Abstract
Genes encoding defense-related proteins have been used to alter the resistance of plants to pathogens and other environmental challenges, but no single fungal gene overexpression has produced broad-spectrum stress resistance in transgenic lines. We have generated transgenic tobacco (Nicotiana tabacum) lines that overexpress the endochitinases CHIT33 and CHIT42 from the mycoparasitic fungus Trichoderma harzianum and have evaluated their tolerance to biotic and abiotic stress. Both CHIT33 and CHIT42, individually, conferred broad resistance to fungal and bacterial pathogens, salinity, and heavy metals. Such broad-range protective effects came off with no obvious detrimental effect on the growth of tobacco plants.
Plants respond to pathogen attacks by expressing a wide array of genes, most of them directly related to defensive molecular mechanisms. Both the hypersensitive response and the systemic acquired resistance that plants exhibit upon biological stress are complex processes in which a network of different signal cascades ends in modulation of the expression of different sets of genes (Ryals et al., 1996; Shirasu et al., 1996). Seemingly separated abiotic stress-signaling pathways also share common elements that are in some cases considered as possible cross-talk points (Ellis and Turner, 2001; Zhu, 2001; Singh et al., 2002).
Both pathogen attacks and abiotic stresses, such as salinity and drought, decrease crop yields worldwide. Many attempts have been made to confer resistance to pathogens and increase tolerance to abiotic stress to plants of agronomic interest. One of the most widely used strategies is to overexpress plant genes that are induced after biotic or abiotic stresses, such as chitinases and glucanases (Alexander et al., 1993; Hong and Hwang, 2006), vacuole and plasma membrane sodium transporters (Apse and Blumwald, 2002; Shi et al., 2003), mitogen-activated protein kinases (Piao et al., 2001; Zhang and Liu, 2001; Xiong and Yang, 2003), transcription factors (Park et al., 2001), peroxidases (Amaya et al., 1999), disease-related R genes, and ferritins (Deak et al., 1999). Alternatively or complementarily, efforts have been made to reinforce the plant array of responsive genes by introducing heterologous genes of well-known antipathogenic effect belonging to other phylla (Lorito et al., 1998; Bolar et al., 2001; Garg et al., 2002; Schutzendubel and Polle, 2002; Kunze et al., 2004). Chitinases are thought to play a dual role, both by inhibiting fungal growth by cell wall digestion and by releasing pathogen-borne elicitors that induce further defense reactions in the host. Transgenic plants overexpressing chitinases of several origins have been shown to exhibit enhanced levels of resistance to fungal infection and delayed disease symptoms when challenged with fungal pathogens (Jach et al., 1995; Lorito et al., 1998; Hong and Hwang, 2006).
The genome of mycoparasites, such as members of the genus Trichoderma, which have specifically evolved to attack other fungi, is a potential source of antipathogenic genes. Trichoderma species can inhibit the growth of other fungal species by means of antibiotics and cell wall-degrading enzymes: chitinases, proteases, glucanases, and mannanases, among others. Transgenic tobacco (Nicotiana tabacum) and potato (Solanum tuberosum) plants overexpressing an endochitinase (CHIT42) from Trichoderma harzianum have been shown to be highly tolerant to the foliar pathogens Alternaria alternata, Alternaria solani, and Botrytis cinerea, and also to the soil-borne pathogen Rhizoctonia solani (Lorito et al., 1998). Overexpression of another endochitinase from Trichoderma (CHIT33) considerably enhances the antifungal activity of T. harzianum strain CECT2413 in in vitro confrontation experiments against R. solani (Limón et al., 1995). The amino acid sequence of CHIT33 shows significant similarity to some pathogenic response-associated class III plant chitinases (Limón et al., 1995) and substantial biochemical differences with other Trichoderma chitinases (de la Cruz et al., 1992). Chitinases CHIT42 and CHIT33 exhibit synergistic in vitro hydrolytic properties when assayed against purified fungal cell walls (de la Cruz et al., 1992).
In this work, we report on the production of tobacco plants overexpressing the Trichoderma endochitinase-encoding gene chit33, alone or in combination with gene chit42, and on the evaluation of their tolerance to a broad range of stress agents. The overexpression of chit33 in tobacco plants not only significantly enhances their tolerance to fungal and bacterial pathogens, but also their resistance to saline stress and high concentrations of heavy metals in the culture medium. We have confirmed previous reports of the enhanced tolerance of chit42-overexpressing plants to fungal pathogens and determined their tolerance to bacterial pathogens and abiotic stresses. Contrary to what has been reported in in vitro experiments with purified proteins, no synergistic effects of CHIT42 and CHIT33 have been observed in planta. The phenotype of the chitinase-overexpressing plants is morphologically indistinguishable from that of control lines with regard to biomass production, fertility, and seed viability. Although some reports exist regarding concomitant enhanced tolerance to biotic and abiotic stress in transgenic plants overexpressing stress response plant determinants, this report outlines broad range effects achieved by the overexpression of a single fungal gene in plants.
RESULTS
Ectopic Expression of the Trichoderma chit33 Gene in Tobacco
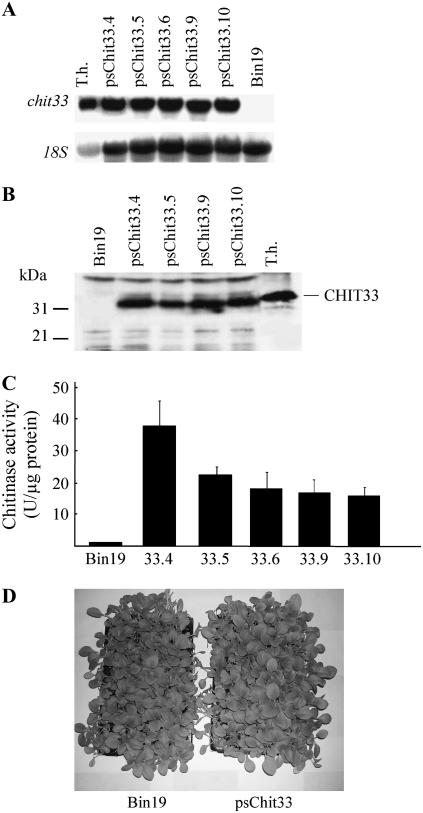
To evaluate the defense potential of endochitinase CHIT33 from T. harzianum strain CECT2413, transgenic tobacco plants that expressed the chit33 gene under the control of the cauliflower mosaic virus 35S promoter were produced. To mediate the export of the protein to the intercellular space, the nucleotide sequence of chit33 encoding its signal peptide was substituted by that of the tomato (Lycopersicon esculentum) pathogenesis-related (PR) protein P1-p14 (Tornero et al., 1994). Nineteen independent tobacco lines harboring the transgene were obtained, and F3 homozygous plants from the five transgenic lines that showed the highest chit33 mRNA accumulation (Fig. 1A) were selected for more extensive functional characterization. The CHIT33 protein was detected by western-blot analysis, and it proved to be identical in size to that produced by Trichoderma, which indicated correct posttranslational processing (Fig. 1B). To assess the functionality of the protein in the transgenic lines, chitinase activity of the total plant protein extracts was quantified by fluorescence assays, using the specific substrate 4-methylumbelliferyl-b-d-N,N′,N″,N′′′-tetraacetylchitotetraoside [4-MU-(GINAc)4] (Fig. 1C). The chitinase activity of each transgenic line correlated with its levels of mRNA chit33 accumulation and of CHIT33 production, whereas that of control plants was almost undetectable. Thus, it can be concluded that the chitinase activity detected in the transgenic chit33 lines is a consequence of chit33 overexpression and responds to its own lytic activity, either alone or in synergistic combination with plant chitinases. No visible phenotypic alteration was detected in transgenic Pseudomonas syringae pschit33 plants grown in soil under greenhouse conditions (Fig. 1D).
Figure 1.
Molecular analysis of representative pschit33 transgenic plants. A, Northern analysis of tobacco pschit33 transgenic lines (33.4, 33.5, 33.6, 33.9, 33.10) and control line (Bin19) grown in standard conditions. As a positive control, total RNA of T. harzianum (T.h.) grown in chit33-inducing conditions was used (Dana et al., 2001). B, Western analysis of total proteins from leaf extracts of control and transgenic tobacco lines. As a positive control, culture supernatant of T.h. grown in chit33-inducing conditions was used. C, Specific chitinase activity in protein-soluble extracts of control and transgenic tobacco lines. 1 U = 1 nmol of 4-methylumbelliferone released per minute. D, Bin19 and pschit33 plants grown for 5 weeks.
Overproduction of chit33 Promotes Disease Resistance of Transgenic Plants to Soil-Borne Pathogens
Transgenic chit33 tobacco plants were tested for resistance to R. solani, an endemic soil-borne pathogen that causes camping-off, seedling blight, and root rot. In infection assays on agar-water plates, the survival rate of the transgenic chit33 plants to Rhizoctonia reached 81%, whereas that of the control plants was 39.6%, a statistically significant difference according to one-way ANOVA (Table I). It has been reported that overexpression in tobacco transgenic lines of a T. harzianum gene (chit42) encoding the endochitinase CHIT42 also improves plant resistance to the same pathogen (Lorito et al., 1998), albeit to a lesser extent. Previous studies have shown that, in some cases, simultaneous expression of different proteins involved in mycoparasitism enhances the resistance of transgenic tobacco lines to fungal pathogens. Trichoderma chit33 and chit42 show synergistic chitinolytic activity in in vitro assays against purified fungal cell walls (de la Cruz et al., 1992). Hybrid lines chit33 × 42 were obtained by crossing the transgenic chit33 and chit42 lines that showed the strongest constitutive expression of each gene; their level of resistance to R. solani was compared with that of the parental chit33 and chit42 lines. The level of resistance conferred by the combined expression of the two transgenes was slightly higher than that of the chit42 parent, and considerably lower than that of chit33 plants, exemplified by the chit33 parental line (Table I).
Table I.
Resistance of pschit plants to R. solani
| Line | Survival Rate |
|---|---|
| % | |
| Bin19 | 39.62 ± 3.37 |
| pschit33 | 81.00 ± 1.70 |
| pschit42 | 56.77 ± 8.50 |
| pschit33 × 42 | 64.58 ± 4.33 |
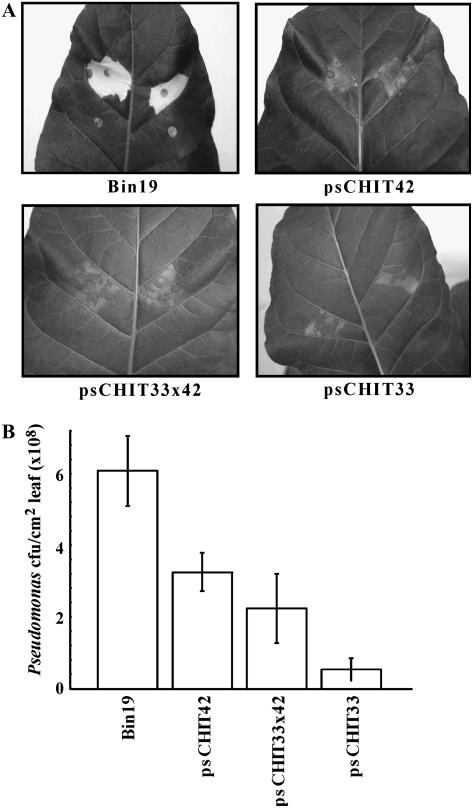
The protective effect of CHIT33 against pathogenic bacteria was also investigated. chit33, chit42, and chit33 × 42 transgenic plants were infiltrated with P. syringae pv tabaci 153, and necrosis was recorded 5 d after inoculation. The necrotic symptoms, defined by the size and density of necrotic lesions in the foliar tissue, were greatly reduced in the pschit33 plants when compared with the controls. A similar reduction of pathogenic effects was observed in pschit42 and pschit33 × 42 transgenic plants (Fig. 2A). Proliferation of P. syringae in the infiltrated plants was determined as colony forming units (cfu)/cm2 of infected leaves. Five days after treatment, the bacterial cell densities in control plants exceeded those in the pschit plants 2- to 10-fold. The pschit33 plants were those that showed maximal inhibition of P. syringae growth in the foliar tissue (Fig. 2B). Thus, the reduction of disease symptoms seems to be associated with the inhibition of bacterial proliferation in the transgenic pschit plants. These results support the hypothesis that chitin hydrolysis is not the primary mechanism that causes the protective effect of pschit33 and pschit42 against plant pathogens.
Figure 2.
Disease resistance against the pathogenic bacteria P. syringae pv tabaci in pschit transgenic tobacco lines. A, Tobacco leaves showing disease symptoms (necrotic areas) 5 d after infection. B, Growth of Pseudomonas in transgenic pschit lines. Fully expanded leaves of 8-week-old tobacco plants were inoculated with 0.5 to 1 × 106 cfu/mL of P. syringae. Five days after inoculation, the infected leaves were collected and the bacterial populations determined. Each experiment was carried out with, respectively, two, three, three, and four independent Bin19, pschit42, pschit33 × 42, and pschit33 lines. Values are the average results from two experiments, and three independent replicas were performed for each experiment.
Chitinase Transgenic Plants Show Increased Accumulation of Peroxidase Activity
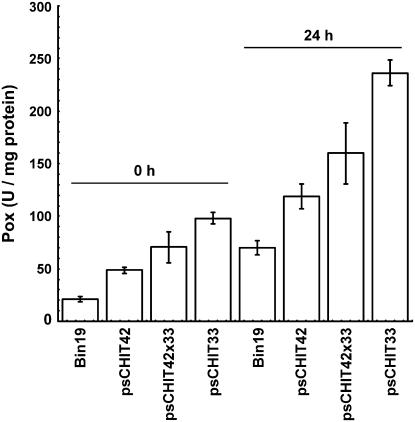
Reactive oxygen species (ROS) are synthesized as signal molecules during the process of plant response to biotic and abiotic stresses (Chamnongpol et al., 1998; Mittler and Rizhsky, 2000; Fath et al., 2002; Neill et al., 2002). To prevent the toxic effect of excess of ROS in plant cells, scavenging mechanisms exist, involving the enhanced enzymatic activity of superoxide dismutase, catalase, plasma membrane-bound NADP oxidases, cytoplasmic and cell wall-bound peroxidases, and amine oxidases in the apoplast (Wojtaszek, 1997; Yoshida et al., 2003). These enzymes produce reactive oxygen intermediates (ROIs), which can also act as signal molecules and play a central role in the defense of plants against pathogen attacks. Anionic peroxidases are involved in the production of ROIs; these act as precursors of lignin and suberin, thus playing a dual role—that of keeping the ROS concentrations at nontoxic levels and also contributing to the reinforcement of the plant cell wall. Previous studies showed that tobacco cell wall-bound anionic peroxidases are induced after pathogen attack and their activity is not inhibited by salicylic acid, as is the case for other ROS-scavenging enzymes, such as ascorbate peroxidase or catalase (Bi et al., 1995; Durner and Klessig, 1995; Takahashi et al., 1997; Mittler and Rizhsky, 2000). The anionic peroxidase activity of pschit33, pschit42, and pschit33 × 42 transgenic plants was determined and found to be significantly higher than that of the control plants in standard growth conditions. As expected, 24 h after infection with Pseudomonas, the levels of peroxidase activity were higher in all lines tested, including control plants, but the differences among lines were maintained (Fig. 3). The enhanced basal and pathogen-induced peroxidase activity of pschit plants correlates with their improved resistance to fungal and bacterial pathogens. It is worth noting that no undesirable effects on root growth and development were observed in the pschit lines, as was previously reported for transgenic tobacco lines overexpressing anionic peroxidase (Lagrimini et al., 1997).
Figure 3.
Peroxidase activity of pschit plants. Fully expanded leaves of 8-week-old tobacco plants were infiltrated with a suspension of P. syringae pv tabaci and collected 24 h after infection. The results shown are the mean of three independent experiments; and the activity measurements were made, respectively, on two, three, three, and four independent Bin19, pschit42, pschit33 × 42, and pschit33 lines.
Enhanced Expression of Pathogen-Response Proteins
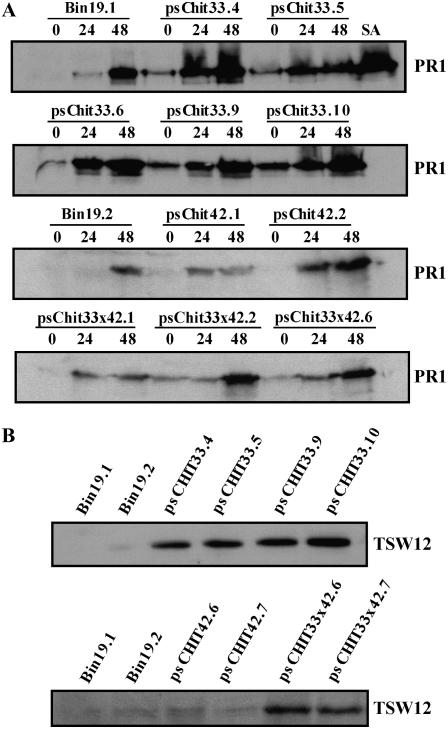
One of the most utilized markers of plant response to pathogenic challenge is PR-1a, a protein of unknown function whose levels under standard conditions are very low and show a very substantial increase in infected plants (Kim et al., 2001). The levels of PR-1a in lines pschit33, pschit42, and pschit33 × 42 were considerably higher than those of control plants, both in nonchallenging conditions and after infection with Pseudomonas, and correlated with those shown for peroxidase activity (Fig. 4A). Analysis of the expression of other PR proteins failed to show any increase in the levels of PR-2, PR-3, PR-4, and PR-5 with regard to those of control plants. Analysis of the expression of other abiotic stress-related defense proteins in basal conditions showed enhanced expression in plants pschit33 of the protein TSW12, a nonspecific lipid transfer protein (Torres-Schumann et al., 1992; Fig. 4B). None of the pschit transgenic lines exhibited spontaneous necrotic lesions, which is a common phenotype for PR-overexpressing mutant or transgenic lines (Mittler and Rizhsky, 2000; Anand et al., 2003; Nishizawa et al., 2003).
Figure 4.
Enhanced defense-related protein expression in pschit transgenic lines. Western analysis of total proteins from leaf extracts of control and transgenic tobacco lines showing the expression levels of PR1 (A) and TSW12 (B). As a positive control, total proteins of salicylic acid-treated wild-type leaves (SA) were used.
Abiotic Stress Tolerance of Fungal Chitinase-Overexpressing Plants
The response of plants to different stresses constitutes a network of interconnected signaling pathways. This is partly due to the fact that the physiological disorders triggered by different stresses might require overlapping protective responses. For example, cold and drought stresses elicit a common array of dehydration response element-responsive genes, and a variety of scavenging enzymes that protect against oxidative damage are part of the plant response to different biological and abiotic stresses, such as fungal pathogen attack and salinity.
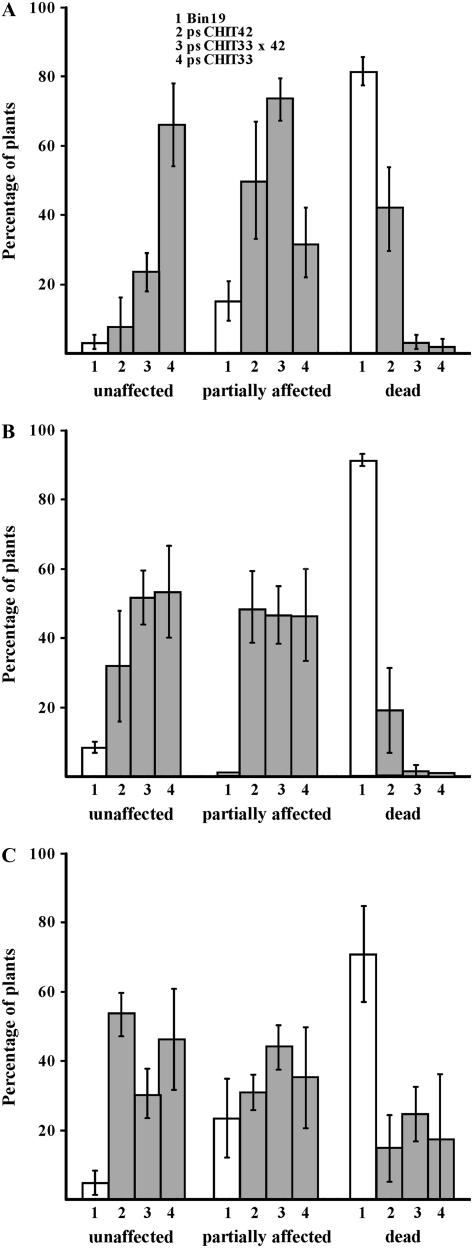
Among other deleterious effects within the plant cell, salt stress causes oxidative damage by enhanced production of ROS (Xiong and Zhu, 2002). Heavy metals such as copper (Cu), mercury (Hg), and cadmium (Cd) also induce the formation and accumulation of ROS in cells (Briat and Lebrun, 1999; Schutzendubel et al., 2001). The tolerance of pschit33, pschit42, and pschit33 × 42 lines to toxic concentrations of salt, Cu, and Cd was evaluated in in vitro assays in conditions that allowed a low, but reproducible, survival rate of the control plants, thus establishing a quantifiable gradient from lethality to survival, and grouping the intermediate states as deleterious effects (Fig. 5). Among the lines tested, pschit33 plants showed the highest survival rate and the lowest rate of stress symptoms and/or death when subjected to salt stress (Fig. 5A). Lines pschit33 × 42 showed similarly low death rates, but, among the surviving plants, a majority presented some symptoms of stress in the form of growth arrest and/or chlorosis. Lines pschit42 showed moderately higher death rates than pschit33 and pschit33 × 42, albeit significantly lower than those of control plants. As is the case for pschit33 × 42 plants, most of the surviving pschit42 plants showed stress symptoms (Fig. 5A). Similar results were obtained when estimating the tolerance of the pschit lines to Cu (Fig. 5B) and Cd (Fig. 5C), although in these two conditions the survival rates and incidence of stress symptoms were similar for all the transgenic pschit lines tested and significantly different from that of control plants. These results confirm that the expression of heterologous chitinases in tobacco plants causes not only a significant increase in resistance to fungal and bacterial pathogens, but also a concomitant tolerance to abiotic stress caused by high concentrations of salt and metal ions in the growth substrate. The differences in the degree of resistance to biotic stress exhibited by the different pschit lines can also be observed with regard to salt stress response. In all cases, the highest levels of salinity tolerance were obtained by overexpressing the Trichoderma gene chit33.
Figure 5.
Abiotic stress tolerance of fungal chitinase-overexpressing plants. A, Salt stress tolerance. Seven-day-old seedlings grown in one-half-strength Murashige and Skoog basal salt medium were transferred to agar-water plates containing 256 mm NaCl. The effects of salt on plant growth and viability after 7 d at 25°C and continuous light allow the division of plant populations of each of the transgenic lines tested into three categories: unaffected plants (no visible effects, sustained growth, and viability), partially affected plants (deleterious effects, marked by growth arrest, and chlorosis symptoms), and dead plants. B and C, Cd and Cu tolerance. The assays were made as in A, substituting NaCl for 300 μm CdSO4 (B) and 300 μm CuSO4 (C) in the selective plates.
DISCUSSION
Chitinases belong to the repertoire of plant defense systems and are nontoxic to plants and higher vertebrates. Hence, the many reported attempts to enhance plant protection against pathogens by homologous and heterologous overexpression of plant chitinases. However, in most cases, the increase of resistance achieved by such a strategy has turned out to be effective within a narrow range of pathogens and is quantitatively modest, leading to the need for using gene combinations to achieve significant levels of plant tolerance.
Here we report the generation of transgenic tobacco plants overexpressing singly, or in combination, two endochitinases from the mycoparasitic fungus T. harzianum. Previous work in our laboratory had shown enhanced tolerance to a wide range of soil-borne and foliar fungal pathogens exhibited by tobacco pschit42 plants overexpressing the Trichoderma endochitinase CHIT42 (Garcia et al., 1994; Lorito et al., 1998). To ensure its correct processing and secretion to the apoplast, the fungal hydrolase was modified by substituting its native signal peptide with a signal peptide of plant origin (Tornero et al., 1994). From the wide array of hydrolases produced by Trichoderma during mycoparasitic interactions, CHIT33 shows significant sequence homology with some defense-related class III plant chitinases. CHIT33 exhibits antifungal activity and synergistic lytic properties with CHIT42 in in vitro assays (de la Cruz et al., 1992; Dana et al., 2001) and its overexpression increases the mycoparasitic activity of transgenic T. harzianum strains (Limón et al., 1999). The chit33 gene was modified in the same way described previously for chit42 (Lorito et al., 1998) and transgenic pschit33 tobacco lines were generated.
Five independent F3 homozygous pschit33 lines were tested and showed significantly enhanced resistance to both fungal (R. solani) and bacterial (P. syringae) pathogens. Transgenic homozygous pschit42 lines also exhibited improved resistance to both pathogens, although to a lesser degree, thus confirming previously reported data on their molecular and physiological characterization (Lorito et al., 1998) and providing new insights on how the defense mechanisms of the pschit plants are modulated by the presence of fungal chitinases. No synergistic effect was observed in the pschit33 × 42 lines tested, their level of resistance being intermediate to that of their parental lines. The intermediate level of resistance achieved by the pschit33 × 42 plants could be interpreted as the result of the competition and/or titration exerted by the less proficient chitinase on the activity of the more active one in any process related to its physiological effects in transgenic plants. Our results suggest that the improved tolerance against pathogens observed in the pschit plants is not the sole consequence of their enhanced chitinolytic activity and that it is very likely that other defense-related mechanisms are being triggered by the presence of either chitinase in the apoplast. Several lines of evidence support this hypothesis. The levels of PR-1a, but not those of PR-2, PR-3, PR-4, and PR-5, are significantly higher in the transgenic pschit lines, both in basal conditions and in plant-pathogen interaction assays. The systemic induction of PR proteins occurs upon stress, pathogen attack, and abiotic stimuli and it is usually accompanied by the development of local and/or systemic resistance to biotic or abiotic challenges. The PR protein expression profile helps to define different physiological responses to environmental challenges (Ryals et al., 1996; Thomma et al., 2001; Hammond-Kosack and Parker, 2003). One of the better-studied plant defense mechanisms is the salicylic acid-dependent pathway involved in systemic acquired resistance, which causes the concomitant activation of PR-1, PR-2, and PR-5. Induced systemic resistance responses dependent on the jasmonic acid pathway enhance expression of another set of PR proteins, PR-3, PR-4, and PR-12. The overexpression in transgenic plants of many components of the plant defense-signaling pathways, such as mitogen-activated protein kinases, transcription factors, and PR chitinases and glucanases, also leads to the activation of PR-1 expression in basal and induced conditions (Cao et al., 1998; Anand et al., 2003; Xiong and Yang, 2003; Park et al., 2004; Luo et al., 2005). Although the function of PR-1 is still unknown, its role in the plant mechanisms of defense against diseases is evident as its level of expression correlates with physiological states of plant-pathogen incompatible interactions, abiotic stress responses, and systemic resistance-like responses.
Cell wall-associated anionic peroxidase activity was considerably higher in the transgenic pschit lines than in control plants, both in standard growth conditions and after Pseudomonas infection. Peroxidase has often been used as an enzymatic marker in studies of defense-related processes (Young et al., 1995). Pathogen recognition by plant cells leads to the production of a variety of ROS that can act as second messengers and activate, in turn, various defense-related genes (Orozco-Cardenas et al., 2001; Bolwell et al., 2002), as well as participating in the strengthening of the cell wall through callose deposition and wall-bound phenolics, suberification, and lignification. The enhanced peroxidase activity shown by the pschit plants can thus be linked to their increased pathogen resistance and to the significant diminution of necrotic symptoms that they exhibit after Pseudomonas infection. It can also account, at least partially, for the abiotic stress resistance observed in pschit plants by counteracting the oxidative stress resulting from salt and heavy metals, and also by favoring water retention in the cell walls, as reported by Amaya et al. (1999) for transgenic tomato lines overexpressing the peroxidase TPX2. Additionally, ROIs produced by the action of anionic peroxidase can trigger other abiotic stress-specific signaling pathways. It is worth noting that lines overexpressing pschit33 and not those overexpressing pschit42 also showed enhanced expression of TSW12, a member of the nonspecific lipid transfer protein family that is induced upon salt and abscisic acid treatment (Torres-Schumann et al., 1992; Molina and Garcia-Olmedo, 1997).
The enhanced tolerance of pschit plants to different stresses could be a result of the liberation of cell wall or apoplastic glycoprotein-derived oligomers due to the action of either of the chitinases, which would act as elicitors, triggering one or more defense-signaling pathways leading to a systemic acquired resistance-like state. It has been reported that apoplastic and cell wall-bound arabinogalactan proteins from carrot (Daucus carota) can contain detectable amounts of glucosamine and N-acetyl-glucosaminyl and are sensitive to endochitinase cleavage (van Hengel et al., 2001). Several oligosaccharines of plant origin also contain N-acetylglucosaminyl units and are thought to be involved in processes of biological relevance, such as modulation of flax (Linum usitatissimum) seedling growth and promotion of tomato fruit ripening. Although the biosynthetic origin of these oligomers is not well established, there is some evidence that they might derive from partial hydrolysis of apoplastic N-linked glycoproteins (Fry et al., 1993). The protein CHIT33, which shares 42% identity with plant chitinases (Limón et al., 1995), could additionally or alternatively act as an elicitor by itself, triggering at least one more mechanism of response exemplified by the overexpression of TSW12.
The results presented in this work identify T. harzianum endochitinases CHIT33 and CHIT42 as physiological determinants capable of generating innate defense responses and enhanced stress tolerance in tobacco transgenic plants without detectable morphological or physiological undesirable side effects.
MATERIALS AND METHODS
Constructs and Plant Transformation
The endochitinase-encoding full-length cDNA chit33 from Trichoderma harzianum strain CECT2431 was modified by substituting its amino-terminal signal peptide (Limón et al., 1995) with the signal peptide of the tomato (Lycopersicon esculentum) PR protein P1-p14 (Tornero et al., 1994). The chimeric ps∷chit33 gene thus obtained was then placed under the control of the cauliflower mosaic virus 35S subunit and the nopaline synthase terminator in the pBIN19 vector. Agrobacterium tumefaciens strain LBA4404 containing the construction pBIN19∷pschit33 was used to transform leaf discs of tobacco (Nicotiana tabacum var Xhanti) following standard protocols (Horsch et al., 1985). Nineteen independent kanamycin-resistant pschit33 tobacco lines were obtained, and five of them were selected for further characterization. Transgenic F3 homozygous plants harboring single-copy integrations of the chit33 or chit42 transgenes were used for biotic and abiotic functional analysis. Transgenic pschit42 × chit33 plants, harboring chit42 and chit33 genes from T. harzianum, were obtained by crossing lines pschit42 (Lorito et al., 1998) and pschit33.5. Line pschit33.5 acted as the female parent. Four F1 hybrid lines, showing constitutive chit42 and chit33 expression, were selected for further analyses.
Molecular Analyses of Transgenic Tobacco Lines
Transgenic plants were propagated on Murashige and Skoog basal salt medium (Sigma) containing 3% Suc and 100 mg/L kanamycin. The presence of the transgenes was detected by PCR amplification using primers 5′-GCCATGCCTTCATTGACTGCTC-3′ (C35) and 5′-CCTCAAAGCATTGACAACCTG-3′ (C33) that amplified the entire open reading frame of the chit33 gene, and primers 5′-GGTTATGCTTTCCATCGG-3′ (EC1) and 5′-CAAGGAGTCAGAGCCAGTCTT-3′ (BB2), which annealed, respectively, at positions 566 and 1,367 from the ATG of the chit42 gene. Northern analyses were performed following standard procedures (Sambrook et al., 1989), using the complete open reading frames of genes chit33, chit42, and the 18S RNA gene of carrot (Daucus carota) as probes on 10 μg of total plant RNA. Probes were labeled with the random primed DNA labeling kit (Amersham), following the manufacturer's instructions. Polyclonal rabbit antibodies raised against GST∷CHIT33, GST∷CHIT42, and PR-1a and TSW12 were used in western-blot assays of total protein extracts from transgenic plants (Torres-Schumann et al., 1992; Garcia et al., 1994; Limón et al., 1995). Protein transfer to nitrocellulose membranes was carried out in a trans-blot semidry transfer cell (Bio-Rad) following the manufacturer's instructions. Protein immunodetection was performed using a secondary antibody conjugated to horseradish peroxidase and the ECL western-blotting analysis system (Amersham).
Enzymatic Assays
Chitinase Activity Assay
Chitinase activity was determined by using the fluorescent-specific substrate [4-MU-(GlNAc)4] (Sigma) as described (Limón et al., 1995). Assay mixes (100 μL) containing 1 μg of total protein extract and 250 μm [4-MU-(GlNAc)4] in 100 mm sodium citrate buffer, pH 3.0, were incubated for 15 min at 30°C in the dark. The reactions were stopped with 2.9 mL of 0.5 m Gly-NaOH buffer, pH 10.4, and fluorescence was measured in a Hoefer TK0100 fluorimeter at 350-nm excitation and 440-nm emission wavelengths. The chitinase activity was expressed as picomoles of 4-methylumbelliferone liberated per minute and micrograms of protein.
Peroxidase Assay
For determination of peroxidase activity, 1 cm2 of foliar tissue was homogenized in 50 mm phosphate buffer, pH 6.0 (1:6 [w/v]). The homogenate was centrifuged at 13,000g for 1 min and protein from the supernatant was used for the assays. Assay mixes (500 μL) contained 0.5 to 1.0 μg of protein, 6 mm guaiacol, and 6 mm hydrogen peroxide in 50 mm sodium acetate buffer, pH 4.5. The reactions were incubated for 10 min and OD470 was measured.
Bacterial Infection and Pseudomonas Resistance Assays
Fully expanded leaves of 8-week-old tobacco plants were inoculated with Pseudomonas syringae pv tabaci 153 according to Thilmony et al. (1995). Each leaf was infiltrated in six points symmetrical with regard to the central nerve with 50 μL of cold bacterial suspension (0.5–1 × 106 cfu/mL 10 mm MgSO4). Mock-infected plants were infiltrated with the same volume of cold 10 mm MgSO4 solution. Pathogen and mock-infected plants were kept at 25°C under 80% relative humidity and 16 h of diurnal light. Twenty-four hours after infection, leaves were sampled and analyzed for peroxidase activity (Polle et al., 1994) and for expression of PR proteins. Necrotic areas were recorded 5 d after infection. To determine bacterial growth in planta, bacteria were extracted 5 d after infiltration from 1 cm2 discs of leaf-infected areas and plated on King's B medium (Martin et al., 1993).
Rhizoctonia Resistance Assays
Mycelium of Rhizoctonia solani pv tabaci 153 was grown in potato (Solanum tuberosum) dextrose broth at 25°C, 150 rpm, for 4 to 5 d, and harvested, weighed, and homogenized in sterile water. Two-week-old tobacco seedlings, germinated on Murashige and Skoog medium, were transferred to water-agar plates (0.7% [w/v]) containing 0.75 g/L of Rhizoctonia mycelium. After 7 d at 25°C and continuous light conditions, survival rates of transgenic and control plants were estimated.
Abiotic Stress Assays
Transgenic tobacco lines were assayed for resistance against saline stress and heavy metals. Each assay was carried out with 2-week-old seedlings (60 per line and per assay; three independent replicas), germinated on Murashige and Skoog medium. Seedlings were transferred to water-agar plates (0.7% [w/v]) containing 256 mm NaCl (saline stress), 300 μm CdSO4, or 300 μm CuSO4. After 7 d at 25°C and continuous light conditions, the effect of each stress-producing agent on transgenic and control plants was estimated by classifying the plant populations of each of the transgenic lines tested into three categories: Unaffected plants (no visible effects, sustained growth, and viability), partially affected plants (deleterious effects, marked by growth arrest and chlorosis symptoms), and dead plants.
Acknowledgments
We are grateful to P. Rodríguez Palenzuela for advice on Pseudomonas resistance assays and K.A. Lawton for providing PR plasmids.
This work was supported by grants from the Spanish Ministerio de Educación y Ciencia and the Dirección General de Universidades e Investigación of the Junta de Andalucía, Spain. M.d.l.M.D. was the recipient of a postgraduate fellowship from the Ministerio de Educación y Ciencia. B.C. is a fellow of Programa Averroes, Junta de Andalucia, Spain.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: José A. Pintor-Toro (pintor@cica.es).
References
- Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E, et al (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc Natl Acad Sci USA 90: 7327–7331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya I, Botella MA, de la Calle M, Medina MI, Heredia A, Bressan RA, Hasegawa PM, Quesada MA, Valpuesta V (1999) Improved germination under osmotic stress of tobacco plants overexpressing a cell wall peroxidase. FEBS Lett 457: 80–84 [DOI] [PubMed] [Google Scholar]
- Anand A, Schmelz EA, Muthukrishnan S (2003) Development of a lesion-mimic phenotype in a transgenic wheat line overexpressing genes for pathogenesis-related (PR) proteins is dependent on salicylic acid concentration. Mol Plant Microbe Interact 16: 916–925 [DOI] [PubMed] [Google Scholar]
- Apse MP, Blumwald E (2002) Engineering salt tolerance in plants. Curr Opin Biotechnol 13: 146–150 [DOI] [PubMed] [Google Scholar]
- Bi YM, Kenton P, Mur L, Darby R, Draper J (1995) Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J 8: 235–245 [DOI] [PubMed] [Google Scholar]
- Bolar JP, Norelli JL, Harman GE, Brown SK, Aldwinckle HS (2001) Synergistic activity of endochitinase and exochitinase from Trichoderma atroviride (T. harzianum) against the pathogenic fungus (Venturia inaequalis) in transgenic apple plants. Transgenic Res 10: 533–543 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53: 1367–1376 [PubMed] [Google Scholar]
- Briat JF, Lebrun M (1999) Plant responses to metal toxicity. C R Acad Sci III 322: 43–54 [DOI] [PubMed] [Google Scholar]
- Cao H, Li X, Dong X (1998) Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc Natl Acad Sci USA 95: 6531–6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H Jr, Van Montagu M, Inze D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 95: 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana MdlM, Limon MC, Mejias R, Mach RL, Benitez T, Pintor-Toro JA, Kubicek CP (2001) Regulation of chitinase 33 (chit33) gene expression in Trichoderma harzianum. Curr Genet 38: 335–342 [DOI] [PubMed] [Google Scholar]
- de la Cruz J, Hidalgo-Gallego A, Lora JM, Benitez T, Pintor-Toro JA, Llobell A (1992) Isolation and characterization of three chitinases from Trichoderma harzianum. Eur J Biochem 206: 859–867 [DOI] [PubMed] [Google Scholar]
- Deak M, Horvath GV, Davletova S, Torok K, Sass L, Vass I, Barna B, Kiraly Z, Dudits D (1999) Plants ectopically expressing the iron-binding protein, ferritin, are tolerant to oxidative damage and pathogens. Nat Biotechnol 17: 192–196 [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF (1995) Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci USA 92: 11312–11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Turner JG (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath A, Bethke P, Beligni V, Jones R (2002) Active oxygen and cell death in cereal aleurone cells. J Exp Bot 53: 1273–1282 [PubMed] [Google Scholar]
- Fry SC, Aldington S, Hetherington PR, Aitken J (1993) Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol 103: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Lora JM, de la Cruz J, Benitez T, Llobell A, Pintor-Toro JA (1994) Cloning and characterization of a chitinase (chit42) cDNA from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 27: 83–89 [DOI] [PubMed] [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Parker JE (2003) Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol 14: 177–193 [DOI] [PubMed] [Google Scholar]
- Hong JK, Hwang BK (2006) Promoter activation of pepper class II basic chitinase gene, CAChi2, and enhanced bacterial disease resistance and osmotic stress tolerance in the CAChi2-overexpressing Arabidopsis. Planta 223: 433–448 [DOI] [PubMed] [Google Scholar]
- Horsch R, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8: 97–109 [DOI] [PubMed] [Google Scholar]
- Kim S, Ahn IP, Lee YH (2001) Analysis of genes expressed during rice-Magnaporthe grisea interactions. Mol Plant Microbe Interact 14: 1340–1346 [DOI] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM, Joly RJ, Dunlap JR, Liu TT (1997) The consequence of peroxidase overexpression in transgenic plants on root growth and development. Plant Mol Biol 33: 887–895 [DOI] [PubMed] [Google Scholar]
- Limón MC, Lora JM, Garcia I, de la Cruz J, Llobell A, Benitez T, Pintor-Toro JA (1995) Primary structure and expression pattern of the 33-kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 28: 478–483 [DOI] [PubMed] [Google Scholar]
- Limón MC, Pintor-Toro JA, Benitez T (1999) Increased antifungal activity of Trichoderma harzianum transformants that overexpress a 33-kDa chitinase. Phytopathology 89: 254–261 [DOI] [PubMed] [Google Scholar]
- Lorito M, Woo SL, Garcia I, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zoina A, et al (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95: 7860–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Song F, Zheng Z (2005) Overexpression in transgenic tobacco reveals different roles for the rice homeodomain gene OsBIHD1 in biotic and abiotic stress responses. J Exp Bot 56: 2673–2682 [DOI] [PubMed] [Google Scholar]
- Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD (1993) Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262: 1432–1436 [DOI] [PubMed] [Google Scholar]
- Mittler R, Rizhsky L (2000) Transgene-induced lesion mimic. Plant Mol Biol 44: 335–344 [DOI] [PubMed] [Google Scholar]
- Molina A, Garcia-Olmedo F (1997) Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2. Plant J 12: 669–675 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53: 1237–1247 [PubMed] [Google Scholar]
- Nishizawa Y, Saruta M, Nakazono K, Nishio Z, Soma M, Yoshida T, Nakajima E, Hibi T (2003) Characterization of transgenic rice plants over-expressing the stress-inducible beta-glucanase gene Gns1. Plant Mol Biol 51: 143–152 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Park CY, Heo WD, Yoo JH, Lee JH, Kim MC, Chun HJ, Moon BC, Kim IH, Park HC, Choi MS, et al (2004) Pathogenesis-related gene expression by specific calmodulin isoforms is dependent on NIM1, a key regulator of systemic acquired resistance. Mol Cells 18: 207–213 [PubMed] [Google Scholar]
- Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13: 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao HL, Lim JH, Kim SJ, Cheong GW, Hwang I (2001) Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J 27: 305–314 [DOI] [PubMed] [Google Scholar]
- Polle A, Otter T, Siefert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol 106: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53: 1351–1365 [PubMed] [Google Scholar]
- Schutzendubel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21: 81–85 [DOI] [PubMed] [Google Scholar]
- Shirasu K, Dixon RA, Lamb C (1996) Signal transduction in plant immunity. Curr Opin Immunol 8: 3–7 [DOI] [PubMed] [Google Scholar]
- Singh K, Foley RC, Onate-Sanchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5: 430–436 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Isobe M, Muto S (1997) An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett 401: 202–206 [DOI] [PubMed] [Google Scholar]
- Thomma BP, Penninckx IA, Broekaert WF, Cammue BP (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- Tornero P, Conejero V, Vera P (1994) A gene encoding a novel isoform of the PR-1 protein family from tomato is induced upon viroid infection. Mol Gen Genet 243: 47–53 [DOI] [PubMed] [Google Scholar]
- Torres-Schumann S, Godoy JA, Pintor-Toro JA (1992) A probable lipid transfer protein gene is induced by NaCl in stems of tomato plants. Plant Mol Biol 18: 749–757 [DOI] [PubMed] [Google Scholar]
- van Hengel AJ, Tadesse Z, Immerzeel P, Schols H, van Kammen A, de Vries SC (2001) N-acetylglucosamine and glucosamine-containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol 125: 1880–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322: 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15: 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25: 131–139 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kaothien P, Matsui T, Kawaoka A, Shinmyo A (2003) Molecular biology and application of plant peroxidase genes. Appl Microbiol Biotechnol 60: 665–670 [DOI] [PubMed] [Google Scholar]
- Young SA, Guo A, Guikema JA, White FF, Leach JE (1995) Rice cationic peroxidase accumulates in xylem vessels during incompatible interactions with Xanthomonas oryzae pv oryzae. Plant Physiol 107: 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y (2001) Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 13: 1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4: 401–406 [DOI] [PubMed] [Google Scholar]