Abstract
IMMUTANS (IM) encodes a thylakoid membrane protein that has been hypothesized to act as a terminal oxidase that couples the reduction of O2 to the oxidation of the plastoquinone (PQ) pool of the photosynthetic electron transport chain. Because IM shares sequence similarity to the stress-induced mitochondrial alternative oxidase (AOX), it has been suggested that the protein encoded by IM acts as a safety valve during the generation of excess photosynthetically generated electrons. We combined in vivo chlorophyll fluorescence quenching analyses with measurements of the redox state of P700 to assess the capacity of IM to compete with photosystem I for intersystem electrons during steady-state photosynthesis in Arabidopsis (Arabidopsis thaliana). Comparisons were made between wild-type plants, im mutant plants, as well as transgenics in which IM protein levels had been overexpressed six (OE-6×) and 16 (OE-16×) times. Immunoblots indicated that IM abundance was the only major variant that we could detect between these genotypes. Overexpression of IM did not result in increased capacity to keep the PQ pool oxidized compared to either the wild type or im grown under control conditions (25°C and photosynthetic photon flux density of 150 μmol photons m−2 s−1). Similar results were observed either after 3-d cold stress at 5°C or after full-leaf expansion at 5°C and photosynthetic photon flux density of 150 μmol photons m−2 s−1. Furthermore, IM abundance did not enhance protection of either photosystem II or photosystem I from photoinhibition at either 25°C or 5°C. Our in vivo data indicate that modulation of IM expression and polypeptide accumulation does not alter the flux of intersystem electrons to P700+ during steady-state photosynthesis and does not provide any significant photoprotection. In contrast to AOX1a, meta-analyses of published Arabidopsis microarray data indicated that IM expression exhibited minimal modulation in response to myriad abiotic stresses, which is consistent with our functional data. However, IM exhibited significant modulation in response to development in concert with changes in AOX1a expression. Thus, neither our functional analyses of the IM knockout and overexpression lines nor meta-analyses of gene expression support the model that IM acts as a safety valve to regulate the redox state of the PQ pool during stress and acclimation. Rather, IM appears to be strongly regulated by developmental stage of Arabidopsis.
Plants with a variegated phenotype display distinct color variation in their vegetative organs, the most common of which being leaves with distinct green/white sectoring (Kirk and Tilney-Bassett, 1978; Rodermel, 2002). Whereas cells in the green sectors contain essentially normal chloroplasts, cells in the white sectors have plastids that are deficient in chlorophyll (Chl) and/or carotenoids (Rodermel, 2001, 2002). The variegated phenotype is most often the result of mutations to distinct genes of either the nuclear or organellar genomes (Tilney-Bassett, 1975) and include the maize (Zea mays) nonchromosomal stripe (ncs) and iojap, as well as Arabidopsis (Arabidopsis thaliana) mutants, chloroplast mutator (chm), pale cress (pac), var1 and var2, cab underexpressed (cue1), and immutans (im; for review, see Rodermel, 2002).
The im mutant was isolated and initially characterized nearly 40 years ago by Rédei and coworkers, who showed that im is the result of a recessive mutation to a nuclear gene and that the resulting variegated phenotype is exacerbated by exposure to elevated temperatures and high light intensities (Rédei, 1963, 1975; Röbbelen, 1968; Wetzel et al., 1994). More recently, Wetzel et al. (1994) found that whereas the green sectors contain the normal allotment of Chls and colored carotenoids, the white sectors showed an accumulation of the colorless carotenoid phytoene, the precursor of the major colored carotenoids. The white sectoring seen when plants are grown under moderate to high irradiance is thus presumed to be the result of the photooxidation of Chl due to the absence of protective carotenoids. Evidence suggests that the IM protein plays a critical role in the desaturation reaction required to convert phytoene into photoprotective carotenoids (Wetzel et al., 1994). Briefly, the desaturation reaction is thought to require the donation of electrons to the plastoquinone (PQ) pool (Norris et al., 1995), a component of the photosynthetic intersystem electron transport chain within the thylakoid membrane. Evidence suggests that subsequent oxidation of PQ involves IM, which acts as a plastid quinol oxidase (Carol et al., 1999; Wu et al., 1999). It follows that the lack of this oxidase in im plants results in the overreduction of PQ, leading to the inhibition of phytoene desaturation. This in turn would lead to phytoene accumulation, Chl photooxidation, and the appearance of white sectors (Wu et al., 1999). Consistent with the notion that white sectoring is triggered by photooxidation is that leaves of im plants are almost indistinguishable from the wild type when plants are grown under low light conditions (Rédei 1963; Wetzel et al., 1994; Aluru and Rodermel, 2004).
In addition to its role in carotenoid biosynthesis, recent in vitro and in vivo evidence indicates that IM may be the sought-after plastid terminal oxidase involved in chlororespiration (Cournac et al., 2000b; Josse et al., 2000, 2003; Joët et al., 2002; Peltier and Cournac, 2002; Fu et al., 2005). Bennoun (1982) proposed that, in the dark, reducing equivalents from a stromal pool of NAD(P)H reduces PQ, which is mediated by a NAD(P)H dehydrogenase. Subsequent oxidation of PQ is thought to require the action of IM. The recent identification of a chloroplastic NAD(P)H dehydrogenase complex (Ohyama et al., 1986, 1988; Guedeney et al., 1996; Sazanov et al., 1996; Burrows et al., 1998; Field et al., 1998; Casano et al., 2000; Horvath et al., 2000) as well as immunological evidence that IM is localized in the stromal lamellae of the thylakoid membrane (Joët et al., 2002; Lennon et al., 2003) have supplied molecular evidence for the existence of a chlororespiratory pathway (Carol et al., 1999; Wu et al., 1999; Cournac et al., 2000a, 2000b, 2002; Carol and Kuntz, 2001; Joët et al., 2002).
IM shows clear sequence similarity to the alternative oxidase (AOX) of the respiratory chain of plant mitochondria, with both proteins being nonheme di-iron carboxylate proteins (Carol et al., 1999; Wu et al., 1999). AOX constitutes the alternative pathway of mitochondrial electron transport, which can be differentiated from the ubiquitous cytochrome pathway by the fact that it is insensitive to cyanide, but rather is inhibited by salicylhydroxamic acid and n-propyl gallate. During alternative pathway respiration, AOX accepts electrons directly from reduced ubiquinone, bypassing the respiratory complexes involved in proton translocation. Because of this, alternative pathway respiration does not contribute to the transmembrane pH gradient used to synthesize ATP. Instead, evidence suggests that, by providing a second pathway of electron flow, the alternative pathway and AOX may serve to prevent the overreduction of electron transport components, which may occur in response to environmental stress (Vanlerberghe and McIntosh, 1997; Maxwell et al., 1999) and which would exacerbate the formation of damaging reactive oxygen species (Møller, 2001; Moore et al., 2002). Similarly to AOX, IM has also been shown to be inhibited by n-propyl gallate (Cournac et al., 2000b; Josse et al., 2000), and it has been hypothesized that IM may be functionally analogous to AOX and serve to keep the photosynthetic electron transport chain relatively oxidized. Exposure of plants to excess irradiation may result in overreduction of electron transport components and may lead to photoinhibition of PSII, which, if not prevented, would result in decreased energy transformation and ultimately a decrease in plant biomass. Increased IM activity under conditions of high light thus may act as a safety valve for excess electrons, preventing the overreduction of the photosynthetic electron transport chain (Niyogi, 2000) and thereby minimizing the aberrant formation of potentially destructive reactive oxygen species within the chloroplast (Melis, 1999; Niyogi, 2000).
Recently, Streb et al. (2005) reported that, compared with other alpine plant species, Ranunculus glacialis acclimated to high light and low temperature exhibited increased levels of IM, which was correlated with a more oxidized electron transport chain as reflected by a lower measure of excitation pressure (EP). Whereas this finding lends support to the hypothesis that IM may serve to keep the photosynthetic electron transport chain more oxidized under environmental stress, direct experimental evidence by measuring the redox state of intersystem electron transport and the redox state of PSI are required to confirm such a role. Using wild-type and im plants as well as transgenics in which IM has been overexpressed six (OE-6×) and 16 (OE-16×) times, we have directly tested this hypothesis by making measurements of Chl a fluorescence as well as measurements of PSI reoxidation kinetics during steady-state photosynthesis using fully expanded all-green leaves of Arabidopsis grown under strictly controlled conditions. We examined the extent to which IM could compete with PSI for photosynthetically generated electrons under controlled growth conditions as well as following a 3-d cold stress period. We hypothesized that plants in which IM has been overexpressed should exhibit increased alternative electron sink capacity, directly competing with PSI for intersystem electrons in comparison to the wild type, and be more resistant to high light-induced photoinhibition. Conversely, plants lacking IM (im) should show greater sensitivity to photoinhibition due to having a more reduced intersystem PQ pool and a lower capacity to keep P700 oxidized.
RESULTS
Plant Phenotype
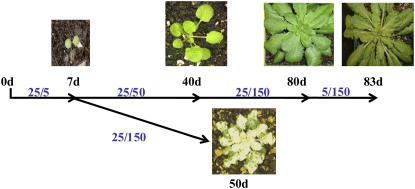
Previous reports investigating the consequence of mutations to IM have compared all green wild-type plants with im plants that display the variegated phenotype (Aluru and Rodermel, 2004; Baerr et al., 2005). However, this approach is problematic because variegated leaves represent a heterogeneous population of photosynthetically competent cells with photooxidized tissue displaying vastly different physiological properties than green tissue. To determine accurately the physiological role of IM requires plants to be grown under conditions where the leaves are all green, regardless of genotype. We found that suppression of the variegated phenotype was possible by first allowing the plants to germinate and grow at 25°C and photosynthetic photon flux density (PPFD) of 5 μmol photons m−2 s−1 (25/5) for 7 d followed by growth at 50 μmol photons m−2 s−1 (25/50) for 35 d. Subsequently, plants were allowed to acclimate fully to the experimental growth condition of 25°C and PPFD of 150 μmol photons m−2 s−1 (25/150) for 40 d prior to exposure to cold stress by shifting plants to 5°C and PPFD of 150 μmol photons m−2 s−1 (5/150) for an additional 3 d (Fig. 1). It should be noted that im plants did develop the variegated phenotype if they were shifted directly to PPFD of 150 μmol photons m−2 s−1 after the initial 7 d at 5 μmol photons m−2 s−1 (Fig. 1).
Figure 1.
Experimental design for the suppression of the variegated phenotype. Seeds from im were germinated and allowed to grow at 25°C with an irradiance of 5 μmol photons m−2 s−1 (25/5) for 7 d. Plants were then shifted from an irradiance of 5 to 50 μmol photons m−2 s−1 (25/50) for 4 weeks. Once the first rosette appeared, the plants were then shifted to 150 μmol photons m−2 s−1 (25/150) for another 4 weeks until the second and third rosettes had completely developed. It is at this stage that fully expanded leaves were used for experimental analysis. Plants were then shifted from 25°C to 5°C for 3 d at an irradiance of 150 μmol photons m−2 s−1 (25/150) to cold stress the plants. Alternatively, when plants were shifted directly from 5 to 150 μmol photons m−2 s−1 after 7 d, im knockout plants exhibited a variegated phenotype when fully developed. All genotypes were grown with an 8/16-h day/night cycle.
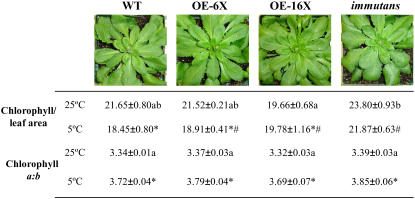
When grown under the same conditions, which suppressed the variegation of im plants, minimal differences where observed in overall morphology (Fig. 2). Plants were similar in size and exhibited minimal differences in total Chl per leaf area compared with the wild type (Fig. 2). Separation of Chl-protein complexes by nondenaturing SDS-PAGE indicated no significant differences in the complement of thylakoid pigment-protein complexes (data not shown) among the four genotypes, which is consistent with the fact that all lines exhibited similar ratios of Chl a/b (Fig. 2; 25/150).
Figure 2.
Morphology, Chl per leaf area (μg Chl cm−2), and Chl a/b ratios of different plant genotypes of Arabidopsis ecotype Columbia. Wild type (WT), OE-6×, OE-16×, and an all-green sectored knockout mutant (im) are shown. All plant genotypes were grown at 25°C at an irradiance of 150 μmol photons m−2 s−1 (25/150). Cold-stressed plants were shifted from 25°C to 5°C for an additional 3 d at the same irradiance (5/150). All plants were grown under an 8/16-h day/night cycle to prevent flowering. Photographs illustrate plants that were grown at 25°C. Letters represent significance between means for leaves grown at 25°C and symbols represent significance between means for leaves that were cold stressed at the 95% confidence interval.
Plant Genotype
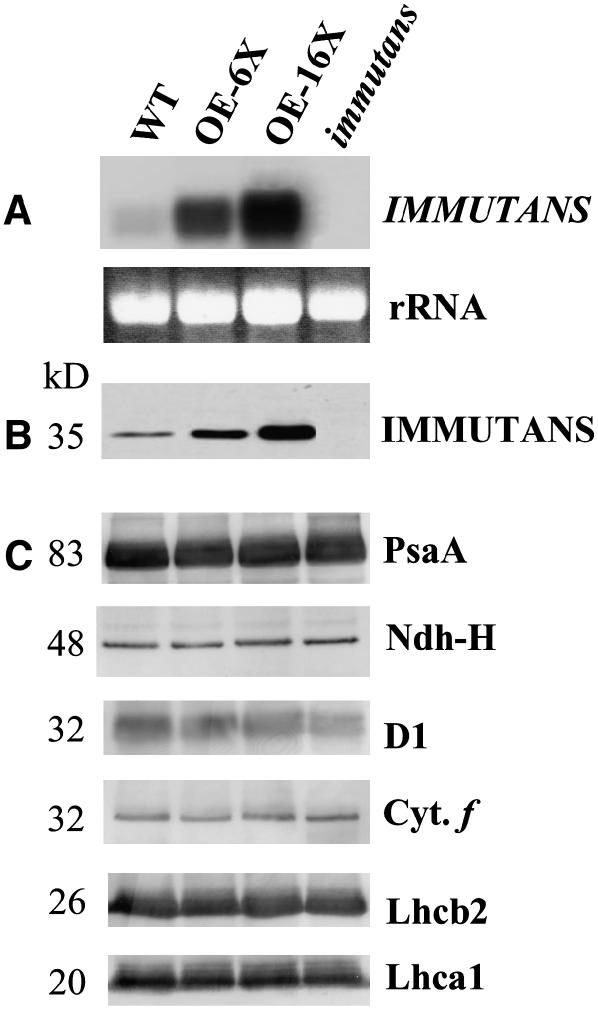
Expression of IM transcript and polypeptide abundance in the four different genotypes was confirmed using RNA-blot analysis and immunoblotting utilizing a polyclonal antibody raised against IM (Rizhsky et al., 2002). Compared to the wild type, the two overexpressing lines had greater IM transcript abundance (Fig. 3A), which correlated with a 6- and 16-fold increase in protein abundance (Fig. 3B). Furthermore, neither the IM transcripts (Fig. 3A) nor polypeptides (Fig. 3B) were detected in the all-green leaves of im plants. In addition, because IM is a thylakoid membrane protein, the relative abundance of specific polypeptides associated with PSI (PsaA), the NAD(P)H dehydrogenase complex (H subunit), PSII (PsbA), the cytochrome b6f (Cyt f) complex, and the major light-harvesting polypeptides associated with PSII (Lhcb2) and PSI (Lhca1) were examined to determine whether the various genotypes exhibited any other major differences in the stoichiometry of these photosynthetic components (Fig. 3C). Minimal differences were observed in the relative abundance of these components of the photosynthetic apparatus in OE-6× and OE-16×, as well as im compared to the wild type (Fig. 3C).
Figure 3.
IM gene expression and protein abundance. mRNA expression of IM (A) relative abundance and of IM protein (B) and immunoblots of polypeptides of the major photosynthetic complexes of isolated thylakoid membranes (C) were performed on leaves obtained from the wild type (WT), OE-6× and OE-16×, and knockout mutant (im) of Arabidopsis. The RNA gel was stained with ethidium bromide to show rRNA and demonstrate equal loading (A). Solubilized thylakoid membranes were loaded equally with 5 μg Chl/lane. Immunoblots were probed using polyclonal antibodies raised against PsaA, H subunit of the NAD(P)H complex, D1, Cyt f, and Lhcb2 and Lhca1. All plant genotypes were grown at 25°C with an irradiance of 150 μmol photons m−2 s−1.
In vivo Chl a fluorescence was used to assess PSII function. All genotypes grown at 25/150 possessed a similar maximal PSII photochemical efficiency (Fv/Fm) of approximately 0.784 (Table I). A second independent measure of PSII photochemistry, thermoluminescence, indicated no significant differences in the peak emission temperatures for either the B-band (29.3°C ± 1.0°C) or the Q-band (13.3°C ± 0.8°C) in all genotypes tested. These measurements show that alterations to IM through mutation or overexpression had little effect on PSII photochemistry.
Table I.
In vivo Chl a fluorescence measurements performed on wild type, OE-6×, OE-16×, and knockout mutant (im) of Arabidopsis
Fluorescence parameters measured include Fv/Fm, the relative reduction state of QA measured as 1 − qP and 1 − qL. Detached leaves were measured at their respective growth temperatures of 25°C (25/150) and 5°C (5/150) for 3-d cold-stressed leaves at an irradiance of 150 μmol photons m−2 s−1. a and b, Significance between means for leaves grown at 25°C; *, #, and ^, significance between means for leaves that were cold stressed at a confidence interval of 95%, where n = 2 with three replicate measurements per experiment ±se.
| Condition | Genotypes | Fv/Fm | 1 − qP | % | 1 − qL | % |
|---|---|---|---|---|---|---|
| 25/150 | Wild type | 0.784 ± 0.01a | 0.106 ± 0.01a | 100 | 0.266 ± 0.01ab | 100 |
| OE-6× | 0.758 ± 0.01a | 0.130 ± 0.01a | 123 | 0.301 ± 0.01a | 113 | |
| OE-16× | 0.790 ± 0.01a | 0.162 ± 0.04a | 153 | 0.294 ± 0.03a | 111 | |
| im | 0.763 ± 0.01a | 0.078 ± 0.02a | 74 | 0.208 ± 0.01b | 78 | |
| 5/150 | Wild type | 0.801 ± 0.02* | 0.244 ± 0.02* | 100 | 0.450 ± 0.02* | 100 |
| OE-6× | 0.755 ± 0.01# | 0.300 ± 0.03* | 123 | 0.496 ± 0.04* | 110 | |
| OE-16× | 0.774 ± 0.01*# | 0.274 ± 0.03* | 112 | 0.452 ± 0.03* | 100 | |
| im | 0.712 ± 0.02^ | 0.275 ± 0.02* | 113 | 0.451 ± 0.01* | 100 |
EP, measured as 1 − photochemical quenching using the puddle model (qP), is a measure of the relative reduction state of quinone A (QA), the first stable quinone acceptor in PSII reaction centers (Dietz et al., 1985; Hüner et al., 1998, 2003; Kramer et al., 2004), and reflects the overall reduction state of the electron transport chain. Whereas both overexpressing lines showed a 23% (OE-6×) and a 53% (OE-16×) higher EP compared to the wild type (Table I), it was interesting to find that im plants exhibited an EP that was approximately 26% lower than wild-type plants (Table I). However, none of the differences in EP between the genotypes tested was statistically significant. Similar trends were observed when EP was calculated using the more recently derived parameter, 1 − photochemical quenching using the lake model (qL; Table I).
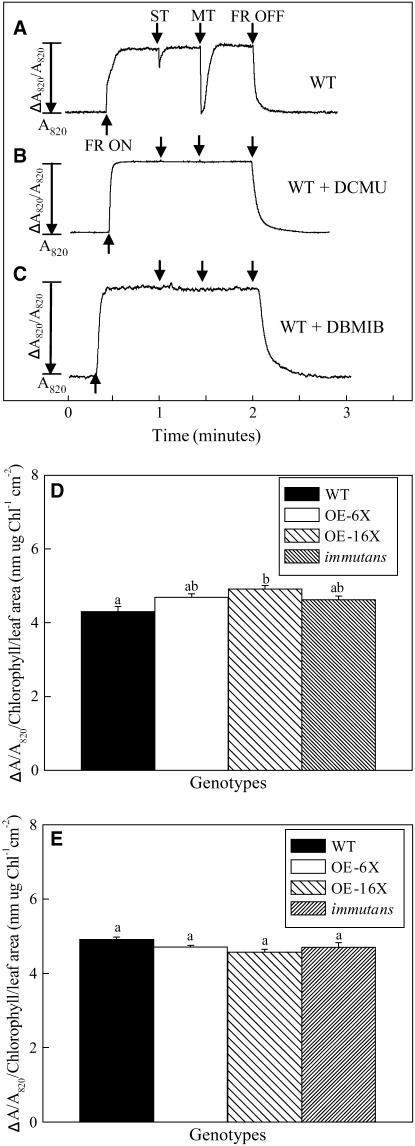
To assess potential functional differences in photosynthetic intersystem electron transport among the genotypes, the oxidation-reduction of P700 was monitored by measuring the absorbance change at 820 nm (ΔA820/A820), which was normalized on a total Chl per leaf area basis (Mi et al., 1992a; Asada et al., 1993; Morgan-Kiss et al., 2001). Upon exposure to far-red (FR) light, wild-type leaves exhibited a rapid change in ΔA820/A820, indicating oxidation of P700 (Fig. 4A). 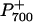 was transiently reduced with either a saturating, single-turnover (ST) flash or a saturating, multiple-turnover (MT) flash of white light in the presence of background FR light (Fig. 4A). When the FR light was turned off,
was transiently reduced with either a saturating, single-turnover (ST) flash or a saturating, multiple-turnover (MT) flash of white light in the presence of background FR light (Fig. 4A). When the FR light was turned off, 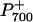 was completely reduced to P700 (Fig. 4A). In the presence of 3-(3′, 4′-dichlorophenyl)-1,1-dimethylurea (DCMU; Fig. 4B), which inhibits PSII at the quinone B (QB) binding site, the ST and MT flashes did not cause any transient reduction of
was completely reduced to P700 (Fig. 4A). In the presence of 3-(3′, 4′-dichlorophenyl)-1,1-dimethylurea (DCMU; Fig. 4B), which inhibits PSII at the quinone B (QB) binding site, the ST and MT flashes did not cause any transient reduction of 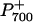 even though the extent of the ΔA820/A820 signal was unchanged. However, in the presence of DCMU, stromal electron donation to the PQ pool could still contribute to the reduction of
even though the extent of the ΔA820/A820 signal was unchanged. However, in the presence of DCMU, stromal electron donation to the PQ pool could still contribute to the reduction of 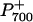 . 2,5-Dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) inhibits intersystem electron transport after the PQ pool at the Cyt f complex. Thus, the maximal ΔA820 /A820 signal under our measuring conditions would be expected in the presence of DBMIB. Figure 4C illustrates that, as expected, DBMIB inhibited the transient reduction of P700+ by the ST and MT flashes. However, the extent of the ΔA820/A820 signal in the presence of DBMIB (Fig. 4C) was 24% greater than either that of the control (Fig. 4A) or that observed in the presence of DCMU (Fig. 4B). This confirms that monitoring the redox state of P700 is a valid measure of intersystem electron transport and that PSII contributed minimally to the ΔA820/A820 signal in leaves of Arabidopsis.
. 2,5-Dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) inhibits intersystem electron transport after the PQ pool at the Cyt f complex. Thus, the maximal ΔA820 /A820 signal under our measuring conditions would be expected in the presence of DBMIB. Figure 4C illustrates that, as expected, DBMIB inhibited the transient reduction of P700+ by the ST and MT flashes. However, the extent of the ΔA820/A820 signal in the presence of DBMIB (Fig. 4C) was 24% greater than either that of the control (Fig. 4A) or that observed in the presence of DCMU (Fig. 4B). This confirms that monitoring the redox state of P700 is a valid measure of intersystem electron transport and that PSII contributed minimally to the ΔA820/A820 signal in leaves of Arabidopsis.
Figure 4.
In vivo measurements of the relative redox state of P700. Detached plant leaves from wild type both untreated (A) and treated with the inhibitor DCMU (B) and treated with the inhibitor DBMIB (C) in Arabidopsis were dark adapted for 20 min prior to the measurement of the oxidation of P700. The steady-state oxidation of P700 (ΔA820/A820) was estimated for plants grown at 25°C (D) and 5°C (E) after the FR light was turned on (FR ON) and the P700 transients were followed after application of the ST and MT flashes of white light. Letters represent statistically significant differences between means at the 95% confidence interval.
Traces for the oxidation of P700 obtained for OE-6× and OE-16× as well as im were qualitatively similar to those obtained for the wild type (data not shown). Overexpression of IM, which was postulated to compete for electrons with PSI, did result in significant differences in the extent of the ΔA820/A820 signal compared to the wild type, where OE-16× exhibited a 14% increase in the extent of the oxidation of P700+, when plants were grown at 25°C and PPFD of 150 μmol photons m−2 s−1 (Fig. 4D). However, OE-6× was not statistically different compared with the wild type in the extent of the ΔA820/A820 signal (Fig. 4D). Unexpectedly, the ΔA820/A820 signal in im was 7% more oxidized than the wild type; considering that these plants lack this oxidase, there should have been a more reduced P700 pool (Fig. 4D). Furthermore, the number of electrons stored in the intersystem electron transport chain (e−/P700) was measured as the ratio of MT to ST. Calculated e−/P700 ranged from 8.6 to 11.1, with no statistical differences between all genotypes tested. These data for e−/P700 were supported by independent measurements of the transient rise in Fo fluorescence after a transition from light to dark during steady-state photosynthesis. No differences in the extent of the transient rise in Fo fluorescence was observed between any of the genotypes tested (data not shown), indicating a comparable reduction state of the intersystem PQ pool (Mills et al., 1979; Endo et al., 1997; Corneille et al., 1998).
Photoinhibition and Recovery of PSII and PSI
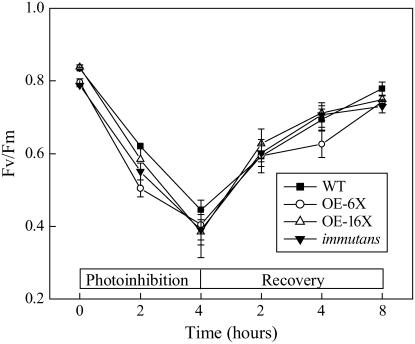
To assess the sensitivity of the four genotypes to photoinhibition of PSII, Fv/Fm was measured in plants exposed to the photoinhibitory treatment of 1,200 μmol photons m−2 s−1 at 5°C (Fig. 5). Prior to photoinhibition, the Fv/Fm was approximately 0.80 for all genotypes examined (Fig. 5) and, after 4 h, all genotypes were photoinhibited approximately to the same extent (Fig. 5). Subsequent recovery from photoinhibition by exposure of plants for 8 h at 25°C and low light (PPFD of 20 μmol photons m−2 s−1; Fig. 5) resulted in nearly 95% recovery of PSII photochemistry in all genotypes. Therefore, we conclude that either the presence or absence of IM has minimal effects on the sensitivity of Arabidopsis to photoinhibition of PSII.
Figure 5.
Fv/Fm of all plant genotypes of Arabidopsis exposed to high light (1,200 μmol photons m−2 s−1) at 5°C and allowed to recover at 25°C with an irradiance of 20 μmol photons m−2 s−1 for up to 8 h. Fv/Fm was measured in detached plant leaves.
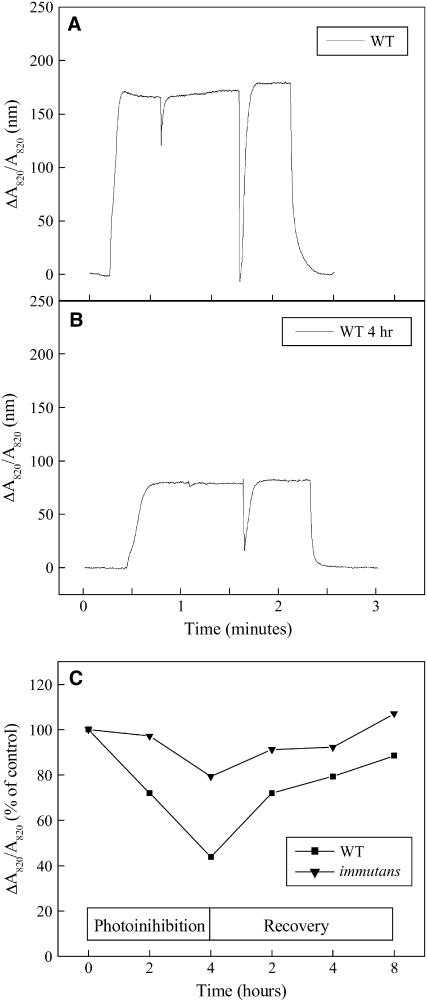
Whereas PSII is considered the main target of photoinhibition, PSI has also been shown to be susceptible to photoinactivation (Ivanov et al., 1998; Terashima et al., 1998; Scheller and Haldrup, 2005). Thus, we also assessed the sensitivity of PSI to photoinhibition in im versus wild-type plants (Fig. 6) under the same conditions used for the photoinhibition and recovery of PSII. The extent of the ΔA820/A820 signal prior to photoinhibition of the wild type (Fig. 6A) decreased by 56% after 4 h of photoinhibition (Fig. 6B), indicating that PSI in wild-type Arabidopsis is sensitive to photoinhibition. A comparison of the kinetics for photoinhibition and recovery of PSI in the wild type and im indicated that PSI in the wild type was more sensitive to photoinhibition than PSI in im (Fig. 6C), contrary to expectations. Both the wild type and im exhibited the capacity to recover from photoinhibition of PSI (Fig. 6C).
Figure 6.
Photoinhibition of PSI. Photooxidation of P700 measured as ΔA820/A820 from detached plant leaves from wild-type control plants grown at 25/150 (A) and after 4 h of photoinhibition at 5°C with PPFD of 1,200 μmol photons m−2 s−1 (B). The steady-state oxidation of P700 was measured as ΔA820/A820 and normalized as a percentage of control. All genotypes of Arabidopsis were exposed to high light (1,200 μmol photons m−2 s−1) at 5°C and allowed to recover at 25°C with an irradiance of 20 μmol photons m−2 s−1 (C). P700 was measured in detached plant leaves. Data presented are of one experimental treatment with three replicate measurements.
Cold Stress
It has been reported that cold acclimation of R. glacialis enhances the accumulation of IM, which may provide protection of these alpine plants from photoinhibition (Streb et al., 2005). To test this in Arabidopsis, all genotypes were shifted to the cold at 5°C and PPFD of 150 μmol photons m−2 s−1 (5/150) for 3 d (Fig. 1). No phenotypic differences between genotypes shifted to 5/150 were observed as reflected in minimal differences in Chl per leaf area and Chl a/b ratios (Fig. 2). Upon exposure to cold stress, the wild type and OE-16× exhibited no statistical differences in Fv/Fm, whereas the knockout mutant exhibited a lower Fv/Fm (0.71; Table I). As shown in Table I, OE-6X× and OE-16× plants grown at 5/150 exhibited higher EP to that of the same plants grown at 25/150, and the values were even higher than cold-stressed wild-type plants. Similar trends were observed when EP was calculated as 1 − qL (Table I). Contrary to expectations, the EP of cold-stressed im plants was not significantly different from those of wild-type plants (Table I).
Upon exposure to cold stress, both OE-6× and OE-16× exhibited a similar ΔA820/A820 signal as the wild type (Fig. 4E). The ΔA820/A820 of cold-stressed im plants was also similar to both overexpression lines as well as the wild type (Fig. 4E). Furthermore, based on the ratio of MT to ST flashes, e−/P700 increased in all genotypes tested upon cold acclimation and varied from 15.0 to 19.4, which was not statistically different at the 95% confidence interval and no obvious trends were observed between any of the genotypes. In addition to cold stress, leaves of the wild type, OE-6×, OE-16×, and im plants, fully expanded at 5/150, exhibited no significant differences with respect to EP, ΔA820/A820 signal, or e−/P700 (data not shown).
Expression of AOX1a and IM
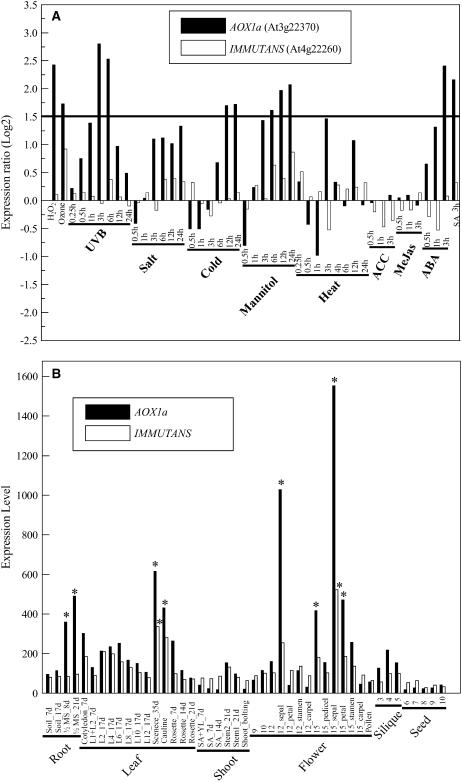
It has been reported that IM displays sequence similarity with AOX, a family of nuclear genes that encode mitochondrial AOX (Carol et al., 1999; Wu et al., 1999). Because AOX transcript, AOX protein, and associated alternative pathway respiration have all been shown to be induced by a wide range of environmental stresses (see Vanlerberghe and McIntosh 1997; Maxwell et al., 1999), it has been proposed that IM functions in a similar capacity within the photosynthetic electron transport chain (Aluru and Rodermel, 2004; Aluru et al., 2006). To compare the extent to which AOX genes and IM are transcriptionally coregulated, expression levels of the major AOX gene from Arabidopsis, AOX1a, were compared to IM using previously published microarray data where wild-type Arabidopsis plants were subjected to various abiotic and hormone stresses. From the data presented in Figure 7A, the expression of IM does not correlate with the expression of AOX1a. Whereas AOX1a transcription abundance increased significantly under various abiotic stresses, such as H2O2, ozone, UV light, cold, and mannitol, as well as hormones such as abscisic acid and salicylic acid (Fig. 7A), the transcript abundance of IM remained at or near control levels. Expression levels of AOX1a and IM were also compared under various stages of Arabidopsis development. In contrast to abiotic stress (Fig. 7A), there did appear to be a correlation between AOX1a and IM expression during development of Arabidopsis (Fig. 7B). Both AOX1a and IM exhibited increased gene expression in cauline leaves and sepals, as well as during leaf senescence (Fig. 7B).
Figure 7.
A, Expression ratio of AOX1a (At3g22370) and IM (At4g22260) transcripts (log base 2) under different abiotic stresses and hormone treatments. H2O2, 100 mm hydrogen peroxide for 3 h; ozone, 200 ppb of ozone for 1 h; UVB, 15-min damaging UVB irradiation; salt, 150 mm NaCl; cold, 4°C shift from room temperature; mannitol, 300 μm mannitol; heat, 38°C shift from room temperature (0.25–3 h) and recovery after 3 h (4–24 h); ACC, 10 μm 1-aminocyclopropane-1-carboxylic acid (ethylene precursor); MeJas, 10 μm methyl jasmonate; ABA, 10 μm abscisic acid; SA, 10 μm salicylic acid. Times indicate hours after treatment initiation. Note significant up-regulation (1.5-fold stringent cutoff) of AOX1a in H2O2, ozone, 3- and 6-h UVB treatments, 12- and 24-h cold treatments, 6-, 12-, and 24-h mannitol treatments, and 3-h treatments in ABA and SA. IM showed no significant up-regulation or down-regulation. B, Differential expression of AOX1a and IM transcripts in different tissues. Expression level based on MAS 5.0 scaling by NASC (see “Materials and Methods”) to give relative expression level (100 units = genomic average). Roots, leaves, shoot apices and stems, flowers, siliques, and seeds in different developmental stages according to Boyes et al. (2001) and Schmid et al. (2005). L, Leaf number (L1 = first appearing leaf); d, days after germination; SA, shoot apex; YL, young leaves; *, significant tissue-specific expression especially in senescing and cauline leaves (AOX1a) and sepals (IM and AOX1a) with more than 2-fold increase in average. Expression was not significantly above background in seeds for IM (stages 8–10) and AOX1a (stages 6–10), and in shoot apices (AOX1a). Roots were collected from soil-grown (Soil) and one-half-strength Murashige and Skoog agar media (MS); note significant increase in AOX1a expression on MS media.
DISCUSSION
A large body of literature proposes models in which IM acts as a plastid terminal oxidase involved in the chlororespiratory pathway (Carol et al., 1999; Wu et al., 1999; Cournac et al., 2000a, 2000b, 2002; Josse et al., 2000, 2003; Joët et al., 2002; Peltier and Cournac, 2002; Fu et al., 2005). By analogy to the AOX of plant mitochondria, the role of the plastid terminal oxidase has been extended to photoprotection of PSII during steady-state photosynthesis by keeping the intersystem PQ pool oxidized under excess excitation (Niyogi, 2000; Streb et al. 2005). If the proposed role of IM in photoprotection is correct, then its overexpression in transgenic plants should keep the PQ pool more oxidized than in the wild type, and, conversely, im plants should exhibit a greater reduction of the PQ pool than the wild type. A corollary to this hypothesis is that the overexpression of IM should increase the competition for intersystem electrons and thereby enhance the capacity to keep PSI more oxidized. Our results indicate that modulation of IM expression and IM polypeptide accumulation does not affect the flux of electrons through the photosynthetic intersystem electron transport chain during steady-state photosynthesis for the following reasons. First, both overexpressing lines exhibited higher excitation pressure than the wild type (Table I). More important, im plants failed to exhibit the expected higher EP than the wild type, but instead exhibited a 23% lower EP as compared with the wild type (Table I). Second, no significant differences were observed in either the intersystem electron pool size (e−/P700) or the reduction state of the PQ pool estimated either as the transient rise in Fo after a light-to-dark transition or as EP (Table I). Third, either a 6- or a 16-fold increase in IM abundance (Fig. 3B) failed to enhance the capacity to keep P700 in the oxidized state as compared with the knockout (Fig. 4, D and E). Conversely, im plants that lack this protein (Fig. 3B) failed to exhibit the expected decrease in capacity to keep P700 oxidized (Fig. 4, D and E). Thus, we conclude that IM lacks the ability to compete with PSI for photosynthetically generated electrons during steady-state photosynthesis, which is consistent with our data indicating that the levels of IM are not correlated with the photoprotection of either PSII or PSI from photoinhibition (Figs. 6 and 7). These results for the sensitivity of PSII to photoinhibition are consistent with those of Joët et al. (2002) and Baerr et al. (2005).
Our functional data from in vivo experiments using two overexpressing lines and an IM mutant (im) in Arabidopsis support the conclusion of Ort and Baker (2002) that IM lacks the capacity to alter electron flux through the intersystem electron transport chain significantly during steady-state photosynthesis. As a consequence, IM cannot provide any significant photoprotection from excess light even when it is overexpressed 16-fold in plants grown either under control (25/150) or cold stress by a sudden shift from 25/150 to 5/150 or after cold acclimation.
Recently, Streb et al. (2005), investigating a high-mountain plant species, R. glacialis, acclimated to high light and low temperature, found that compared with other alpine species it had greater IM protein abundance, which was correlated with having lower EP. The authors suggested that IM provides enhanced photoprotection through its capacity to keep the PQ pool oxidized. Data from our in vivo experiments where we genetically manipulated IM levels (Fig. 3) from complete absence (im) to 16-fold higher (OE-16×) than the wild type do not support this conclusion. We believe that this discrepancy can best be explained by the fact that acclimation to low temperature can significantly enhance the photosynthetic capacity in many cold-tolerant herbaceous plants, such as rye (Secale cereale), wheat (Triticum aestivum; Gray et al., 1996; Savitch et al., 2002), Arabidopsis (Savitch et al., 2001), and Brassica napus (Savitch et al., 2005), which would prevent overreduction of the electron transport chain independent of IM abundance. Furthermore, acclimation to high light has been shown to enhance the Mehler reaction in wheat (Savitch et al., 2000), an alternative method of keeping the electron transport chain oxidized. Thus, given the wide array of acclimatory strategies that prevent overreduction of electron transport, the higher levels of IM and the lower EP reported for cold-acclimated R. glacialis may not reflect a cause-and-effect relationship, but rather a simple correlation.
How can im plants exhibit the tendency to keep PSII more oxidized than either the wild type or the two overexpressors (Table I)? Because im plants grown under the specific conditions described here exhibit no obvious phenotype (Fig. 2), we suggest that this may be due to the fact that inactivating IM has induced the expression and biosynthesis of yet another plastoquinol oxidase in the chloroplast, which is more effective than IM. Several authors have concluded that a cyanide-sensitive chloroplast oxidase (Buchel and Garab, 1995; Lajko et al., 1997; Joët et al., 2002) and a thylakoid hydroquinone peroxidase may be involved in the oxidation of the PQ pool (Casano et al., 2000). Alternatively, im may be able to keep PSII more oxidized as compared to the wild type due to an up-regulation of the metabolic electron sink capacity at the acceptor side of PSI. Upon investigating the rates of photosynthesis in green-leaf sectors compared with white-leaf sectors in im variegated leaves, Aluru et al. (2001) reported that the green-leaf sectors have increased rates of photosynthesis relative to the wild type to compensate for a lack of photosynthesis in the white-leaf sectors. Therefore, the lack of phenotype in im plants may be caused by functional redundancy due to the ability of networks to buffer the effects of perturbations by related pathways (Cutler and McCourt, 2005). Our analyses indicate that extreme caution must be exercised in the interpretation of experimental results for the role of IM based solely on either expression levels or immunoblotting without concomitant functional measurements. We show that even large changes in either gene expression or protein accumulation are not necessarily associated with any enhancement in the proposed function of IM as a plastid terminal oxidase during steady-state photosynthesis.
Because IM shows sequence homology with AOX (Carol et al., 1999; Wu et al., 1999), it has been suggested that IM is a stress-induced protein related to oxidative stress (Aluru et al., 2001, 2006; Aluru and Rodermel, 2004; Mittler et al., 2004). AOX1a has been previously reported as a stress-induced gene (Vanlerberghe and McIntosh, 1997; Maxwell et al., 1999; Molen et al., 2006). However, we have shown through a direct comparison of IM to AOX1a that there is no correlation in gene expression between these two genes due to stress, including oxidative stress (Fig. 7A). In contrast to environmental stress, IM does appear to be correlated to AOX1a expression during development, especially in cauline leaves and sepals (Fig. 7B) and during senescence, which has also been reported elsewhere (Aluru et al., 2001). We, therefore, conclude that IM expression is modulated minimally by abiotic stress, which is consistent with our in vivo functional measurements. However, IM expression is modulated in concert with AOX1a in response to development (Fig. 7B). Consistent with previously published data (Wu et al., 1999; Aluru et al., 2001, 2006), as well as preliminary results indicating the regulation of variegation by excitation pressure during the early stages of leaf development in im (D. Rosso, S.R. Rodermel, and N.P.A. Hüner, unpublished data), we suggest that IM plays an important role in keeping the PQ pool oxidized during chloroplast biogenesis and the assembly of the photosynthetic apparatus. However, once chloroplast development is complete and photosynthetic competence is attained, IM has a minimal impact on the flux of electrons between PSII and PSI. Thus, neither our in vivo functional measurements of photosynthetic intersystem electron transport nor our meta-analyses of Arabidopsis microarrays support the proposed analogous roles for IM and AOX as stress-induced safety valves during steady-state photosynthesis. Therefore, care must be exercised in the putative assignment of function based solely on DNA sequence analyses.
MATERIALS AND METHODS
Plant Growth
Arabidopsis (Arabidopsis thaliana ecotype Columbia) wild type, OE-6×, and OE-16×, as well as the im mutant of IM, were germinated and grown under controlled environmental conditions at 25°C with a PPFD of 5 μmol photons m−2 s−1 for 1 week. Plants were thinned to one plant per pot and grown under controlled environmental conditions at 25°C and with a PPFD of 50 μmol photons m−2 s−1 for an additional 4 weeks until the first rosette was fully developed (see Fig. 1). After the first rosette had developed, plants were shifted to 25°C with a PPFD of 150 μmol photons m−2 s−1 for an additional 40 d (Fig. 1). All measurements were made on fully expanded leaves from the second rosette and all plants were kept under an 8-h photoperiod to prevent flowering. Plants were cold stressed by shifting them from 25/150 to 5/150 for 3 d prior to making measurements (Fig. 1).
Total Chl per Leaf Area
Chl was extracted with buffered 80% (v/v) aqueous acetone containing 2.5 mm sodium phosphate buffer, pH 7.8, and measured by the method of Porra et al. (1989). The absorbance was measured at 663.6 nm and 646.6 nm and corrected to 750 nm for light scattering in a Beckman DU-640 spectrophotometer (Beckman Coulter). Leaf area was measured with a LI-COR area meter (LI-3100C; LI-COR Biosciences).
Plasmid Constructs and Transformations
To generate IM overexpression plants, a full-length IM cDNA (Wu et al., 1999) was cloned in the Xho1 and Sst1 sites of the binary vector pBI121; the gene was driven by the cauliflower mosaic virus 35S promoter. The IM construct was transferred into Agrobacterium tumefaciens, and wild-type Arabidopsis (ecotype Columbia) plants were then transformed by the floral-dip method (Clough and Bent, 1998). Kanamycin-resistant plants were selected at the T1 generation on plates containing 1× MS salts, 1% (v/v) Suc, 0.8% (v/v) agar, pH 5.7, with 50 μg/mL kanamycin. PCR and Southern blotting were performed to verify that the plants were transformed. RNA and protein analyses were performed using T2 generation plants.
RNA Extraction and Gel-Blot Analysis
Total RNA was isolated from all genotypes of Arabidopsis using hot phenol followed by LiCl precipitation (Maxwell et al., 1999). RNA was separated on 1.2% (w/v) agarose gels, with equal amounts of RNA per gel lane, containing formaldehyde and transferred to Hybond N membrane (Amersham-Pharmacia Biotech) as previously described (Maxwell et al., 1999). Radiolabeled DNA probes were made using the high-prime labeling kit (Roche). The blots were probed with the full-length IM cDNA (Wu et al., 1999).
Thylakoid Preparation and Immunoblotting
Thylakoid membranes were isolated according to the method of Harrison and Melis (1992). Proteins were separated on 15% (w/v) SDS-PAGE in the presence of 6 m urea according to the method of Laemmli (1970). Immunoblotting was performed by electrophoretically transferring the proteins from SDS-PAGE gel to a nitrocellulose membrane (Bio-Rad Laboratories). Immunodetection was performed using horseradish peroxidase-conjugated secondary antibodies (Sigma) and enhanced chemiluminescence according to the manufacturer (ECL; Amersham-Pharmacia Biotech). Proteins were immunodetected with specific polyclonal antibodies raised against the PsaA polypeptide of the PSI reaction center (1:1,000 dilution), the H subunit of the NAD(P)H dehydrogenase complex (1:500 dilution; D. Rumeau, unpublished data), D1 polypeptide of the PSII reaction center (1:1,000 dilution), Cyt f (1:1,000 dilution), Lhca1 and Lhcb2, respectively (1:1,000 dilution; Agri-sera), and IM (1:1,000 dilution).
Thylakoid Isolation and Pigment Protein Analysis
Leaf material was ground in cold isolation buffer (50 mm Tricine, 0.4 m sorbitol, 10 mm NaCl, 5 mm MgCl2 hexahydrate, pH 7.8) in a mortar and pestle on ice, filtered through two layers of miracloth (typical pore size 22–25 μm; Calbiochem), and centrifuged for 5 min (5,000g). The pellet was either resuspended in isolation buffer for thermoluminescence measurements (as described above) or in cold 50 mm Tricine, pH 8.0, wash buffer before Chl determination in 80% (v/v) acetone according to Porra et al. (1989).
Thylakoids were centrifuged at 10,000g for 5 min, solubilized in an SDS/dodecylmaltoside (DM) buffer with a 20:1 (w/w) DM + SDS:Chl ratio (0.1% [w/v] SDS, 0.45% [w/v] DM, 0.3 m Tris, 13% [v/v] glycerol), and then centrifuged to remove the insoluble material. Separation of Chl proteins was undertaken by nondenaturing electrophoresis in a 5% to 10% (w/v) linear gradient polyacrylamide gel according to Laemmli (1970), as modified by Komenda (2000), where the gel contained no detergent and the SDS was replaced by 0.2% (v/v) Deriphat 160 in electrophoretic buffer. To determine relative band intensity, the green gels were scanned at 671 nm (DU-540; Beckman).
P700 Photooxidation
The relative redox state of P700 was estimated in vivo as ΔA820/A820 using a PAM-101 modulated fluorometer (Heinz Walz) equipped with an ED-800DT emitter-detector and PAM-102 units, following the procedure of Schreiber et al. (1988), as described by Ivanov et al. (1998). FR light (λmax = 715 nm, 10 W m−2; Schott filter RG 715) was provided by the FL-101 light source. MT (50 ms) and ST half-peak width of 14-μs saturating flashes were applied with XMT-103 and XST-103 power/control units, respectively. Leaves were vacuum infiltrated with 20 μm DCMU in darkness. One-way ANOVA was performed to determine statistical significance between genotypes (P ≤ 0.05) followed by a Bonferroni test to test for differences between group means at a 95% confidence interval (Microcal Origin 7.5; Origin Lab Corporation).
Chl a Fluorescence and Relative Redox State of QA
Steady-state fluorescence measurements were made using a PAM Chl fluorometer (PAM-101, 103; Heinz Walz). Two Schott lamps (KL 1500) provided saturating flashes and actinic illumination for photosynthesis. Samples were dark adapted for 20 min prior to all measurements. Fluorescence parameters, such as the Fv/Fm, qP, qL, and quenching coefficient of the nonphotochemical quenching (qN), were calculated (Bradbury and Baker, 1981; Schreiber et al., 1986; van Kooten and Snell, 1990; Kramer et al., 2004). A post hoc test followed by a Student-Newman-Keuls test was performed to determine statistical differences between means at a confidence interval of 95% (SPSS; Systat Software).
Photoinhibition and Recovery
Detached leaves were photoinhibited either at 25°C and PPFD of 500 μmol photons m−2 s−1, or at 5°C and 1,200 μmol photons m−2 s−1. Recovery from photoinhibition took place at 25°C with an irradiance of 20 μmol photons m−2 s−1 for an additional 8 h. Photoinhibition was measured as the decrease in Fv/Fm using a PAM fluorometer (PAM-103; Heinz Walz). A post hoc test followed by a Student-Newman-Keuls test was performed to determine statistical differences between means at a confidence interval of 95% (SPSS; Systat Software).
Microarray Expression Analysis
The microarray analysis followed Geisler-Lee et al. (2006). Arabidopsis oligo microarray data were downloaded from the Nottingham Arabidopsis Stock Center (NASC; http://affymetrix.arabidopsis.info/narrays/experimentbrowse.pl). Stress series and developmental series were provided by the AtGene Express project (http://www.uni-frankfurt.de/fb15/botanik/mcb/AFGN/atgenex.htm). The oligo microarrays were based on unique oligonucleotides for the Arabidopsis genome, which did not cross-hybridize closely related genes. We totaled the Microarray Suite, version 5.1, (http://www.affymetrix.com/products/software/specific/mas.affx), scaled and normalized signal values for AOX1a (At3g22370) and IM (At4g22260) genes, and then determined relative expression in different Arabidopsis tissue. AFGN and Affymetrix did all methods for microarray handling and normalization and published them online within the NASC database.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF098072 (IM) and AF370166 (AOX1a).
Acknowledgments
We thank Dr. Maneesha Aluru for her help and expertise. We would also like to thank Dr. Dominique Rumeau for the gift of antibody against the H subunit of the NDH complex, and Dr. Jean-Marc Ducruet for providing and assisting with the signal analysis software for thermoluminescence.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC; grant to D.P.M., and N.P.A.H.) and by the U.S. Department of Energy (Energy Biosciences; grant no. DF–FG02–94ER20147 to S.R.R.). D.R. is the recipient of an NSERC postgraduate scholarship.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Norman P.A. Hüner (nhuner@uwo.ca).
References
- Aluru MR, Bae H, Wu D, Rodermel SR (2001) The Arabidopsis immutans mutation affects plastid differentiation and the morphogenesis of white and green sectors in variegated plants. Plant Physiol 127: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru MR, Rodermel SR (2004) Control of chloroplast redox by the IMMUTANS terminal oxidase. Physiol Plant 120: 4–11 [DOI] [PubMed] [Google Scholar]
- Aluru MR, Yu F, Fu A, Rodermel SR (2006) Arabidopsis variegation mutants: new insights into chloroplast biogenesis. J Exp Bot 57: 1871-1881 [DOI] [PubMed] [Google Scholar]
- Asada K, Heber U, Schreiber U (1993) Electron flow to the intersystem chain from stromal components and cyclic electron flow in maize chloroplasts, as detected in intact leaves by monitoring redox change of P700 and chlorophyll fluorescence. Plant Cell Physiol 34: 39–50 [Google Scholar]
- Baerr JN, Thomas JD, Taylor BG, Rodermel SR, Gray GR (2005) Differential photosynthetic compensatory mechanisms exist in the immutans mutant of Arabidopsis thaliana. Physiol Plant 124: 390–402 [Google Scholar]
- Bennoun P (1982) Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA 79: 4352–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M, Baker NR (1981) Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta 63: 542–551 [DOI] [PubMed] [Google Scholar]
- Buchel C, Garab G (1995) Evidence for the operation of a cyanide-sensitive oxidase in chlororespiration in the thylakoids of the chlorophyll c-containing alga Pleurochloris meiringensis (Xanthophyceae). Planta 197: 69–75 [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ (1998) Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J 17: 868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Kuntz M (2001) A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci 6: 31–36 [DOI] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano LM, Zapata JM, Martin M, Sabater B (2000) Chlororespiration and poising of cyclic electron transport. J Biol Chem 275: 942–948 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Corneille S, Cournac L, Guedeney G, Havaux M, Peltier G (1998) Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts—characterization of a NAD(P)H-plastoquinone oxidoreductase activity. Biochim Biophys Acta 1363: 59–69 [DOI] [PubMed] [Google Scholar]
- Cournac L, Josse EM, Joët T, Rumeau D, Latouche G, Redding K, Kuntz M, Peltier G (2000. a) Flexibility in photosynthetic electron transport: a newly identified chloroplast oxidase involved in chlororespiration. Philos Trans R Soc Lond B Biol Sci 355: 1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Latouche G, Cerovic Z, Redding K, Ravenel J, Peltier G (2002) In vivo interactions between photosynthesis, mitorespiration, and chlororespiration in Chlamydomonas reinhardtii. Plant Physiol 129: 1921–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse EM, Kuntz M, Peltier G (2000. b) Electron flow between photosystem ΔII and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem 275: 17256–17262 [DOI] [PubMed] [Google Scholar]
- Cutler S, McCourt P (2005) Dude where's my phenotype? Dealing with redundancy in signaling networks. Plant Physiol 138: 558–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Schreiber U, Heber U (1985) The relationship between the redox state of QA and photosynthesis in leaves at various carbon-dioxide, oxygen and light regimes. Planta 166: 219–226 [DOI] [PubMed] [Google Scholar]
- Endo T, Mi HL, Shikanai T, Asada K (1997) Donation of electrons to plastoquinone by NAD(P)H dehydrogenase and by ferredoxin-quinone reductase in spinach chloroplasts. Plant Cell Physiol 38: 1253–1258 [Google Scholar]
- Field TS, Nedbal L, Ort DR (1998) Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. Plant Physiol 116: 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Park S, Rodermel SR (2005) Sequences required for the activity of IM (IMMUTANS), a plastid terminal oxidase. In vitro and in planta mutagenesis of iron-binding sites and a conserved sequence that corresponds to exon 8. J Biol Chem 280: 42489–42496 [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, Geisler M, Coutinho PM, Segerman B, Nishikubo N, Takahashi J, Asperborg H, Djerbi S, Master E, Andersson-Gunneras S, et al (2006) Poplar carbohydrate-active enzymes (CAZymes). Gene identification and expression analyses. Plant Physiol 140: 946–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR, Savitch LV, Ivanov AG, Huner NPA (1996) Photosystem II excitation pressure and development of resistance to photoinhibition. II. Adjustment of photosynthetic capacity in winter wheat and winter rye. Plant Physiol 110: 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedeney G, Corneille S, Cuine S, Peltier G (1996) Evidence for an association of ndhB, ndhJ, gene products and ferredoxin-NADP-reductase as components of a chloroplastic NAD(P)H dehydrogenase complex. FEBS Lett 378: 277–280 [DOI] [PubMed] [Google Scholar]
- Harrison MA, Melis A (1992) Organization and stability of polypeptides associated with the chlorophyll a-b light-harvesting complex of photosystem II. Plant Cell Physiol 33: 627–637 [Google Scholar]
- Horvath EM, Peter SO, Joët T, Rumeau D, Cournac L, Horvath GV, Kavanagh TA, Schäfer C, Peltier G, Medgyesy P (2000) Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol 123: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüner NPA, Öquist G, Melis A (2003) Photostasis in plants, green algae and cyanobacteria: the role of light harvesting antenna complexes. In BR Green, WW Parson, eds, Light-Harvesting Antennas in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 401–421
- Hüner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3: 224–230 [Google Scholar]
- Ivanov AG, Morgan RM, Gray GR, Velitchkova MY, Huner NPA (1998) Temperature/light dependent development of selective resistance to photoinhibition of photosystem I. FEBS Lett 430: 288–292 [DOI] [PubMed] [Google Scholar]
- Joët T, Genty B, Josse EM, Kuntz M, Cournac L, Peltier G (2002) Involvement of a plastid terminal oxidase in plastoquinone oxidation as enhanced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem 277: 31623–31630 [DOI] [PubMed] [Google Scholar]
- Josse EM, Alcaraz JP, Labouré AM, Kuntz M (2003) In vitro characterization of a plastid terminal oxidase (IM). Eur J Biochem 270: 3787–3794 [DOI] [PubMed] [Google Scholar]
- Josse EM, Simkin AJ, Gaffé J, Labouré AM, Kuntz M, Carol P (2000) A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol 123: 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JTO, Tilney-Bassett RAE (1978) The Plastids: Their Chemistry, Structure, Growth, and Inheritance. Elsevier Press, Amsterdam
- Komenda J (2000) Role of two forms of the D1 protein in the recovery from photoinhibition of photosystem II in the cyanobacterium Synechococcus PC 7942. Biochim Biophys Acta 1457: 243–252 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79: 209–218 [DOI] [PubMed] [Google Scholar]
- Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lajko F, Kadioglu A, Borbely G, Garab G (1997) Competition between the photosynthetic and the (chloro)respiratory electron transport chains in cyanobacteria, green algae and higher plants. Effect of heat stress. Photosynthetica 33: 217–226 [Google Scholar]
- Lennon AM, Prommeenate P, Nixon PJ (2003) Location expression and orientation of the putative chlororespiratory enzymes, Ndh and IMMUTANS, in higher plant-plastids. Planta 218: 254–260 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A (1999) Photosystem II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo. Trends Plant Sci 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Mi H, Endo T, Schreiber U, Asada K (1992. a) Donation of electrons from the cytosolic components to intersystem chain in the cyanobacterium Synechococcus sp. PCC 7002, as determined by reduction of P700+. Plant Cell Physiol 33: 1099–1105 [Google Scholar]
- Mills JD, Crowther D, Slovacek RE, Hind G, McCarthy RE (1979) Electron transport pathways in spinach chloroplasts. Reduction of the primary acceptor of photosystem II by reduced nicotinamide adenine dinucleotide phosphate in the dark. Biochim Biophys Acta 547: 127–137 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Molen TA, Rosso D, Piercy S, Maxwell DP (2006) Characterization of the alternative oxidase of Chlamydomonas reinhardtii in response to oxidative stress and a shift in nitrogen source. Physiol Plant 127: 74–86 [Google Scholar]
- Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591 [DOI] [PubMed] [Google Scholar]
- Moore AL, Albury MS, Crichton PG, Affourtit C (2002) Function of the alternative oxidase: Is it still a scavenger? Trends Plant Sci 7: 478–481 [DOI] [PubMed] [Google Scholar]
- Morgan-Kiss RM, Ivanov AG, Huner NPA (2001) The Antarctic psychrophile, Chlamydomonas subcaudata, is deficient in state I-state II transitions. Planta 214: 435–445 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Norris SR, Barette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7: 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, et al (1986) Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322: 572–574 [Google Scholar]
- Ohyama K, Kohchi T, Sano T, Yamada Y (1988) Newly identified groups of genes in chloroplasts. Trends Biochem Sci 13: 19–22 [DOI] [PubMed] [Google Scholar]
- Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53: 523–550 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rédei GP (1963) Somatic instability caused by a cysteine-sensitive gene in Arabidopsis. Science 139: 767–769 [DOI] [PubMed] [Google Scholar]
- Rédei GP (1975) Arabidopsis as a genetic tool. Annu Rev Genet 9: 111–127 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel SR, Inzé D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32: 329–342 [DOI] [PubMed] [Google Scholar]
- Röbbelen G (1968) Genbedingte rotlicht-empfindlichkeit der chloroplastendifferenziering bei Arabidopsis. Planta 80: 237–254 [Google Scholar]
- Rodermel SR (2001) Pathways of plastid to nucleus signaling. Trends Plant Sci 6: 471–478 [DOI] [PubMed] [Google Scholar]
- Rodermel SR (2002) Arabidopsis variegation mutants. In CR Somerville, ER Myerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, pp 1–28 [DOI] [PMC free article] [PubMed]
- Savitch LV, Allard G, Seki M, Robert LS, Tinker NA, Huner NPA, Shinozaki K, Singh J (2005) The effect of over-expression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and freezing tolerance in Brassica napus. Plant Cell Physiol 46: 1525–1539 [DOI] [PubMed] [Google Scholar]
- Savitch LV, Barker-Astrom J, Ivanov AG, Hurry V, Oquist G, Huner NPA, Gardestrom P (2001) Cold acclimation of Arabidopsis thaliana results in incomplete recovery of photosynthetic capacity which is associated with an increased reduction of the chloroplast stroma. Planta 214: 295–301 [DOI] [PubMed] [Google Scholar]
- Savitch LV, Leonardos ED, Krol M, Jansson S, Grodzinski B, Huner NPA, Oquist G (2002) Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ 25: 761–771 [Google Scholar]
- Savitch LV, Massacci A, Gray GR, Huner NPA (2000) Acclimation to low temperature or high light mitigates sensitivity to photoinhibition: roles of the Calvin cycle and the Mehler reaction. Aust J Plant Physiol 27: 253–264 [Google Scholar]
- Sazanov LA, Burrows P, Nixon PJ (1996) Detection and characterization of a complex I-like NADH-specific dehydrogenase from pea thylakoids. Biochem Soc Trans 24: 739–743 [DOI] [PubMed] [Google Scholar]
- Scheller HV, Haldrup A (2005) Photoinhibition of photosystem I. Planta 221: 5–8 [DOI] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C, Neubauer C (1988) Measuring P700 absorbance changes around 830 with a new type of pulse modulation system. Z Naturforsch C 43: 686–698 [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62 [DOI] [PubMed] [Google Scholar]
- Streb P, Josse EM, Gallouët E, Baptist F, Kuntz M, Cornic G (2005) Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant Cell Environ 28: 1123–1135 [Google Scholar]
- Terashima I, Noguchi K, Itohnemoto T, Park YM, Kubo A, Tanaka K (1998) The cause of PSI photoinhibition at low temperatures in leaves of Cucumis sativus, a chilling-sensitive plant. Physiol Plant 103: 295–303 [Google Scholar]
- Tilney-Bassett RAE (1975) Genetics of variegated plants. In CW Birky, PS Perlman, TJ Byers, eds, Genetics and Biogenesis of Mitochondria and Chloroplasts. Ohio State University Press, Columbus, OH, pp 268–308
- van Kooten O, Snell JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48: 703–734 [DOI] [PubMed] [Google Scholar]
- Wetzel CM, Jiang CZ, Meehan LJ, Voytas DF, Rodermel SR (1994) Nuclear-organelle interactions: the immutans variegation mutant of Arabidopsis is plastid autonomous and impaired in carotenoid biosynthesis. Plant J 6: 161–175 [DOI] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel CM, Voytas DF, Rodermel SR (1999) The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11: 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]