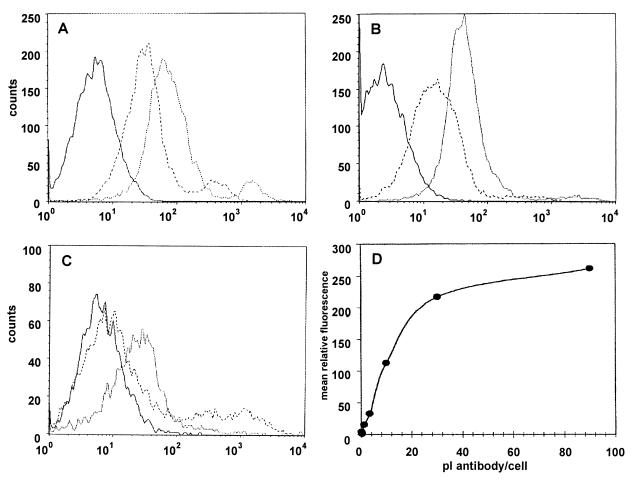
To further confirm the surface localization of the ATP synthase, HUVEC were analyzed by flow cytometry and immunofluorescence microscopy. A rabbit polyclonal antiserum raised against the α-subunit of ATP synthase from E. coli reacted with the cell membranes of HUVEC as determined by fluorescence-assisted flow cytometry (Fig. 4A). Control flow cytometry studies were performed by using A549 cells, which are known to express the α/β-subunits of ATP synthase (13) (Fig. 4B). A549 cells also were analyzed with anti-α-subunit ATP synthase antibody preincubated with a 5-fold molar excess of recombinant α-subunit of ATP synthase protein and showed a decreased affinity for binding (Fig. 4C). HUVEC were incubated with increasing concentrations of antibody to determine saturation. Fig. 4D demonstrates specific, saturable binding of antibody directed against the α-subunit of ATP synthase on HUVEC membranes.
Figure 4.
Binding of antibody directed against the α-subunit of ATP synthase on the surface of HUVEC by flow cytometry. HUVEC were analyzed by FACScan flow cytometry as described in Materials and Methods. Histogram plots are shown for HUVEC (A) and A549 (B) where dotted lines represent cells incubated with antibody directed against the α-subunit of ATP synthase, dashed lines preimmune serum, and solid lines secondary antibody only. Histogram plot of A549 shown in C are similar with dotted lines representing antibody incubated with a 5-fold molar excess α ATP synthase protein. (D) HUVEC demonstrate specific, saturable binding of antibodies directed against the α-subunit of ATP synthase. The mean relative fluorescence of HUVEC incubated with preimmune rabbit serum subtracted from the mean relative fluorescence of HUVEC incubated with the same volume of anti-α ATP synthase gave the mean relative fluorescence resulting from the specific binding of antibodies directed against the α-subunit of ATP synthase on the HUVEC surface.
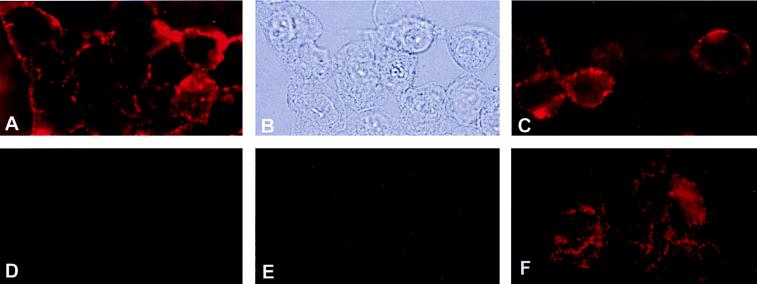
Immunofluorescence microscopy of HUVEC confirmed the surface-associated immunoreactivity of α-subunit ATP synthase antibody on HUVEC cell membranes (Fig. 5A). Control experiments were performed with secondary antibody alone (Fig. 5D), preimmune serum (Fig. 5E), and permeabilized HUVEC in the presence of anti-α-subunit ATP synthase antibody (Fig. 5F). Human dermal microvascular endothelial cells also reacted with antiserum raised against the α-subunit of ATP synthase (Fig. 5C).
Figure 5.
Immunofluorescence microscopy of ATP-synthase on HUVEC surface. HUVEC were incubated with rabbit polyclonal antiserum raised against the α-subunit of ATP synthase from E. coli as described in Materials and Methods. (A) HUVEC under epi-illumination showing immunofluorescent surface staining for the α-subunit of ATP synthase. (B) Same field of HUVEC under visible light. (C) Human dermal microvascular endothelial cells also showed immunofluorescent surface staining for the α-subunit of ATP synthase. Control experiments were performed with (D) preimmune serum and (E) secondary antibody alone. (F) HUVEC were permeabilized by acetone fixation before adding antibodies for the α-subunit of ATP synthase.
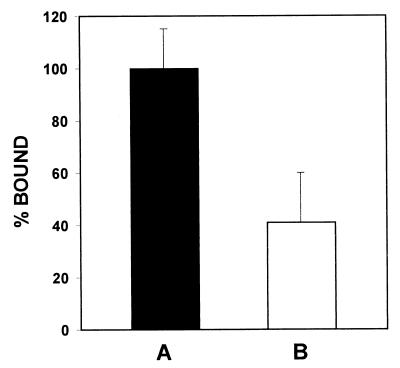
Inhibition of Angiostatin Binding in the Presence of the Antibody to the α-Subunit of ATP Synthase.
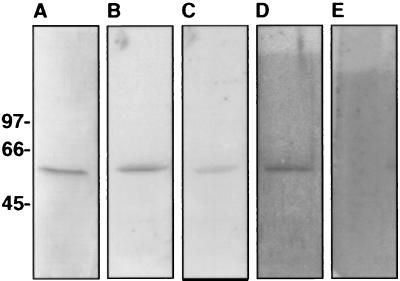
The rabbit polyclonal antiserum raised against the α-subunit ATP synthase blocked binding of angiostatin to HUVEC by 59%, demonstrating that this protein functions in angiostatin binding (Fig. 6). In addition, 125I-labeled angiostatin bound to purified recombinant α-subunit from human ATP synthase (Fig. 7B), and binding was inhibited ≈56% in the presence of a 250-fold molar excess of unlabeled angiostatin (Fig. 7C). Complete inhibition of binding was not obtained and may be caused in part by nonspecific binding, improper folding of the recombinant protein, or binding epitopes only found in the presence of the α/β-heterodimer. Furthermore, binding to the recombinant α-subunit ATP synthase was not inhibited by a 2,500-fold molar excess of unlabeled plasminogen (Fig. 7D). 125I-labeled plasminogen did not bind to the recombinant α-subunit ATP synthase (Fig. 7E), but did bind to annexin II (Fig. 3D).
Figure 6.
Competition binding assay between angiostatin and the antibody against the α-subunit of ATP synthase from E. coli. HUVEC were plated at a constant density of 10,000 cells/well and incubated with 0.5 μM 125I-labeled angiostatin in the presence of 1:10 dilution of antibody against the α-subunit of ATP synthase from E. coli for 1 h at 4°C. Cells were washed and remaining bound radioactivity was quantified by γ-counting. Nonspecific binding was measured in the presence of excess unlabeled angiostatin and was subtracted from total binding. (A) Total binding of 0.5 μM 125I-labeled angiostatin was designated as 100%. (B) Angiostatin binding is inhibited by 59% in the presence of a 1:10 dilution of anti-α-subunit ATP synthase antibody. Competition studies also were performed simultaneously by using rabbit preimmune serum to account for nonspecific inhibition. Error bars represent SD. A one-tailed homoscedastic t test was used for statistical analysis; P < 0.10.
Figure 7.
Angiostatin binding to the recombinant α-subunit of human ATP synthase. The α-subunit of human ATP synthase was cloned and expressed in E. coli and purified by using Qiagen’s nickel-Sepharose protein purification system before dialyzing in PBS, pH 7.0. Recombinant protein was electrophoresed on 5–15% SDS/PAGE, electroblotted onto Immobilon membrane, and incubated 18 h in 10 mM Tris⋅HCl/0.15 M NaCl/0.05% Nonidet P-40, pH 7.5 (TSN buffer) containing 125I-angiostatin. For competition studies unlabeled ligand was added 4 h before radiolabeled ligand. Blots were washed in TSN buffer containing 0.05% Tween80 and bound radioactivity was quantified on a Molecular Dynamics PhosphorImager. (A) Coomassie stain of Immobilon membrane containing the α-subunit of human ATP synthase. (B) Binding of 0.5 μM 125I-labeled angiostatin. (C) Binding of 0.5 μM 125I-labeled angiostatin in the presence of a 250-fold molar excess of unlabeled angiostatin. Binding of angiostatin is inhibited by ≈56%. (D) Binding of 0.5 μM 125I-labeled angiostatin in the presence of a 2,500-fold molar excess of unlabeled plasminogen. Binding of angiostatin is not inhibited. (E) Binding of 0.5 μM 125I-labeled plasminogen to the α-subunit of human ATP synthase. Plasminogen did not bind to the recombinant α-subunit of ATP synthase; however, it did bind the annexin II control (as shown in Fig. 3).
Inhibition of Proliferation in the Presence of Antibody to the α-Subunit of Human ATP Synthase.
To determine whether the antiproliferative effects of angiostatin were mediated by ATP synthase binding, cell proliferation assays were performed in the presence of antiserum raised against the α-subunit of ATP synthase from E. coli. The inhibitory effects of angiostatin on HUVEC proliferation were abrogated by ≈81% in the presence of antibody to the α-subunit of ATP synthase (Table 2), providing direct evidence that angiostatin binding to the α-subunit of ATP synthase functions as a mechanism for inhibition of endothelial cell growth. Based on these data we speculate that this binding site could serve as a receptor for angiostatin.
Table 2.
The antiproliferative effect of angiostatin is reversed by anti-α subunit ATP synthase antibody
| Concentration angiostatin added, μM | Percent proliferation inhibited, ±SEM
|
||
|---|---|---|---|
| Without antibody | With antibody | % Recovery | |
| 0 | 0 | 0 | 0 |
| 0.5 | 10 ± 1.4 | 1 ± 0.2 | 90 |
| 0.75 | 25 ± 4.2 | 5 ± 4.1 | 80 |
| 1.0 | 23 ± 9.0 | 6 ± 0.8 | 74 |
HUVEC were plated at a density of 5,000 cells/well in media containing angiostatin at a final concentration of 0.5, 0.75, and 1.0 μM. Anti-α subunit ATP synthase antibody derived from E. coli was added concomitantly at a dilution of 1:10. MTS/PMS solution was added and absorbance of formazan was quantitated according to the manufacturer’s specifications. Results represent three separate experiments performed in duplicate with SEM. Percent recovery represents the ability of the anti-α-subunit ATP synthase antibody to block the antiproliferative effect of angiostatin, and thereby restore proliferation to an average of 81% of that obtained with the control cells.
ATP synthase is composed of two functional domains termed F1 and F0. The F1 portion contains multiple subunits (α3β3γδɛ) and acts as the catalytic site for ATP synthesis, whereas the membrane-embedded F0 portion is a proton channel (33). Isolated α- and β-subunits bind ATP and have weak ATPase activity; however, ATP synthesis requires all F1 and F0 subunits (34).
Endothelial cells play a strategic role within the vasculature, serving as a barrier between the intravascular compartment and the underlying tissues, and often are exposed to hypoxic stress. Relative to other cell types, endothelial cells are more resistant to hypoxic challenge by their ability to maintain a high level of intracellular ATP (35). It is interesting to speculate that a plasma membrane-associated ATP synthase may produce extracellular ATP, which can diffuse back into the cell, providing an additional, albeit limited, ATP source (36, 37). Angiostatin, by binding to the α/β-subunits of plasma membrane-localized ATP synthase, may disrupt this production of ATP, rendering endothelial cells more vulnerable to hypoxic challenge and eventual irreversible cell damage. In the microenvironment of a growing tumor, tissue hypoxia provides a powerful stimulus for the production of angiogenic growth factors such as vascular endothelial growth factor, basic fibroblast growth factor, and angiopoetin. The ability of host endothelial cells to respond to these growth factors by increased proliferation likely depends on their ability to survive hypoxic challenge. By abolishing the ability to resist low oxygen tension, angiostatin may decrease endothelial cell survival in the tumor microenvironment. It recently has been reported that angiostatin also may function by inducing endothelial cell apoptosis, providing an additional independent mechanism for the antiangiogenic action of this polypeptide (38).
Acknowledgments
We thank Zuzana Valnickova and Ida Thøgersen for amino-terminal sequence analysis. The anti-α-subunit of ATP synthase rabbit serum was the generous gift of Dr. A. E. Senior, Rochester Medical Center, Rochester, NY. We also thank Dr. David Howell, Duke University Medical Center, Durham, NC for his help with the immunofluorescence microscopy and flow cytometry. This work was supported by Glaxo Wellcome Research Grants (S.V.P. and T.L.M.).
ABBREVIATION
- HUVEC
human umbilical vein endothelial cells
References
- 1.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 2.Sottrup-Jensen L, Claeys H, Zajdel M, Petersen T E, Magnusson S. Prog Chem Fibrinolysis Thrombolysis. 1978;3:191–209. [Google Scholar]
- 3.Gately S, Twardowski P, Stack M S, Patrick M, Boggio L, Cundiff D L, Schnaper H W, Madison L, Volpert O, Bouck N, et al. Cancer Res. 1996;36:4887–4890. [PubMed] [Google Scholar]
- 4.Dong Z, Kumar R, Yang X, Fidler I J. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 5.Lijnen H R, Ugwu F, Collen D. Biochemistry. 1998;37:4699–4702. doi: 10.1021/bi9731798. [DOI] [PubMed] [Google Scholar]
- 6.Gately S, Twardowski P, Stack M S, Cundiff D, Grella D, Castellino F J, Enghild J, Kwaan H C, Lee F, Kramer R A, et al. Proc Natl Acad Sci USA. 1997;94:10868–10872. doi: 10.1073/pnas.94.20.10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stathakis P, Fitzgerald M, Matthias L J, Chesterman C N, Hogg P J. J Biol Chem. 1997;272:20641–20645. doi: 10.1074/jbc.272.33.20641. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly M S, Holmgren L, Chen C, Folkman J. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 9.Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yah P, Lu H. Proc Natl Acad Sci USA. 1998;95:6367–6372. doi: 10.1073/pnas.95.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajjar K A, Harpel P C, Jaffe E A, Nachman R L. J Biol Chem. 1986;261:11656–11662. [PubMed] [Google Scholar]
- 11.Hajjar K A, Jacovina A T, Chacko J. J Biol Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- 12.Miles L A, Dahlberg C M, Plescia J, Felez J, Kato K, Plow E F. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 13.Das B, Mondragon M O H, Sadeghian M, Hatcher V B, Norin A J. J Exp Med. 1994;180:273–281. doi: 10.1084/jem.180.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virgilio F D, Pizzo P, Zanovello P, Bronte V, Collavo D. Immunol Today. 1990;11:274–277. doi: 10.1016/0167-5699(90)90111-l. [DOI] [PubMed] [Google Scholar]
- 15.Rozengurt E, Heppel L A, Friedberg I. J Biol Chem. 1977;252:4584–4590. [PubMed] [Google Scholar]
- 16.Rozengurt E, Heppel L. J Biol Chem. 1979;254:708–714. [PubMed] [Google Scholar]
- 17.Chahwala S B, Cantley L C. J Biol Chem. 1984;259:13717–13722. [PubMed] [Google Scholar]
- 18.Saribas A S, Lustig K D, Zhang X K, Weisman G A. Anal Biochem. 1993;209:45–52. doi: 10.1006/abio.1993.1080. [DOI] [PubMed] [Google Scholar]
- 19.Virgilio F D, Bronte V, Collavo D, Zanovello P. J Immunol. 1989;143:1955–1960. [PubMed] [Google Scholar]
- 20.Zanovello P, Bronte V, Rosato A, Pizzo P, Virgilio F D. J Immunol. 1990;145:1545–1550. [PubMed] [Google Scholar]
- 21.Deutsch D, Mertz E T. Science. 1970;170:1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Gronow M, Robbins K C. Biochemistry. 1984;23:190–194. doi: 10.1021/bi00297a003. [DOI] [PubMed] [Google Scholar]
- 23.Castellino F J. Chem Rev. 1981;81:431–436. [Google Scholar]
- 24.Morales D E, McGowan K A, Grant D S, Maheshwari S, Bhartiya D, Cid M C, Kleinman H K, Schnaper H W. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 25.Perlin D S, Senior A E. Arch Biochem Biophys. 1985;236:603–611. doi: 10.1016/0003-9861(85)90664-2. [DOI] [PubMed] [Google Scholar]
- 26.Rao R, Perlin D S, Senior A E. Arch Biochem Biophys. 1987;255:309–315. doi: 10.1016/0003-9861(87)90398-5. [DOI] [PubMed] [Google Scholar]
- 27.Young T N, Pizzo S V, Stack M S. J Biol Chem. 1995;270:999–1002. doi: 10.1074/jbc.270.3.999. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Matsudaira P. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 30.Mann M, Højrup P, Roepstrorff P. Biol Mass Spectrom. 1993;22:338–345. doi: 10.1002/bms.1200220605. [DOI] [PubMed] [Google Scholar]
- 31.Pappin D J C, Højrup P, Bleasby A J. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 32.Matsui N M, Smith D M, Clauser K R, Fichmann J, Andrews L E, Sullivan C M, Burlingame A L, Epstein L B. Electrophoresis. 1997;18:409–417. doi: 10.1002/elps.1150180315. [DOI] [PubMed] [Google Scholar]
- 33.Penefsky H S, Cross R L. Adv Enzymol Relat Areas Mol Biol. 1991;64:173–214. doi: 10.1002/9780470123102.ch4. [DOI] [PubMed] [Google Scholar]
- 34.Boyer P D. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 35.Graven K K, Farber H W. Kidney Int. 1997;51:426–437. doi: 10.1038/ki.1997.57. [DOI] [PubMed] [Google Scholar]
- 36.Unno N, Menconi M J, Salzman A L, Smith M, Hagen S, Ge Y, Ezzell R M, Fink M P. Am J Physiol. 1996;270:G1010–G1021. doi: 10.1152/ajpgi.1996.270.6.G1010. [DOI] [PubMed] [Google Scholar]
- 37.Unno N, Menconi M J, Smith M, Hagen S J, Brown D A, Aquirre D E, Fink M P. Surgery. 1997;121:668–680. doi: 10.1016/s0039-6060(97)90056-8. [DOI] [PubMed] [Google Scholar]
- 38.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O’Reilly M O, Folkman J. Proc Natl Acad Sci USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]