Abstract
Ascorbic acid (Asc) is a major antioxidant in plants that detoxifies reactive oxygen species (ROS) and maintains photosynthetic function. Expression of dehydroascorbate reductase (DHAR), responsible for regenerating Asc from an oxidized state, regulates the cellular Asc redox state, which in turn affects cell responsiveness and tolerance to environmental ROS. Because of its role in Asc recycling, we examined whether DHAR is important for plant growth. Suppression of DHAR expression resulted in a preferential loss of chlorophyll a, a lower steady state of Rubisco as measured by the amount of the large subunit of Rubisco (RbcL), and a lower rate of CO2 assimilation. As a consequence, a slower rate of leaf expansion and reduced foliar dry weight were observed. In addition, an accelerated rate of loss of chlorophyll, RbcL, light-harvesting complex II, and photosynthetic functioning was observed in mature leaves, resulting in premature leaf aging. Reduced growth rate as measured by plant height and leaf number was consistent with the DHAR-mediated reduction of photosynthetic function. Increasing DHAR expression maintained higher levels of chlorophyll, RbcL, light-harvesting complex II, and photosynthetic functioning, resulting in delayed leaf aging. The effect of DHAR expression on leaf aging inversely correlated with the level of lipid peroxidation, indicating that DHAR functions to protect against ROS-mediated damage. These observations support the conclusion that through its Asc recycling function, DHAR affects the level of foliar ROS and photosynthetic activity during leaf development and as a consequence, influences the rate of plant growth and leaf aging.
Ascorbic acid (Asc) is a major antioxidant that serves many functions in plants. Asc is involved in the detoxification of reactive oxygen species (ROS), e.g. superoxide, singlet oxygen, ozone, and hydrogen peroxide (H2O2), which are produced during aerobic metabolic processes such as photosynthesis or respiration (Asada and Takahashi, 1987). Asc also participates in the regeneration of α-tocopherol (vitamin E) from the tocopheroxyl radical (Asada, 1994). In addition, Asc functions as a cofactor for enzymes such as prolyl and lysyl hydrolases, violaxanthin deepoxidase, and ethylene-forming enzyme (Davies et al., 1991; McGarvey and Christoffersen, 1992; Smith et al., 1992; Eskling et al., 1997) as well as for 2-oxoacid-dependent dioxygenases required for the synthesis of abscisic acid (ABA) and gibberellic acid (Arrigoni and De Tullio, 2000, 2002; Smirnoff, 2000). Asc is involved in the regulation of cell elongation and progression through the cell cycle (for review, see Smirnoff, 1996; Horemans et al., 2000).
Once used, Asc is oxidized to the monodehydroascorbate (MDHA) radical that can be reduced to Asc in the chloroplast or cytosol by MDHA reductase (MDHAR) in an NAD(P)H-dependent reaction (Asada, 1999). In the chloroplast, the MDHA radical can also be reduced to Asc by thylakoid-associated, reduced ferredoxin that is more effective than reduction by MDHAR (Miyake and Asada, 1994; Asada, 1999). MDHA produced in the thylakoid lumen by violaxanthin deepoxidase or following donation of electrons from Asc to PSII or PSI (Mano et al., 1997; Mano, 1999), however, is not available as a substrate for reduction by ferredoxin or MDHAR but rapidly disproportionates to Asc and dehydroascorbate (DHA) when the pH of the lumen is low (Asada, 1999). DHA is then reduced to Asc by DHA reductase (DHAR) in a reaction requiring glutathione. Because the apoplast contains little glutathione or DHAR, DHA, which predominates in the apoplast, must reenter the cell for reduction to Asc. In the absence of sufficient DHAR, however, DHA undergoes irreversible hydrolysis to 2,3-diketogulonic acid.
Given that Asc is present in most cellular compartments and that several pathways exist to ensure that Asc is recycled, it might be expected that perturbations in one recycling pathway would be offset by the activity of the remaining pathways to maintain the cellular Asc redox state. However, changes in DHAR expression result in substantial alterations in the cytosolic and apoplastic Asc redox state; overexpression of DHAR in tobacco (Nicotiana tabacum) leaves increased the Asc redox state (i.e. was more reduced), whereas suppression of DHAR had the opposite effect (Chen et al., 2003; Chen and Gallie, 2004, 2005). These observations suggest that DHAR is expressed in rate-limiting amounts and that DHAR contributes significantly to establishing the cellular Asc redox state, at least in leaves.
H2O2 functions as a signaling intermediate downstream of ABA to promote stomatal closure (Price et al., 1994, 2000; Murata et al., 2001; Schroeder et al., 2001a, 2001b; Zhang et al., 2001). Guard cells of plants expressing DHAR exhibit a higher (i.e. more reduced) Asc redox state, reduced levels of H2O2, decreased responsiveness to H2O2 or ABA signaling, and greater stomatal opening (Chen and Gallie, 2004). Suppression of DHAR expression results in higher levels of H2O2 in guard cells and enhanced stomatal closure under normal growth conditions or following water stress. Thus, the level of DHAR expression is important for appropriate signaling in guard cells, because it establishes the efficiency of H2O2 scavenging. Increasing DHAR expression also provided enhanced tolerance to environmental ROS, e.g. ozone, despite the increase in stomatal conductance (Chen and Gallie, 2004).
One means by which Asc reduces photoinhibition is by promoting conversion of violaxanthin to zeaxanthin in the xanthophyll cycle to dissipate excess excitation energy as part of nonphotochemical quenching (NPQ). The importance of the Asc pool size in supporting growth was shown with the Arabidopsis (Arabidopsis thaliana) vitamin C1 (vtc1) mutant that is defective in GDP-Man pyrophosphorylase and accumulates only 25% to 30% of the wild-type level without altering the Asc redox state (Conklin et al., 1996, 1999; Veljovic-Jovanovic et al., 2001). In addition to being hypersensitive to ozone, sulfur dioxide, or UVB light, vtc1 plants exhibit slower shoot growth, smaller leaves, and reduced shoot fresh weight and dry weight (Veljovic-Jovanovic et al., 2001). vtc1 plants exhibited no significant difference in the light saturation curves for CO2 assimilation or chlorophyll fluorescence under these growth conditions, suggesting that the effect on growth was not due to decreased photochemical efficiency or reduced photosynthetic capacity (Veljovic-Jovanovic et al., 2001). No change in the amount of H2O2 and only a slight reduction in NPQ were observed in vtc1 leaves (Veljovic-Jovanovic et al., 2001). Expression of cytosolic ascorbate peroxidase (APX) increased and that of chloroplast APX isoforms was either unchanged or slightly decreased, suggesting that the level of Asc is involved in the regulation of the compartmentalization of the antioxidant system in Arabidopsis (Veljovic-Jovanovic et al., 2001). The vtc2 mutant has only 10% to 30% of wild-type levels of Asc and is slightly deficient in the feedback deexcitation component of NPQ that causes the dissipation of excess light as heat (Muller-Moule et al., 2003). The vtc2 mutant experiences chronic photooxidative stress in high light and photobleaches when transferred from low to high light accompanied by increased lipid peroxidation and photoinhibition (Muller-Moule et al., 2003, 2004).
Because changes in DHAR expression result in substantial changes in the Asc redox state not seen in the vtc mutants, we examined whether the efficiency of Asc recycling as determined by DHAR influenced plant growth and leaf function. Plants suppressed in DHAR expression exhibited a slower rate of leaf expansion, slower shoot growth, delayed flowering time, and reduced foliar dry weight. These phenotypes correlated with reduced leaf function as measured by the preferential loss of chlorophyll a, a reduced level of Rubisco large subunit (RbcL), and a lower rate of CO2 assimilation in mature leaves. The effect of DHAR expression on leaf aging inversely correlated with the level of lipid peroxidation, indicating that the efficiency of Asc recycling was important in regulating ROS-mediated damage. These results suggest that DHAR contributes to plant growth by maintaining photosynthetic functioning through efficient Asc recycling that limits ROS-mediated damage that slows leaf aging.
RESULTS
Chlorophyll Pool Size, CO2 Assimilation, and Plant Growth Rate Correlate with the Level of Foliar DHAR Activity
To investigate how the level of DHAR activity correlates with leaf function at the whole plant level, DHAR activity and protein levels were measured in every second leaf of mature tobacco plants just prior to flowering. DHAR activity was highest in the youngest leaves and declined with leaf age (Fig. 1A). DHAR protein levels also were highest in the youngest leaves and declined with leaf age, although the decline in DHAR protein was not as great as the decline in DHAR activity, suggesting that DHAR activity may be posttranslationally regulated. The changes in DHAR activity largely correlated with the change in the chlorophyll a and b pool sizes (Fig. 1B) as well as the rate of CO2 assimilation (Fig. 1C). The only exception to this was that DHAR activity was near maximum in the youngest leaves when the chlorophyll a and b pool size and the rate of CO2 assimilation were not yet at maximum.
Figure 1.
DHAR activity, chlorophyll pool size, and rate of CO2 assimilation during leaf development. A, DHAR activity was measured from every second leaf of adult tobacco just prior to flowering. DHAR protein levels were measured by western analysis. B, Chlorophyll a (♦) and b (○) levels and rate of CO2 assimilation (C) were determined from every second leaf of adult tobacco. Leaf 2 was the youngest leaf and leaf 20 the oldest leaf tested.
In its antioxidant function, Asc acts to maintain photosynthetic function through the detoxification of ROS as well as to maintain electron flow through PSI and PSII (Asada, 1999). Because DHAR contributes substantially to the regulation of the Asc redox state (Chen et al., 2003; Chen and Gallie, 2004), we examined whether alterations in DHAR expression affected components of the photosynthetic machinery or photosynthetic activity. To investigate this possibility, we used DHAR-overexpressing (DOX) or DHAR-suppressed (DKD) tobacco that have been described previously (Chen et al., 2003; Chen and Gallie, 2004). DOX lines were generated following the introduction of a wheat (Triticum aestivum) DHAR (DHARTa) cDNA under the control of the cauliflower mosaic virus (CaMV) 35S promoter. A wheat DHAR cDNA was used because introduction of a tobacco DHAR cDNA under the control of the CaMV 35S promoter resulted only in suppression of endogenous tobacco DHAR expression. Although the nucleotide sequence of the wheat DHAR cDNA has diverged enough that it did not silence endogenous tobacco DHAR expression, at the protein level, wheat DHAR shares 73% identity and 85% similarity with the tobacco ortholog. Therefore, expression of wheat DHAR was used to generate tobacco with increased DHAR activity (i.e. the aggregate of wheat DHAR activity and endogenous tobacco DHAR), whereas expression from the tobacco DHAR transgene was used to generate tobacco with reduced DHAR activity. In each case, it is the level of total DHAR activity present within a line that is correlated with changes in leaf growth and function. The wheat and tobacco DHAR genes used encode a cytosolic DHAR. Of the nine independent DOX lines initially characterized (three of which have been previously described; Chen et al., 2003) and six DKD lines (one of which has been previously described; Chen and Gallie, 2004), a reduction in DHAR activity correlated with reduced chlorophyll content and rate of growth, whereas an increase in DHAR activity correlated with higher chlorophyll content (data not shown). Detailed growth measurements were then performed on one representative DOX and DKD line.
To examine leaf function and growth at different leaf ages, expanding, mature, and presenescent leaves of DOX, DKD, and control plants were used for the analysis. An expanding leaf is defined as one that has achieved approximately 50% of its final size but has not reached a maximum rate of CO2 assimilation (e.g. leaf 4, Fig. 1C). A mature leaf is defined as a fully expanded leaf that exhibits a maximum rate of CO2 assimilation (e.g. leaf 6, Fig. 1C). Thus, each leaf type collected from the three lines examined was defined by its developmental stage, in the same position with regard to the apical leaf whorl, and of similar chronological age. Presenescent leaves exhibited reduced rates of CO2 assimilation relative to the maximum exhibited by a mature leaf (e.g. leaf 18, Fig. 1C) but still contained chlorophyll, albeit at significantly reduced levels. Presenescent leaves were defined according to their position (numbering from the plant base) so that leaves of equivalent chronological age could be collected from all lines to permit a comparison of various parameters of leaf aging, e.g. chlorophyll content, level of Rubisco or light-harvesting chlorophyll-protein complex, lipid oxidation, and leaf dry weight. All measurements were made at the same time each day during the course of an experiment to avoid any possible diurnal effects.
Overexpression of wheat DHAR resulted in a substantial increase in DHAR activity in expanding, mature, and presenescent leaves (Fig. 2A). The level of endogenous DHAR protein (DHARNt) remained unaffected in expanding and mature leaves of DOX plants as quantitated from the western analysis but was 77% higher in presenescent leaves than in control leaves (Fig. 2B). This is consistent with the prolonged maintenance of leaf function in DOX plants (see below). In contrast, DKD tobacco exhibited substantially reduced levels of DHAR activity in leaves at the same developmental stages (Fig. 2A). The level of endogenous DHARNt in expanding, mature, and presenescent leaves of DKD plants was reduced to 47%, 35%, and 44%, respectively, of the level in the corresponding leaves of control plants (Fig. 2B), data that are in good agreement with the reduction in DHAR activity (Fig. 2A). Although the level of DHAR activity declined with leaf age in DOX, DKD, and control plants, the level of activity was always higher in DOX leaves and lower in DKD leaves relative to the control (Fig. 2A).
Figure 2.
DHAR activity correlates with ROS-mediated damage and the level of RbcL and LHCII. A, The level of DHAR activity, lipid peroxidation (as measured by the TBARS assay), and total APX was measured in expanding, mature, and presenescent leaves of control (C), DOX, and DKD tobacco. The average and sd from three replicates are reported. B, The level of DHAR (DHARNt, DHARTa), RbcL, and LHCII were measured by western analysis. C, RT-PCR analysis of CP1 expression (top) in mature leaves, presenescent leaves, senescent stage 1 leaves (approximately 2 weeks older than presenescent leaves), and senescent stage 2 leaves (approximately 4 weeks older than presenescent leaves) of control, DOX, and DKD plants. RT-PCR analysis of actin expression (bottom) was also performed as a control using the same leaf samples.
The level of lipid peroxidation, as measured by thiobarbituric acid reactive substances (TBARS) assay, increased with leaf age; however, it was consistently higher in mature and presenescent leaves of DKD plants than in the corresponding leaves of DOX plants (P < 0.05 and P < 0.005; n = 3, respectively) when grown in moderate light (approximately 500 μmol m−2 s−1; Fig. 2A). Lipid peroxidation in presenescent DKD leaves was also significantly higher than in the corresponding leaves of control plants (P < 0.05; n = 3), indicating that the extent of lipid peroxidation may be affected by the level of foliar DHAR activity. Although the level of lipid peroxidation was consistently lower in DOX leaves relative to the control, the difference was not significant. No significant difference in lipid peroxidation was observed in expanding leaves among the three lines, suggesting that the differences in lipid peroxidation accumulated over time. Although total APX activity declined with leaf age in DOX, DKD, and control plants, no consistent change in its activity with respect to DHAR activity was observed, in good agreement with previous results (Chen et al., 2003). The level of Rubisco, as measured by the amount of RbcL and the level of light-harvesting complex II (LHCII) declined with leaf age, but their relative abundance in expanding or mature leaves of DOX, DKD, and control plants was not substantially different. In presenescent leaves, however, the level of RbcL and LHCII in DKD plants was reduced to 64% and 38%, respectively, of the level present in the corresponding leaves of control plants, and the level of LHCII in DOX plants was 29% higher than in the control (Fig. 2B). The reduction in the amount of LHCII in DKD plants was not observed in vtc2 mutant plants (Muller-Moule et al., 2004). The correlation between DHAR activity and the abundance of RbcL and LHCII suggests that the level of DHAR activity may be important in maintaining these proteins during leaf aging.
To examine whether changes in the level of DHAR activity influenced the induction of a senescence-related gene, expression of tobacco CP1, the ortholog of the senescence-associated gene SAG12 of Arabidopsis, was examined in DOX, DKD, and control plants using reverse transcription (RT)-PCR. Little to no CP1 expression was detected in mature or presenescent leaves from DOX, DKD, and control plants (Fig. 2C), supporting the conclusion that the presenescent leaves used in this study had not initiated senescence. CP1 expression was detected in senescent stage 1 DKD leaves, which were approximately 2 weeks older than the presenescent leaves used in this study (Fig. 2C). No CP1 expression was detected, however, in senescent stage 1 leaves of DOX or control plants. CP1 expression was also detected in senescent stage 2 DKD leaves (approximately 4 weeks older than the presenescent leaves used in this study), whereas expression was just detectable in senescent stage 2 DOX or control leaves (Fig. 2C). These data suggest that a reduction in the level of DHAR activity accelerates the onset of senescence.
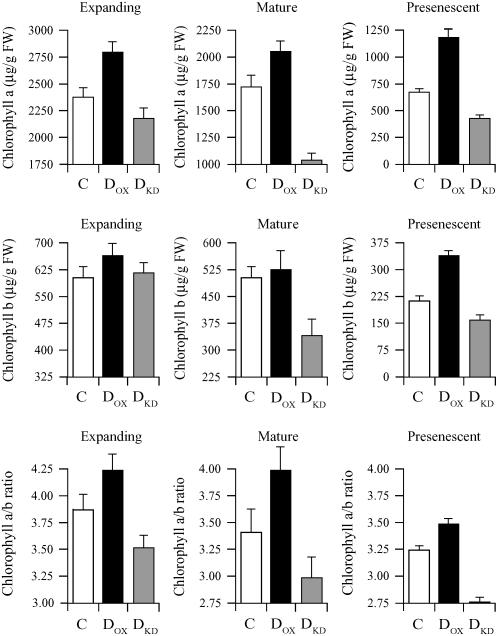
To determine whether the level of DHAR activity may affect the pool size of chlorophyll a and b, chlorophyll levels were measured in expanding, mature, or presenescent leaves of DOX, DKD, and control plants. The level of chlorophyll a was significantly higher in expanding leaves of DOX plants than in control plants (P < 0.005; n = 4) as it was in mature or presenescent leaves (P < 0.05 and P < 0.005; n = 4, respectively; Fig. 3), whereas it was significantly lower in DKD mature and presenescent leaves (P < 0.005 and P < 0.005; n = 4, respectively) but not in expanding DKD leaves (P = 0.085; n = 4) relative to the control. However, the level of chlorophyll a was significantly lower in expanding DKD leaves relative to expanding DOX leaves (P < 0.005; n = 4). Although a small increase in the level of chlorophyll b was observed in expanding DOX leaves relative to the control, this difference was only significant in presenescent leaves (P < 0.005; n = 4; Fig. 3). No significant difference in the level of chlorophyll b was observed in expanding DKD leaves relative to the control (P = 0.797; n = 4), but its level was significantly reduced in mature or presenescent leaves of DKD plants relative to the control (P < 0.05 and P < 0.05; n = 4, respectively). The chlorophyll a to b ratio was significantly lower in expanding, mature, and presenescent leaves of DKD plants than in DOX leaves (P < 0.05, P < 0.05, and P < 0.005; n = 4, respectively). The chlorophyll a to b ratio was also significantly lower in presenescent DKD leaves than in control leaves (P < 0.01; n = 4; Fig. 3).
Figure 3.
DHAR activity correlates with the chlorophyll pool size and chlorophyll a/b ratio. Chlorophyll a, chlorophyll b, and the chlorophyll a/b ratio were measured in expanding, mature, and presenescent leaves of control (C), DOX, and DKD tobacco. The average and sd from four replicates are reported.
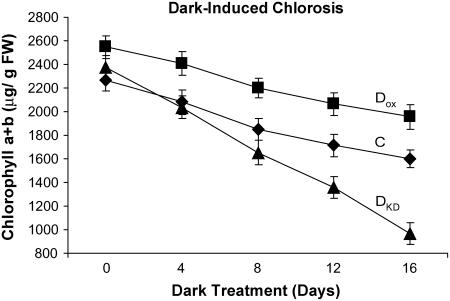
The chlorophyll pool size is determined by rates of its synthesis and degradation. To investigate whether the level of DHAR activity influences the rate of chlorophyll loss, young leaves from DOX, DKD, and control plants were dark treated, and the rate of chlorophyll loss was followed over time. Although the chlorophyll pool size in DOX leaves was larger than in control leaves, the rate of chlorophyll loss from DOX leaves (i.e. loss of 38.1 μg/g fresh weight/d) was only slightly lower than that from control leaves (i.e. loss of 42.4 μg/g fresh weight/d; Fig. 4). The rate of chlorophyll loss from DKD leaves was more than 2-fold greater (i.e. loss of 87.1 μg/g fresh weight/d) than that from control leaves despite the use of DKD leaves with an initial chlorophyll pool size similar to control leaves.
Figure 4.
Suppression of DHAR expression accelerates loss of chlorophyll. Chlorophyll (a + b) was measured every 4 d from dark-treated leaves of control (C, ♦), DOX (▪), and DKD tobacco (▴). The average and sd from three replicates are reported.
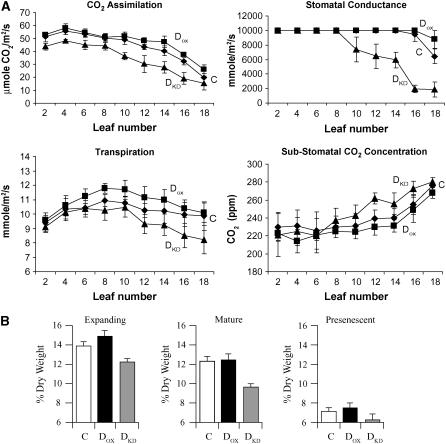
To examine whether the observed differences among DOX, DKD, and control leaves correlated with differences in CO2 assimilation, gas exchange was measured from every second leaf. The rate of CO2 assimilation in expanding and mature leaves of DOX plants was similar to those of control plants despite a higher rate of transpiration (Fig. 5A). The rate of stomatal conductance was too high to measure differences in these same leaves between DOX and control plants. In older, fully expanded leaves as well as in presenescent leaves, a higher rate of CO2 assimilation was observed in DOX plants. Although this correlated with a higher rate of transpiration, a lower substomatal CO2 concentration was also observed, suggesting a higher rate of CO2 assimilation (Fig. 5A). The rate of CO2 assimilation was consistently lower in all leaves of DKD plants relative to the control (Fig. 5A). In mature and presenescent leaves, this correlated with reduced rates of transpiration and stomatal conductance previously reported for DKD plants (Chen and Gallie, 2004), but the higher level of substomatal CO2 concentration in these DKD leaves indicated that the internal concentration of CO2 was not limiting and thus suggested that DKD leaves were less efficient in assimilating CO2. In young DKD leaves, the rate of transpiration was similar to that of control leaves, and therefore the observed reduced rate of CO2 assimilation in the young DKD leaves supported the conclusion of less efficient CO2 assimilation. These data suggest that the reduced rate of CO2 assimilation in young DKD leaves is likely not a consequence of changes in the level of RbcL or LHCII but do correlate with changes in the chlorophyll pool size and chlorophyll a/b ratio. The reduced rate of CO2 assimilation in older DKD leaves and higher rate of CO2 assimilation in older DOX leaves correlate with similar changes in the levels of RbcL, LHCII, and chlorophyll pool size as well as the chlorophyll a/b ratio (Figs. 2 and 3).
Figure 5.
DHAR activity affects the efficiency of CO2 assimilation and leaf dry weight. A, The rates of CO2 assimilation, transpiration, and stomatal conductance, and the substomatal CO2 concentration were measured from every second leaf of control (C, ♦), DOX (▪), and DKD tobacco (▴). Leaf 2 was the youngest leaf and leaf 18 the oldest leaf tested. Expanding leaves include leaves 2 to 4, mature leaves include leaves 6 to 8, and presenescent leaves include leaves 16 to 18. B, The dry weight as measured as percent of fresh weight was determined for expanding, mature, and presenescent leaves. The average and sd from three replicates are reported.
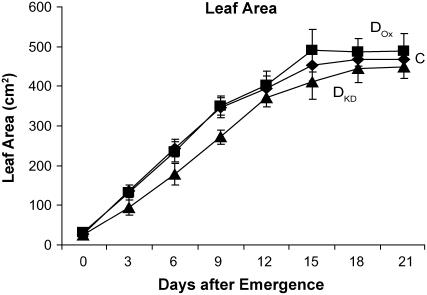
The reduced rate of CO2 assimilation in DKD leaves correlated with a reduced leaf dry weight of expanding and mature leaves relative to the control (P < 0.005 and P < 0.01; n = 3, respectively), although the difference was not significant in presenescent leaves (P = 0.11; n = 3; Fig. 5B). Leaf dry weight in expanding, mature, and even presenescent DKD leaves was significantly lower than DOX leaves (P < 0.01, P < 0.05, and P < 0.05; n = 3, respectively; Fig. 5B). No significant difference in leaf dry weight was observed between expanding, mature, or presenescent leaves of DOX and control plants (P = 0.19, P = 0.83, and P = 0.08; n = 3, respectively; Fig. 5B), correlating with their similar rates of CO2 assimilation during leaf growth (Fig. 5A). To examine whether the differences in CO2 assimilation observed in DOX and DKD plants are accompanied by other changes in growth, parameters of plant growth were measured. Plant growth as measured by height over time was little altered in DOX plants relative to the control, and no change in leaf number was observed (Fig. 6). DOX plants flowered at the same time as control plants (Table I). DKD plants exhibited a significantly reduced rate of growth (P < 0.005 at weeks 5–7; n = 8) but caught up during late growth (P = 0.130 and P = 0.161 at weeks 8 and 9, respectively; n = 8). DKD plants had significantly fewer leaves during growth (P = 0.05, P < 0.005, and P < 0.05 at weeks 5, 6, and 7, respectively; n = 8) but eventually produced the same number of leaves as the control (Fig. 6). Consistent with this, DKD plants flowered significantly later (Table I) than control plants (P = 0.001; n = 8). The average leaf internode distance of fully grown DKD plants was slightly less than that of control plants, although the difference was not significant (P = 0.9; n = 18). Although no significant difference in leaf area was observed between DOX and control plants, leaves from DKD plants were consistently smaller in size relative to the control (P = 0.0014; Fig. 6). These data indicate that the growth rate but not final plant height is reduced in DKD tobacco, suggesting that efficiency of Asc recycling is important in maintaining maximum growth. Because increasing DHAR expression did not increase plant growth, the endogenous level of DHAR activity appears to be sufficient to support a maximum growth rate.
Figure 6.
The level of DHAR activity affects the rate of plant growth. Plant height and leaf number were measured in control (C, ♦), DOX (▪), and DKD tobacco (▴) beginning at 4 weeks after germination until the appearance of the inflorescence. Leaf area was measured following the vegetative to floral transition when all leaves had reached their final size. Leaf 1 was the youngest leaf and leaf 9 was the oldest leaf measured. The average and sd from eight replicates are reported.
Table I.
DHAR is required for correct internode length, leaf growth, and flowering time
| Internode Length | Leaf Expansiona | Flowering Time | |
|---|---|---|---|
| cm | cm2/d | d | |
| Control | 9.32 ± 0.68 | 36.5 | 60.25 ± 1.09 |
| DOX | 9.42 ± 0.70 | 36.6 | 60.13 ± 1.27 |
| DKD | 8.89 ± 0.74 | 29.7 | 64.38 ± 2.18 |
Expansion from the sixth true leaf was measured.
The Level of DHAR Activity Correlates with the Maintenance of Chlorophyll a and Leaf Growth Rate
The previous analyses provided insight into how DHAR activity affects growth at the level of the whole plant. However, it is not valid to assume that expanding, mature, and presenescent leaves from a single plant are representative of the developmental stages that a specific leaf undergoes during its development, as not all leaves on a given plant are biochemically or functionally equivalent (e.g. leaf size, chlorophyll content). Therefore, to investigate how DHAR activity affects growth of a specific leaf, we followed the growth of the sixth true leaf (as numbered from the plant base) in DOX, DKD, and control plants. We first measured the area of the sixth true leaf when the leaf was fully expanded, a value that remained constant for a given line when the plants were grown under identical conditions. From the area of the sixth true leaf in its fully expanded state, sixth true leaves could be analyzed during their growth when they were 8%, 50%, or 100% of the fully expanded size. DHAR activity and protein level on a fresh weight basis declined during leaf expansion in control plants as it did in DOX and DKD plants (Fig. 7, A and B). Correlating with the decline in DHAR was a decrease in the Asc pool size and redox state (i.e. more oxidized) during the expansion of control leaves (Fig. 7A). The decrease in protein and Asc pool size may be explained in part by the dilution during the cell expansion that occurs during leaf growth. In contrast, dilution does not account for the oxidation of the Asc redox state. As reported previously (Chen and Gallie, 2004), increasing DHAR expression in DOX plants results in an increase in the Asc pool size and redox state, both of which decline during leaf expansion although always remaining higher than control leaves (Fig. 7A). Suppressing DHAR expression in DKD plants results in higher levels of DHA (Chen and Gallie, 2004), consistent with reduced Asc recycling, although the total ascorbate pool size (i.e. Asc + DHA) shows slight to no reduction depending on leaf age (Fig. 7A). As with control and DOX leaves, the Asc pool size and redox state in DKD plants declined with leaf expansion and were consistently lower than in control leaves. The relative level of RbcL was not substantially different between DOX and control plants during leaf expansion. The relative level of RbcL was also not substantially different between DKD and control plants when leaves were 8% of their fully expanded size, but when DKD leaves reached 50% or 100% of their fully expanded size, the level of RbcL was reduced to 87% and 64% of that present in control leaves (Fig. 7B). No consistent change in the relative level of LHCII was observed in DOX, DKD, and control leaves during their expansion (Fig. 7B).
Figure 7.
The level of DHAR activity correlates with the foliar Asc redox state and level of RbcL and LHCII during leaf expansion. A, The level of DHAR activity, Asc (black bars), DHA (white bars), and the Asc redox state (Asc/DHA) were measured in the sixth true leaf during its growth from 8%, 50%, and 100% of fully expanded size of control (C), DOX, and DKD tobacco. The average and sd from three replicates are reported. B, The level of DHAR, RbcL, and LHCII were measured in the same leaves as in A by western analysis.
The pool size of chlorophyll a and b in control leaves declined concomitantly during leaf expansion such that the chlorophyll a/b ratio was largely unaltered (Fig. 8A). Although the pool size of chlorophyll a in DKD leaves was not significantly different from control leaves when the leaves were 8% of their fully expanded state (P = 0.437; n = 4), it was significantly lower than the control when the leaves reached 50% or 100% of the fully expanded state (P < 0.05 and P < 0.05; n = 4; Fig. 8A). In contrast, the pool size of chlorophyll a in DOX leaves was significantly higher than in control leaves at 8%, 50%, and 100% of the fully expanded state (P < 0.05, P < 0.05, and P < 0.01, respectively; n = 4), although the pool size of chlorophyll b was not significantly different from the control (Fig. 8A). Thus, although the chlorophyll a/b ratio in DOX and DKD leaves was not significantly different when the leaves were 8% of their fully expanded state (P = 0.189; n = 4), it was significantly lower in DKD leaves when the leaves reached 50% or 100% of the fully expanded state (P < 0.05 and P < 0.01; n = 4; Fig. 8A). These data suggest that increasing DHAR activity increases the pool size of chlorophyll a from early leaf development up to senescence, whereas decreasing DHAR activity does not affect the initial pool size of chlorophyll a but does result in a preferential loss of chlorophyll a during subsequent leaf expansion and aging.
Figure 8.
The level of DHAR activity correlates with the chlorophyll a pool size, the chlorophyll a/b ratio, and leaf dry weight during leaf expansion. A, Chlorophyll a, chlorophyll b, and the chlorophyll a/b ratio were measured in the sixth true leaf during its growth from 8%, 50%, and 100% of fully expanded size of control (C), DOX, and DKD tobacco. B, The dry weight as measured as the percent of fresh weight was determined at the same stages of leaf growth as in A. The average and sd from four replicates are reported.
To examine how alterations in the efficiency of Asc recycling resulting from changes in DHAR expression affected CO2 assimilation as a leaf ages, gas exchange was measured in expanding leaves every 3 d. The rate of CO2 assimilation in DOX leaves was similar to control leaves during leaf expansion but declined at a slower rate as the leaf aged, correlating with a higher stomatal conductance (Fig. 9). The rate of CO2 assimilation was consistently lower in DKD leaves throughout its expansion and subsequent aging. The reduced level of CO2 assimilation in DKD leaves could not be explained by a corresponding reduction in stomatal conductance but did correlate with a higher level of substomatal CO2 concentration (Fig. 9), suggesting less efficient assimilation of CO2.
Figure 9.
The level of DHAR activity affects the efficiency of CO2 assimilation during leaf expansion. The rates of CO2 assimilation, transpiration, and stomatal conductance, and the substomatal CO2 concentration were measured in the sixth true leaf during its growth from control (C, ♦), DOX (▪), and DKD tobacco (▴). Measurements were taken every third day, starting when the leaves were 8% of fully expanded size until the leaves were 100% of fully expanded size (approximately day 9) and continued for an additional 9 d. The average and sd from three replicates are reported.
The rate of leaf expansion was followed for the same cohort of leaves and revealed that DOX and control leaves expanded at the same rate (P = 0.91; n = 8), whereas the expansion of DKD leaves was significantly delayed relative to the control (P = 0.028; n = 8; Fig. 10; Table I). The reduced rate of CO2 assimilation and slower rate of expansion of DKD leaves correlated with a reduced leaf dry weight throughout leaf expansion (Fig. 8B) that was similar to the reduced leaf dry weight observed for all leaves of adult plants (Fig. 5B). No significant difference in leaf dry weight during leaf expansion was observed between DOX and control leaves (Fig. 8B), correlating with their similar rates of CO2 assimilation during early leaf growth (Fig. 9).
Figure 10.
The level of DHAR activity affects the rate of leaf growth. Leaf area was measured for the sixth true leaf during its growth in control (C, ♦), DOX (▪), and DKD tobacco (▴) every 3 d beginning at leaf emergence. The average and sd from eight replicates are reported.
DISCUSSION
In this study, we present evidence suggesting that the level of foliar DHAR activity influences the rate of leaf aging, and, as a consequence, the rate of plant growth. We were able to take advantage of the fact that DHAR is expressed in limiting amounts with regard to the Asc redox state (Chen et al., 2003; Chen and Gallie, 2004) to test whether alterations in DHAR expression would perturb plant growth. Suppressing DHAR expression resulted in less efficient Asc recycling and as a consequence, a lower (i.e. more oxidized) Asc redox state. The effect that suppressing DHAR expression had on Asc recycling increased with leaf age and correlated with an increase in ROS (Chen and Gallie, 2004), lipid peroxidation, and slower leaf and plant growth. This reduced growth rate correlated with a smaller chlorophyll pool size and a lower chlorophyll a/b ratio resulting from the preferential loss of chlorophyll a during leaf expansion. The lower chlorophyll a/b ratio correlated with a reduced rate of CO2 assimilation in all leaves. Although reducing DHAR expression has been shown to reduce stomatal conductance, which might be expected to limit CO2 diffusion into the leaf interior, a reduced rate of CO2 assimilation was also observed in expanding and newly expanded leaves in which stomatal conductance was not substantially affected. Moreover, despite the lower stomatal conductance in older DKD leaves, the substomatal CO2 concentration was higher than it was in control leaves, indicating that CO2 diffusion into the leaf interior was not limited but rather CO2 was not being used efficiently. The preferential loss of chlorophyll a in DKD leaves may have been a consequence of the higher level of ROS present and may account for the reduced photosynthetic activity observed in DKD leaves. In addition to the lower chlorophyll a/b ratio in older DKD leaves, the reduced rate of CO2 assimilation correlated with reduced levels of RbcL and LHCII. Although it is not known which of these may be rate limiting in older leaves, these data suggest that the efficiency of Asc recycling as determined by the level of DHAR activity plays an important role in leaf aging. The reduced rate of leaf expansion observed in DKD plants is likely a consequence of the accelerated loss of photosynthetic activity in mature leaves that may have prematurely reduced their photosynthate that they could provide to sink tissues. This would also explain the slower plant growth rate as measured by plant height and rate of leaf production over time. Changes in the level of DHAR expression affect the Asc pool size and redox state within the cytosol and apoplast (Chen and Gallie, 2005), and as Asc is readily transported throughout the cell (Horemans et al., 2000), changes in chloroplastic Asc would also be expected. Whether changes in chloroplastic or cytosolic Asc are responsible for the observed effects on leaf aging remain to be determined.
The observation that increasing DHAR expression correlated with higher levels of RbcL and chlorophyll and a higher rate of CO2 assimilation in presenescent leaves, whereas reducing DHAR expression in the same leaves correlated with a lower level of RbcL and LHCII, a smaller pool size of chlorophyll, and reduced rate of CO2 assimilation indicates that the level of foliar DHAR activity can affect the rate of loss of leaf function. The progressive loss of DHAR as a function of leaf age, correlating with a loss of chlorophyll and CO2 assimilation rate, is consistent with a potential role for this enzyme in influencing the rate of leaf aging. The fact that reducing DHAR expression correlated with slower growth is also consistent with a role of DHAR in influencing leaf aging. The observation that increasing the level of DHAR activity correlated with reduced lipid peroxidation whereas reducing DHAR activity correlated with increased lipid peroxidation particularly during leaf aging is consistent with previous observations demonstrating an inverse relationship between the level of DHAR expression and the foliar level of ROS (Chen and Gallie, 2004). It also suggests that the level of DHAR activity may affect leaf function and aging by regulating the level of ROS and, as a consequence, affect the amount of ROS-generated damage that occurs to the photosynthetic machinery. Reducing DHAR expression in DKD leaves also resulted in an accelerated onset of senescence as revealed by the premature induction of expression of the SAG12 ortholog, CP1. These observations support the conclusion that through its Asc recycling function, the level of DHAR activity affects the basal level of ROS during leaf development and, as a consequence, influences the rate of leaf aging. In addition, the observation that increasing DHAR expression did not substantially increase leaf expansion or plant growth indicates that the endogenous level of DHAR expression provides a level of Asc recycling sufficient to support maximum growth. This is in contrast to the role of DHAR in regulating guard cell function, where increasing DHAR expression results in substantial changes in stomatal behavior as a result of changes in ROS levels in the guard cells (Chen and Gallie, 2004).
Because no change in the photosynthetic capacity or in H2O2 levels was observed in the Arabidopsis vtc1 mutant, it was suggested that the low Asc pool size did not impair photosynthetic functioning or result in oxidative stress (Veljovic-Jovanovic et al., 2001). The observation that vtc1 plants continued to exhibit a slower shoot growth rate when grown under conditions of high CO2 that suppress oxidative stress resulting from photorespiration supported the conclusion that the slow growth phenotype was unrelated to a higher oxidative load (Veljovic-Jovanovic et al., 2001). vtc2 mutants that contain only 10% to 30% of the wild-type level of Asc exhibit sensitivity to high light and photobleach when transferred from low to high light (Muller-Moule et al., 2002, 2003). When grown in high light, vtc2 plants contain about 40% of the wild-type level of Asc and are able to grow despite signs of oxidative stress, such as lower electron transport, a lower rate of oxygen evolution, and lower PSII quantum efficiency (Muller-Moule et al., 2004). These mutants demonstrate that although some degree of photooxidative stress may result from the reduced levels of Asc, a significant reduction in Asc does not prevent growth under conditions of high light.
In contrast to vtc mutants, DKD plants showed reduced photosynthetic function and reduced growth in moderate light. Plants suppressed for DHAR are not substantially Asc deficient but rather have a decreased Asc redox state (i.e. more oxidized) that may explain the differences observed between the present plants and the vtc mutants. However, the fact that perturbations to the Asc pool through changes in Asc biosynthesis (vtc mutants) or recycling (DHAR-silenced lines) can affect photosynthetic function under certain growth conditions suggests that the Asc pool is important to leaf function. The correlations between changes in DHAR activity and changes in the foliar level of H2O2 (Chen and Gallie, 2004), rate of CO2 assimilation, and the level of RbcL, LHCII, and chlorophyll are consistent with the conclusion that the slow growth phenotype exhibited by plants suppressed for DHAR is related to reduced photosynthetic functioning and increased oxidative load. The reduced photosynthetic function in DKD leaves may lead to an earlier onset of carbon starvation that can induce autophagy (Moriyasu and Ohsumi, 1996; Brouquisse et al., 1998). The higher rate of chlorophyll loss in carbon-starved (i.e. dark-treated) DKD leaves relative to control leaves (Fig. 4) is consistent with this possibility. The higher level of ROS (such as H2O2) present in DKD leaves (Chen and Gallie, 2004) may also promote leaf aging and senescence through increased signaling that induces expression of senescence-related genes known to be enhanced in leaves experiencing a higher oxidative load (Navabpour et al., 2003). It is also possible that a lower (i.e. more oxidized) Asc redox state in plants suppressed for DHAR contributes to slower rate of cell division and/or elongation through mechanisms that are separate from a lower fixed carbon supply resulting from a lower rate of photosynthesis. Asc promotes G1 to S progression of cells within the onion (Allium cepa) root meristem and pericycle (for review, see Smirnoff, 1996) and reversed the inhibition of cell division caused by lycorine treatment, which reduces Asc content (Arrigoni, 1994). However, any such effect would be limited to growing tissues and would not account for the observed reduction in chlorophyll, RbcL, or CO2 assimilation in expanding DKD leaves or the accelerated loss of chlorophyll, RbcL, LHCII, or CO2 assimilation from mature DKD leaves. Moreover, the prolonged maintenance of leaf function in plants overexpressing DHAR supports a role for this enzyme in leaf aging. Thus, these observations support the conclusion that an important function of DHAR in leaves is to maintain photosynthetic function by limiting ROS-mediated damage.
MATERIALS AND METHODS
Plant Transformation and Growth Conditions
Full-length wheat (Triticum aestivum) and tobacco (Nicotiana tabacum) DHAR cDNAs (accession nos. AY074784 and AY074787, respectively) were isolated as described previously (Chen et al., 2003). Transgenic tobacco (cv Xanthi) expressing the His-tagged wheat DHAR from the CaMV 35S promoter (in the binary vector, pBI101) was generated using Agrobacterium tumefaciens as described (Chen et al., 2003). Transgenic tobacco plants suppressed for DHAR were identified following the introduction of a tobacco DHAR construct in pBI101.
All plants were grown in commercial soil in a glasshouse supplied with charcoal-filtered air. Experiments were carried out and repeated from fall to spring to avoid excessive heat or light. Plants were watered to saturation twice/day (7 am and 1 pm) to ensure even soil moisture. Plants were grown under natural light conditions in a 10-h light and 14-h dark cycle. The average temperature during the day was 25.9°C ± 0.6°C and 20.2°C ± 0.5°C during the night. The average light intensity in the morning (9 am) was 514 ± 206 μmol m−2 s−1 and in the afternoon (1 pm) was 1,191 ± 244 μmol m−2 s−1.
To determine leaf area during leaf expansion, digital images were taken at 3-d intervals. Leaf area was calculated using Adobe Photoshop (version 6.0) by normalizing total leaf size to an internal standard included in each image. Eight leaves at each developmental stage were measured, and the data were processed using Microsoft Excel. Leaf area of whole plants was determined using the same approach by measuring the area of every other leaf. For leaf expansion studies, we used the sixth true leaf as numbered from the plant base. The area of the sixth true leaf of DOX, DKD, and control plants measured when the leaf was fully expanded remained constant for a given line when the plants were grown under identical conditions. Knowing the area of the sixth true leaf in its fully expanded state, corresponding leaves were collected from subsequently grown plants when they were 8%, 50%, or 100% of the size of the fully expanded state.
Western Analysis
Anti-DHAR antiserum raised against DHAR purified from wheat seedlings was used for western analysis. Protein extracts were resolved using standard SDS-PAGE and the protein transferred to 0.22 μm polyvinylidene difluoride membrane by electroblotting. Following transfer, the nitrocellulose membranes were blocked in 5% milk in TPBS (0.1% Tween 20, 137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 1.4 mm KH2PO4, pH 7.4) followed by incubation with primary antibodies diluted typically 1:1,000 to 1:2,000 in TPBS with 1% milk for 1.5 h. The blots were then washed twice with TPBS and incubated with goat anti-rabbit horseradish peroxidase-conjugated antibodies (Southern Biotechnology Associates) diluted to 1:5,000 to 1:10,000 for 1 h. The blots were washed twice with TPBS and the signal detected typically between 1 to 15 min using chemiluminescence (Amersham). Each western was repeated three to four times and representative results presented. The results from the western analysis were quantitated using MicroComputer Imaging Device Elite image processing software (version 7.0, Imaging Research).
Enzyme Assays
DHAR activity was assayed essentially as described (Hossain and Asada, 1984). Tobacco leaves were ground in extraction buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 2 mm EDTA, 1 mm MgCl2) and soluble protein obtained following a 5-min centrifugation at 13,000 rpm. DHAR was assayed from an equal amount of protein as described (Bradford, 1976) in 50 mm K2HPO4/KH2PO4 pH 6.5, 0.5 mm DHA, and 1 mm reduced glutathione and its activity followed by an increase in A265. APX activity was determined as described (de Pinto et al., 2000). Each experiment was repeated two to three times and representative results presented.
Asc and DHA Measurements
Asc was measured as described (Foyer et al., 1983). Leaves were ground in 2.5 m HClO4 and centrifuged at 13,000 rpm for 10 min. Two volumes of 1.25 m Na2CO3 were added to the supernatant and following centrifugation, 100 μL was added to 895 μL 100 mm K2HPO4/KH2PO4, pH 5.6. Asc was determined by the change in A265 following the addition of 0.25 units ascorbate oxidase. The total amount of reduced and oxidized Asc (i.e. Asc and DHA) was determined by reducing DHA to Asc (in a reaction containing 100 mm K2HPO4/KH2PO4, pH 6.5, 2 mm reduced glutathione, and 0.1 μg recombinant wheat DHAR protein incubated at 25°C for 20 min prior to measuring Asc). The amount of DHA was determined as the difference between these two assays.
TBARS Assay
TBARS were measured following the approach essentially described by Larkindale and Knight (2002). A total of 0.5 g of leaf was ground in liquid nitrogen in a 1.5-mL microfuge tube using a micropestle. Then, 0.5 mL of 0.5% (w/v) thiobarbituric acid in 20% (w/v) trichloroacteic acid and 0.5 mL of buffer (50 mm Tris-HCl, pH 8.0, 175 mm NaCl) was added. Following heating at 95°C for 25 min and pelleting the cell debris, the absorbance of the supernatant was measured at 532 nm, with the A600 subtracted to account for nonspecific turbidity. The amount of malonaldehyde was calculated using an exciting coefficient of 155 mm−1 cm−1, in agreement with a standard curve relating malonaldehyde concentrations to absorbance. The measurements were repeated two times and representative results presented.
Chlorophyll Measurements
Chlorophyll a and b were measured spectrophotometrically as described (Jeffrey and Humphrey, 1975). In brief, leaf samples were ground in liquid nitrogen and extracted with 90% (v/v) acetone. The A664 and 647 nm was determined and used to calculated chlorophyll a and b content by the equations: Chl a = 11.93A664 − 1.93A647 and Chl b = 20.36A647 − 5.50A664, respectively. Each experiment was repeated two to three times and representative results presented.
Leaf Function Assays
For all experiments, plants were grown under daylight conditions of approximately 500 μmol m−2 s−1 in the morning and 1,200 μmol m−2 s−1 in the afternoon. For all experiments, measurements were collected at the same time each day (between 10:30 am and 11:30 am) during the course of the experiment. In situ rates of CO2 assimilation, transpiration, and stomatal conductance were measured with TPS-1 portable photosynthesis system. For whole plant assays, every second leaf on a plant was measured. Each experiment was repeated two times and representative results presented.
RT-PCR Analysis
Total nucleic acid was isolated from whole leaves using the RNeasy Plant Mini kit (Qiagen). Residual genomic DNA was removed by on-column DNAse I digestion, using RQ1 RNase free DNase I (Promega). We used 0.5 μg total RNA for cDNA synthesis using Omniscript RT (Qiagen) with oligo(dT)20 as the primer. PCR reactions contained 1 μL of the RT reaction, and 1.5 μg of forward and primers in a total reaction volume of 25 μL and were performed with the following conditions: initial denaturing step, 94°C/15 min; 35 cycles of 94°C/15 s, 52°C/30 s, 72°C/60 s, and a final extension step of 72°C/5 min. PCR products were visualized on an ethidium bromide-stained 1.2% agarose gel. Primers used were: actin (X63603) forward, 5′-CGCGAAAAG-ATGACTCAAATC-39 and reverse, 5′-AGATCCTTTCTGATATCCACG-3′; tobacco CP1 (AY881011) forward, 5′-CTTTATCAGAGCAAGAGCTTG-3′ and reverse, 5′-TTTTGATGCGCATATATCCAC-3′.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY074784 and AY074787.
Acknowledgments
We thank Dr. Tadahiko Mae for the gift of anti-Rubisco antiserum and Dr. Bruce Kohorn for the gift of anti-LHCII antiserum
This work was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service (grant no. 2002–35100–12469) and by the University of California Agricultural Experiment Station.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Daniel R. Gallie (drgallie@citrus.ucr.edu).
References
- Arrigoni O (1994) Ascorbate system in plant development. J Bioenerg Biomembr 26: 407–419 [DOI] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157: 481–488 [Google Scholar]
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569: 1–9 [DOI] [PubMed] [Google Scholar]
- Asada K (1994) Mechanisms for scavenging reactive molecules generated in chloroplasts under light stress. In NR Baker, JR Bowyer, eds, Photoinhibition of Photosynthesis. From Molecular Mechanisms to the Field. Bios Scientific Publishers, Oxford, pp 129–142
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In DJ Kyle, CB Osmond, CJ Arntzen, eds, Photoinhibition. Elsevier, Amsterdam, pp 227–287
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brouquisse R, Gaudillere JP, Raymond P (1998) Induction of a carbonstarvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiol 117: 1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16: 1143–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR (2005) Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol 138: 1673–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Young TE, Ling J, Chang S-C, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100: 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96: 4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93: 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MB, Austin J, Partridge DA (1991) Vitamin C: Its Chemistry and Biochemistry. Royal Society of Chemistry, Cambridge
- de Pinto MC, Tommasi F, De Gara L (2000) Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow 2. Plant Physiol Biochem 38: 541–550 [Google Scholar]
- Eskling M, Arvidsson P, Akerlund HE (1997) The xanthophyll cycle, its regulation and components. Physiol Plant 100: 806–816 [Google Scholar]
- Foyer CH, Rowell J, Walker D (1983) Measurements of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157: 239–244 [DOI] [PubMed] [Google Scholar]
- Horemans N, Foyer CH, Asard H (2000) Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci 5: 263–267 [DOI] [PubMed] [Google Scholar]
- Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25: 85–92 [Google Scholar]
- Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae, and natural phytoplankton. Biochem Physiol Pflanz 167: 191–194 [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J (1999) Photooxidation of ascorbate on the donor side of photosystem I in the thylakoid lumen. Plant Cell Physiol (Suppl) 40: 37 [Google Scholar]
- Mano J, Ushimaru T, Asada K (1997) Ascorbate in thylakoid lumen as an endogenous electron donor to photosystem II: protection of thylakoids from photoinhibition and regeneration of ascorbate in stroma by dehydroascorbate reductase. Photosynth Res 53: 197–204 [Google Scholar]
- McGarvey DJ, Christoffersen RE (1992) Characterization and kinetic parameters of ethylene-forming enzyme from avocado fruit. J Biol Chem 267: 5964–5967 [PubMed] [Google Scholar]
- Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction of the monodehydroascorbate radical in spinach thylakoids. Plant Cell Physiol 35: 539–549 [Google Scholar]
- Moriyasu Y, Ohsumi Y (1996) Autophagy in tobacco suspension-cultured cells in response to sucrose starvation. Plant Physiol 111: 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Moule P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Moule P, Golan T, Niyogi KK (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol 134: 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Moule P, Havaux M, Niyogi KK (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133: 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabpour S, Morris K, Allen R, Harrison E, A-H-Mackerness S, Buchanan-Wollaston V (2003) Expression of senescence-enhanced genes in response to oxidative stress. J Exp Bot 54: 2285–2292 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6: 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001. a) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ (2001. b) Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot (Lond) 78: 661–669 [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-faceted molecule. Curr Opin Plant Biol 3: 229–235 [PubMed] [Google Scholar]
- Smith JJ, Ververidis P, John P (1992) Characterization of the ethylene forming enzyme partially purified from melon. Phytochemistry 31: 1485–1494 [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426–435 [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]