Abstract
Plants are able to integrate exogenous 1-deoxy-d-xylulose (DX) into the 2C-methyl-d-erythritol 4-phosphate pathway, implicated in the biosynthesis of plastidial isoprenoids. Thus, the carbohydrate needs to be phosphorylated into 1-deoxy-d-xylulose 5-phosphate and translocated into plastids, or vice versa. An enzyme capable of phosphorylating DX was partially purified from a cell-free Arabidopsis (Arabidopsis thaliana) protein extract. It was identified by mass spectrometry as a cytosolic protein bearing d-xylulose kinase (XK) signatures, already suggesting that DX is phosphorylated within the cytosol prior to translocation into the plastids. The corresponding cDNA was isolated and enzymatic properties of a recombinant protein were determined. In Arabidopsis, xylulose kinases are encoded by a small gene family, in which only two genes are putatively annotated. The additional gene is coding for a protein targeted to plastids, as was proved by colocalization experiments using green fluorescent protein fusion constructs. Functional complementation assays in an Escherichia coli strain deleted in xk revealed that the cytosolic enzyme could exclusively phosphorylate xylulose in vivo, not the enzyme that is targeted to plastids. xk activities could not be detected in chloroplast protein extracts or in proteins isolated from its ancestral relative Synechocystis sp. PCC 6803. The gene encoding the plastidic protein annotated as “xylulose kinase” might in fact yield an enzyme having different phosphorylation specificities. The biochemical characterization and complementation experiments with DX of specific Arabidopsis knockout mutants seedlings treated with oxo-clomazone, an inhibitor of 1-deoxy-d-xylulose 5-phosphate synthase, further confirmed that the cytosolic protein is responsible for the phosphorylation of DX in planta.
In plants, the biosynthesis of the active isoprene units (Δ2- and Δ3-isopentenyl diphosphates) and common precursors for isoprenoid biosynthesis involves two distinct pathways (Lichtenthaler, 1999; Rohmer, 1999; Rohdich et al., 2001). It is commonly accepted that, under normal physiological conditions, mevalonate (MVA) is utilized for the biosynthesis of nonplastidial isoprenoids (phytosterols, prenylated proteins, sesquiterpenoids, etc.), whereas plastidial isoprenoids (carotenoids, plastoquinone, diterpenes, monoterpenes, etc.) are synthesized via the alternative 2C-methyl-d-erythritol 4-phosphate (MEP) pathway (Fig. 1). 1-Deoxy-d-xylulose 5-phosphate synthase (DXS) is a transketolase-like enzyme, catalyzing the thiamine diphosphate-dependent reaction that yields 1-deoxy-d-xylulose 5-phosphate (DXP) from pyruvate and d-glyceraldehyde 3-phosphate (Fig. 1). It was originally identified in bacteria (Sprenger et al., 1997; Lois et al., 1998) and represents the first enzyme involved in plastidial isoprenoid biosynthesis (Bouvier et al., 1998; Lange et al., 1998). When the pathway is defective or inhibited, isoprenoids can also be synthesized starting from exogenous 1-deoxy-d-xylulose (DX) or 2C-methyl-d-erythritol (ME), added to the culture medium, but both compounds need subsequently to be phosphorylated in vivo (Fig. 1). ME could only be used for incorporation into bacteria (Duvold et al., 1997; Kuzuyama et al., 1999; Fontana et al., 2001) and was found toxic to plant cells (Hemmerlin et al., 2003a), though the free tetrol or the corresponding lactone was shown present in many plants (Dittrich and Angyal, 1988). While there is only weak evidence that DX has a functional role in vivo, it was already substantially used as a stable or radioactive isotope-labeled precursor for studies of the biosynthetic pathway in plants (Schwender et al., 1997; Sagner et al., 1998; Arigoni et al., 1999; Hoeffler et al., 2002; Luan and Wüst, 2002), as well as in bacteria (Hill et al., 1989; Broers, 1994; Giner et al., 1998; Rosa-Putra et al., 1998b; Spiteller et al., 2002). The assumption that in plants the MVA and the MEP pathways somehow conspire was based on incorporation studies (Schwarz, 1994; Adam et al., 1999; Kasahara et al., 2002; Hemmerlin et al., 2003a; Hampel et al., 2005), which also revealed some catalytical properties of enzymes involved in the MEP pathway. Furthermore, DX has been successfully used to partially complement the CLA1 phenotype, an Arabidopsis (Arabidopsis thaliana) DXS knockout mutant (Estévez et al., 2000), which shows an albino phenotype (Mandel et al., 1996).
Figure 1.
Isopentenyl diphosphate synthesis via the MEP pathway. Intermediates involved in the biosynthesis are as follows: 1, pyruvate; 2, d-glyceraldehyde 3-phosphate; 3, DXP; 4, MEP; 5, 4-diphosphocytidyl-2C-methyl-d-erythritol; 6, 2-phospho-4-(cytidine 5′-diphospho)-2C-methyl-d-erythritol; 7, 2C-methyl-d-erythritol 2,4-cyclodiphosphate; 8, (E)-4-hydroxy-3-methylbut-2-enyl diphosphate; 9, isopentenyl diphosphate; 10, dimethylallyl diphosphate; 11, DX; and 12, ME. Genes encoding the enzymes of the pathway are as follows: dxs, 1-deoxy-d-xylulose 5-phosphate synthase; dxr, 1-deoxy-d-xylulose 5-phosphate reductoisomerase; ygbP, 4-diphosphocytidyl-2C-methyl-d-erythritol synthase; ychB, 2-phospho-4-(cytidine 5′-diphospho)-2C-methyl-d-erythritol kinase; ygbB, 2C-methyl-d-erythritol 2,4-cyclodiphosphate synthase; gcpE, (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase; lytB, (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase; and idi, isopentenyl diphosphate isomerase.
Previously, an enzyme-catalyzed formation of DX (1-deoxy-d-threo-2-pentulose) was described in several bacteria (Yokota and Sasajima, 1984). The E1 component (pyruvate decarboxylase) of the pyruvate dehydrogenase complex was identified to catalyze the decarboxylation of pyruvate and the condensation of the enzyme-bound hydroxyethyl-thiamine diphosphate with glyceraldehydes (Yokota and Sasajima, 1986). In plants, the localization of this complex is mitochondrial and plastidial (Tovar-Méndez et al., 2003); however, the physiological relevance or even presence of these secondary activities remains obscure. Along with DX, cell-free extracts from Escherichia coli and Klebsiella planticola are able to catalyze the formation, from pyruvate and d-glyceraldehyde, of 5-hydroxypentane-2,3-dione (laurencione), a product resulting from the dehydration of DX (Rosa Putra et al., 1998a). This latter compound is a natural product that accumulates, among others, in the red alga Laurencia spectabilis (Bernart et al., 1992), but was also proposed to serve as a pyridoxol precursor (Wolf et al., 1997).
It has been established that the nonphosphorylated DX is not a substrate for 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR), the next enzyme downstream in the isoprenoid pathway (Kuzuyama et al., 2000). This suggests that for integration into the plastidial MEP pathway, a phosphorylation is needed (Fig. 1) by a DX kinase (DXK) and a transport across the plastidial envelope. The sequence of these two steps remained unknown, as well as the identities of the enzyme and/or of the transporter implied in this process. Recently, Flügge and Gao (2005) showed that a plastidial d-xylulose 5-phosphate (XP) transporter is able to accept DXP as a substrate. These findings argue in favor of a phosphorylation of DX in the cytosol, followed by transport of DXP across the plastidial envelope. In addition, it has been reported that d-xylulose kinase (XK; EC 2.7.1.17), an enzyme that participates in the pentose phosphate pathway, phosphorylates DX in E. coli (Wungsintaweekul et al., 2001). In a more recent study, Hemmi et al. (2002) identified a nonannotated gene encoding a protein possessing high homologies with the IIC component of the phospho(enol)pyruvate-dependent phosphotransferase system. Its expression in E. coli led to an increase in the incorporation of DX by E. coli cells, but, nonetheless, the identity of the enzyme really responsible for DX phosphorylation in planta still remained enigmatic.
Here, we describe the isolation and characterization of a cytosolic enzyme from Arabidopsis having DXK activity. The identified corresponding gene belongs to a family of only two members, encoding a cytosolic form and a plastidial one. To verify or not the hypothesis that this second enzyme could also be involved in DX phosphorylation, different strategies were applied: among others, functional complementation in E. coli or analysis of Arabidopsis knockout mutants. In this way, we could irrevocably validate the identity of DXK as corresponding to the protein initially purified from crude plant extracts.
RESULTS
Identification of 1-Deoxy-d-Xylulokinase Purified from Arabidopsis Protein Extracts
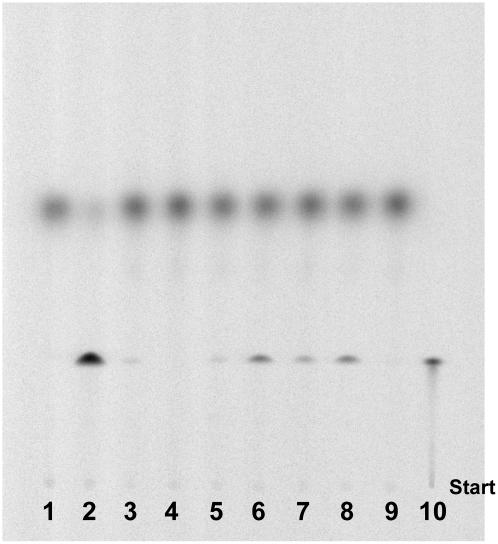
Enzyme-synthesized [2-14C]DX (Hemmerlin et al., 2003a) was used to detect DXK activity in crude cell extracts isolated from different organisms (Sphingobacterium multivorum, Staphylococcus aureus, Saccharomyces cerevisiae, Arabidopsis, tobacco [Nicotiana tabacum] BY-2, and radish [Raphanus sativus]). A recombinant His-tagged version of E. coli XK (Tritsch et al., 2004), shown to bear DXK activity (Wungsintaweekul et al., 2001), was used as a positive control (Fig. 2, lane 2). DXK activity could not be detected in S. cerevisiae, an organism synthesizing isoprenoids exclusively via MVA, while all other protein extracts did catalyze the formation of DXP (Fig. 2). As DXP is incorporated into the plastidial MEP pathway, chloroplast protein extracts were tested as well, but no activity could be evidenced (Fig. 2, lane 9). A primary screening of various enzyme sources allowed us to identify Arabidopsis and radish total protein extracts as the best candidates to start with plant DXK purification (Fig. 2, lanes 6 and 8). To facilitate the later cloning of the gene encoding DXK and its characterization, Arabidopsis was chosen as the source of proteins.
Figure 2.
Screening for DXK activity in protein extracts from different plants and microorganisms. Proteins were incubated for 4 h in the presence of 50 mm Tris-HCl, 20 mm MgCl2, 10 mm KF, 20 mm ATP, and 3.5 mm [2-14C]DX. After incubation, an aliquot (2 μL) of the reaction mixture was directly spotted on a silica plate, and the product DXP was separated from the substrate DX by TLC. The plate was developed in (6:1:3, v/v/v) n-propyl alcohol:ethyl acetate:water and analyzed by phosphor imaging. Lane 1, Negative control without enzymes; lane 2, positive control with purified E. coli xylulose kinase; lane 3, S. multivorum; lane 4, S. cerevisiae; lane 5, S. aureus; lane 6, Arabidopsis; lane 7, tobacco BY-2; lane 8, radish; lane 9, radish chloroplast protein extract; lane 10, [2-14C]DXP.
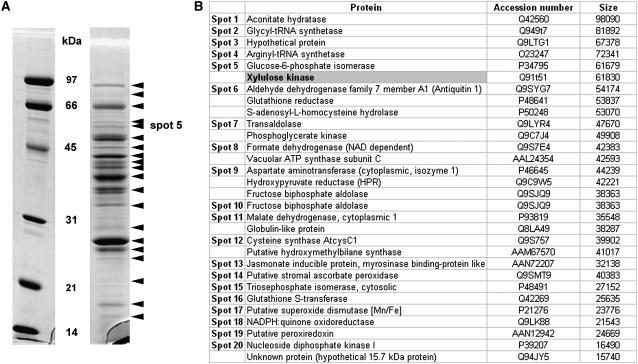
For purification, stability of apparent enzyme activity was tested in preliminary experiments. DXK from Arabidopsis was revealed to be heat stable. Further attempts to purify the enzyme to a higher degree after the Sepharose-Q column were unsuccessful and led to loss of activity. Therefore, we decided to identify all proteins contained in the mixture by a proteomic approach. The Coomassie Blue-stained gel revealed about 20 major bands (Fig. 3A). All spots, identified by MALDI-TOF-MS, are listed in Figure 3B and corresponded to proteins encoded by the Arabidopsis genome (Arabidopsis Genome Initiative, 2000). However, only one (in spot no. 5) contained the conserved motifs FGGY-N (pfam00370.11) and FGGY-C (pfam02782.11) characteristic of a carbohydrate pentose kinase. This enzyme corresponds to a putative XK (accession no. Q91T51) with a calculated molecular mass of 61,830 D (Fig. 3B).
Figure 3.
Arabidopsis DXK protein purification analyzed by SDS-PAGE. A, The partially purified protein extract was concentrated, then separated by SDS-PAGE on a 12% acrylamide gel and stained with Coomassie Blue. Black arrowheads indicate sections of the gel, which were cut off and treated for protein identification by mass spectrometry. Identified proteins are listed in B. The position of spot number 5, in which the presence of a protein containing a pentose kinase motif was identified, is highlighted. Molecular mass standards (Bio-Rad; low-range SDS-PAGE standards) are as indicated (phosphorylase B, 97 kD; bovine serum albumin, 66 kD; ovalbumin, 45 kD; carbonic anhydrase, 31 kD; soybean trypsin inhibitor, 21 kD; and lysozyme, 14 kD).
Enzymatic Characterization of a Recombinant Deoxy-d-Xylulose Kinase from Arabidopsis
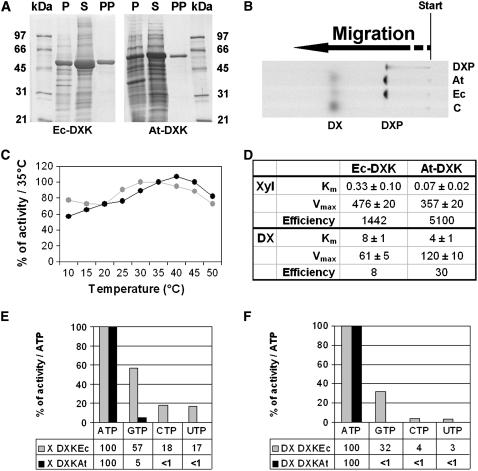
To confirm that the identified protein bears DXK activity, a recombinant His-tag enzyme (His6-At-DXK) and as its homolog from E. coli (His6-Ec-DXK) were purified using Ni2+ columns. The purity of both recombinant enzymes was greater than 98% as judged by SDS-PAGE (Fig. 4A), with apparent molecular masses being close to the calculated values (53,414 D for the E. coli enzyme and 62,119 for the Arabidopsis enzyme, respectively). Following the technique described in Figure 2, a DXK enzyme assay was performed (Fig. 4B). In the presence of ATP and Mg2+, the recombinant enzymes were able to catalyze the conversion of DX into a more polar compound migrating with authentic DXP on a thin-layer chromatography (TLC) plate. The formation of DXP was proven by a coupled spectrometric assay with DXR (Fig. 1), as described by Kuntz et al. (2005).
Figure 4.
Some enzymatic characteristics of DXK activity from Arabidopsis (At-DXK) and E. coli (Ec-DXK). A, Recombinant His-tagged proteins were overexpressed in E. coli and purified with Ni2+ columns. P, Pellet; S, supernatant; PP, purified protein. Molecular mass standards are as indicated in the previous figure. B, Catalytical activity of purified At-DXK and Ec-DXK. Proteins were incubated for 4 h in the presence of 50 mm Tris-HCl, pH 8.0, 20 mm MgCl2, 10 mm KF, 20 mm ATP, and 3.5 mm [2-14C]DX. Aliquots were spotted on a silica TLC plate, which was developed in (6:1:3, v/v/v) n-propyl alcohol:ethyl acetate:water and analyzed by phosphor imaging. An authentic sample of [2-14C]DXP and an aliquot of an assay in which protein was omitted (control C) comigrated as references. C, Activities as a function of increasing temperature. Dark gray dots represent the activity measured with Ec-DXK, and light gray dots correspond to the activity measured with At-DXK. D, Kinetic properties. Details of conditions were described in “Materials and Methods.” Km values are indicated in mm and Vmax in mm mg−1 min−1 (mean of three independent experiments). E and F, Nucleotide preference was calculated using initial velocity measurements. Dark gray bars represent the activity measured with His6-Ec-DXK, and light gray bars correspond to the activity measured with His6-At-DXK. The comparison was made using d-xylulose (E) or DX (F) as substrate.
To compare His6-At-DXK with His6-Ec-DXK, some enzymatic properties were determined. We observed that His6-At-DXK, initially purified from protein extracts, was heat stable. Thus, in a first series of experiments, using [2-14C]DX, we evaluated enzyme stability of recombinant His6-Ec-DXK versus His6-At-DXK at various temperatures (Fig. 4C). Both enzymes showed more than 50% of the optimum activity between 10°C and 50°C, confirming temperature stability within a broad range. Overall, in comparison to His6-Ec-DXK, His6-At-DXK is the more efficient enzyme (Fig. 4D). His6-At-DXK showed specific XK activity in the presence of ATP as a cosubstrate but also accepted DX, albeit at greatly reduced efficiency (175-fold), like its homolog from E. coli (Fig. 4D). A 5-fold higher affinity was observed for the plant enzyme when d-xylulose was used as a substrate, and 2-fold when DX was used (Fig. 4D). While enzyme activity remained stable for several weeks, it has to be noted that the Km values have to be determined with freshly prepared enzyme fractions, as the values slightly increased over storage time. Next, substrate specificities for nucleoside triphosphate were tested (Fig. 4, E and F). The given activities were obtained using the radiometric assay, and values represent initial velocities calculated from time-stop measurements. Generally, each nucleotide was accepted as a cosubstrate when d-xylulose was used as a substrate; however, their relative efficiency appears quite different. The plant enzyme showed a clear preference for ATP, as compared to His6-Ec-DXK. In the presence of DX instead of d-xylulose, the activity decreased for both enzymes. However, as compared to His6-Ec-DXK, His6-At-DXK used exclusively ATP as a substrate. It has to be noted that after a longer incubation time (>16 h), DXP can be formed even if Vi is low (<1%).
A Small Gene Family Encodes XK Proteins with Differential Subcellular Localization in Arabidopsis
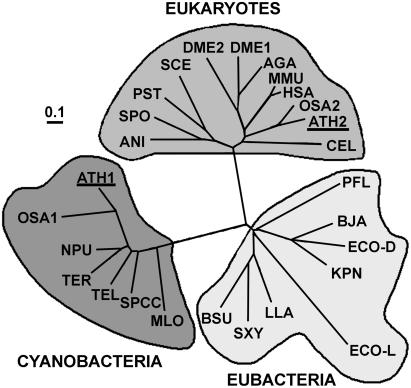
A second gene (At2g21370), encoding a putative XK (Swiss-Prot accession no. P42826), was found in the Arabidopsis genome. Alignment of protein sequences having homologies with XK from different organisms, by the aid of ClustalX software (Thompson et al., 1997; downloaded at ftp://ftp-igbmc.u-strasbg.fr), indicated there is no significant similarity between both XKs. Furthermore, this protein is more closely related to the well-characterized E. coli XK (Wungsintaweekul et al., 2001) than the enzyme we purified from plant extracts. Consequently, the corresponding gene was referred to as Atxk-1, in comparison to the E. coli gene, while the gene encoding the purified DXK was named Atxk-2. A phylogenetic tree (Fig. 5), constructed with ClustalX using the neighbor-joining method, showed that they have evolved independently. The analyses distinguished three distant groups: the first one assembled eukaryotes, the second grouped mainly cyanobacteria, and the third one joined eubacteria. The Arabidopsis proteins confine to two divergent groups: AtXK-1 clusters with the cyanobacteria group, while AtXK-2 clusters with proteins present in higher eukaryotic cells.
Figure 5.
Phylogenetic tree of xylulose kinases revealing two evolutionary distinct sequence clusters for the Arabidopsis genes. Sequences from Arabidopsis were compared with other annotated xylulose kinases. Alignment was performed with ClustalX. The tree was calculated by neighbor-joining distance method and is represented in an unrooted form. Line lengths indicate the relative distances between nodes. MLO, Mesorhizobium loti (NP_104677); ATH1, Arabidopsis (NP_179732); OSA1, Oryza sativa (NP_912678); TEL, Thermosynechococcus elongatus (NP_682081); SPCC, Synechocystis sp. PPC (NP_442817); NPU, Nostoc punctiforme (NP_488696); TFL, Trichodesmium erythraeum (ZP_00071393); PFL, Pseudomonas fluorescens (ZP_00088158); ECOL, E. coli l-xylulose kinase (NP_418037); ECOD, E. coli xk (AAN32628); KPN, Klebsiella pneumoniae (AAC26499); BJA, Bradyrhizobium japonicum (NP_767759); LLA, Lactococcus lactis (AAD20250); BSU, Bacillus subtilis (NP_389643); SXY, Staphylococcus xylosus (P27155); SPO, Schizosaccharomyces pombe (NP_587930); ANI, Aspergillus niger (CAC83746); SCE, S. cerevisiae (NP_011710); PST, Pichia stipitis (AAF72328); ATH2, Arabidopsis (NP_199776); OSA2, O. sativa (XP_479289); HSA, Homo sapiens (NP_005099); MMU, Mus musculus (AAH25442); DME2, Drosophila melanogaster (NP_608543); AGA, Anopheles gambiae (EAA06371); DME1, D. melanogaster (NP_650582); CEL, Caenorhabditis elegans (NP_498988).
In addition, Atxk-1 bears a 5′ extension in the open reading frame, which encodes an additional peptide of 43 amino acids as compared to the Synechocystis protein. It was identified as a potential targeting peptide by Predotar (http://urgi.infobiogen.fr/predotar/predotar.html) for prediction of subcellular location, with a probability of 40% for chloroplastic targeting and only 2% for mitochondrial targeting. The Psort software (http://psort.ims.u-tokyo.ac.jp/form.html) predicted the protein to be targeted to the stroma of the chloroplast with a probability of 51%, 36% to the mitochondrium and 29% to peroxisomes. Indeed, the protein contained at its C terminus a SGL motif, similar to a SKL-like peroxisome targeting signal type 1 motif. Finally, Target P1.1 (http://www.cbs.dtu.dk/services/TargetP/) estimated at 43.5% AtXK-1 as a plastidial enzyme and 64.5% as a mitochondrial protein.
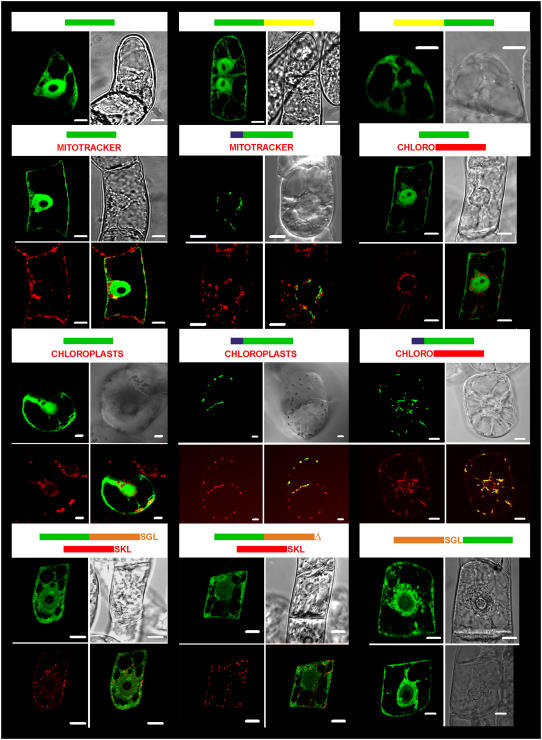
To confirm the function of the putative transit peptide, the DNA sequence coding for the N-terminal propeptide was fused to the 5′ end of the gene coding for green fluorescent protein (GFP). Transient expression of the fused GFP in tobacco (cv xanthi) cells verified that the peptide was sufficient to target the reporter protein to chloroplasts (Fig. 6). The green fluorescence colocalized with chloroplasts, while control cells expressing GFP solely evidenced a cytosolic localization surrounding them (Fig. 6). For comparison, a known chloroplast-specific transit peptide (Hemmerlin et al., 2003b) fused to red fluorescent protein (RFP) was used for colocalization in tobacco BY-2 cells and led to the same conclusion (Fig. 6). A possible mitochondrial targeting function of the peptide was disproved by transient expression in tobacco BY-2 cells, followed by staining of the cells with the MitoTracker Red CMXRos probe. The photographs illustrated that the observed punctuated structures obtained after expression of PTARA1-GFP did not colocalize with the MitoTracker (Fig. 6), demonstrating that the peptide's only role is targeting into the plastids. In addition, the hypothesis of AtXK-1 being targeted to peroxisomes was investigated by colocalization experiments, either with the truncated form of AtXK-1 fused to the C-terminal side of GFP or its mutated version in which the SGL motif was removed (Fig. 6). None of these chimeric proteins colocalized with an expressed RFP-SKL protein (Hemmerlin et al., 2006), which was taken as reference for peroxisomal labeling (Fig. 6). When fused to the N terminus of GFP, the truncated form of the protein (without transit peptide), starting with MSGNKGTN, was found in the cytosol (Fig. 6), and some cells contained a few protein aggregates within the cytosol. Furthermore, GFP fusion proteins at either the N terminus or C terminus of AtXK-2 confirmed that the protein was cytosolic, as was expected by the sequence analysis and the purification experiment (Fig. 6). In summary, the colocalization experiments proved that AtXK-1 is a plastidial protein, whereas AtXK-2 corresponds to a cytosolic one. Further, AtXK-1 presents no membrane domains and apparently corresponds to a stromal protein.
Figure 6.
Subcellular localization of At-XK isoforms fused to GFP. The constructs used for colocalization experiments are represented at the top of the pictures. Bars represent the gene contained in the construct used for bombardment experiments (green: gfp; yellow: Atxk-2; blue: Atxk-1 transit peptide; red: rfp; orange: truncated form of Atxk-1; chloro: coding for PT5-RFP targeting specially the RFP into chloroplasts). The truncated form of Atxk-1 was fused either with its C terminus SGL motif or without (symbolized by an orange triangle). Pictures, depicting 5- to 16-h-old cells after transformation of tobacco cv xanthi (containing chloroplasts) or cv Bright Yellow-2 cells (all other cases), are documented. Green pictures are representative of images recordings made with a LSL510 confocal microscope after excitation at 488 nm and recording of the emission between 505 and 530 nm (top right picture) for the detection of GFP. Red pictures are representative of acquisitions made after excitation at 543 nm and emission of the natural red fluorescence of chlorophyll or RFP. The bright-field image is represented in the top right picture. When indicated, the bottom right picture is a superposition of both green and red channels, which shows colocalization of the fusion protein with the red standard, if any. The white scale bars represent 10 μm.
Functional Complementation of an E. coli XK Deletion Mutant
If we consider Atxk-1 to putatively encode a protein with XK activity, the question comes up as to why we did not identify this protein as a DXK. Furthermore, the possibility of DX phosphorylation by this enzyme would change the sequence of events compared to those expected with the isolated AtXK-2. In this case, DX would first be transported into the chloroplasts and then become phosphorylated. Does this enzyme bear higher substrate specificity for d-xylulose than for DX or is even the catalytic activity completely different?
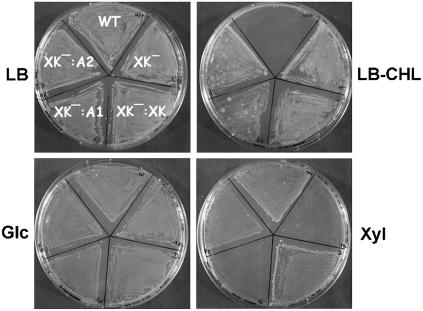
In vitro activity assays with the recombinant enzyme did not provide evidence for any carbohydrate kinase activity. We tested nearly 40 sugars (monosaccharides and disaccharides, including d-xylulose and DX) as potential substrate for AtXK-1, but none of them was significantly phosphorylated (data not shown). Because this might have been due to the lack of some unknown cofactor(s) or interaction with other proteins, the capacity of AtXK-1 to phosphorylate d-xylulose in vivo was investigated by functional complementation assays. We took advantage of the fact that E. coli contains a unique gene (xylB) encoding XK (Rosenfeld et al., 1984). A strain was created in which xylB was deleted and replaced by a CAT cassette. The deletion did not affect Xyl isomerase activity in vivo (data not shown), which was essential for complementation assays. While no obvious phenotype could be detected on rich Luria-Bertani (LB) medium, the growing capacity of the mutant strain was strongly affected on minimum M9 medium supplemented with 0.2% Xyl (Fig. 7). As a control, when the medium was supplemented with 0.2% Glc, the mutant grew, as did the wild-type DY329 strain (Fig. 7).
Figure 7.
Functional complementation of an E. coli xylB deletion mutant by At-XK isoforms. DY329-xylB∷cat E. coli cells were transformed with pET (XK−), pET-Ec-XK (XK−:XK), pET-At-XK1 (XK−:A1), or pET-At-XK2 (XK−:A2), and, along with a nontransformed DY329 strain (WT), plated on LB, LB supplemented with chloramphenicol (LB-CHL), minimum M9 supplemented with 0.05% yeast extract and with 0.2% Glc (Glc), or minimum M9 medium supplemented with 0.05% yeast extract and 0.2% Xyl (Xyl) medium. All plates were supplemented with 1 mm IPTG and were incubated at 30°C until colonies appeared.
Functional complementation assays were performed with the Arabidopsis isogenes Atxk-1, Atxk-2, and xylB (positive control). With d-Xyl as a carbon source and in the presence of 1 mm IPTG, AtXK-2 and XylB could rescue the xylB deficiency, whereas the strain transformed with the construct coding for AtXK-1 did not grow, like the mutant strain transformed with an empty vector (Fig. 7). These results suggest that Atxk-1 does not code for a xylulose kinase. To prove that AtXK-1 is not responsible for the phosphorylation of DX into DXP in planta, additional strategies had to be developed, as described below.
Chloroplasts and Synechocystis sp. PCC 6803 Protein Extracts Do Not Phosphorylate Xylulose or DX
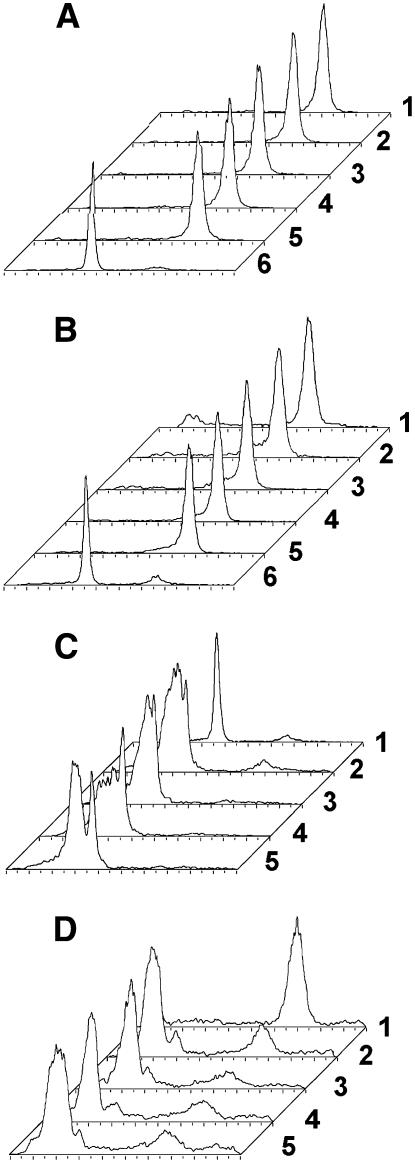
Because our studies suggested that At-XK1 corresponds to a plastidial enzyme, we tested the hypothesis that chloroplast protein extracts catalyze the conversion of d-xylulose into XP or DX into DXP. Being genetically close to Arabidopsis, radish, which produces considerable plant material within a few days, was chosen to verify this hypothesis. Stromatal protein extracts were assayed, but neither apparent-DXK (Fig. 2) nor XK activity (data not shown) could be evidenced. As AtXK-1 bears strong homologies with the Synechocystis sp. PCC 6803 protein (NP_442817) annotated as XK, we tested protein extracts from this cyanobacterium for enzyme activities. Neither XK (Fig. 8A) nor DXK (Fig. 8B) activities could be detected, but enzymatically synthesized [14C]d-XP was converted into other compounds with distinct Rf and, therefore, different polarities (Fig. 8, C and D). Different NTPs were tested, but none of them could be utilized to catalyze the formation of DXP or XP (Fig. 8, A and B). These results demonstrate that in cyanobacteria, as well as in chloroplasts, there is no XK activity, at least not in form of a soluble enzyme. However, it cannot be excluded that the protein is not expressed or that it is regulated by an unknown mechanism, even though the transcripts are detected by reverse transcription (RT)-PCR in wild-type plants (Fig. 9C).
Figure 8.
Measurement of XK and DXK activities in Synechocystis sp. PCC 6803 protein extracts. The protein extracts were incubated for 4 h in the presence of assay buffer (Tris-HCl [50 mm], MgCl2 [20 mm], KF [10 mm], NTP [20 mm]) and 3.5 mm (1.1 mCi mmol−1) [2-14C]DX or 3.05 mm [U-14C]Xyl (1.4 mCi mmol−1). After incubation, a fraction (2 μL) of the reaction mixture was directly spotted on silica plates, and the product DXP (or XP) was separated from the substrate DX (or d-xylulose) by developing in (6:1:3, v/v/v) n-propyl alcohol:ethyl acetate:water. The plates were analyzed with a radioactivity TLC scanner (Berthold). From the back to the front are represented each time: 1, the substrate; 2, an assay with ATP; 3, GTP; 4, CTP; 5, UTP; and 6, the reference product, if known. A, DXK products separated on a silica plate. B, XK products separated on a silica plate. C, Metabolism of XP developed on silica plates. The assays (15 μL) were carried out in the assay buffer at 37°C. The concentration of added NTP was 6 mm and that of [14C]xp 5 mm (0.25 mCi/mmol). After 4 h of incubation, an aliquot (3 μL) was analyzed by TLC on silica plates (eluent: n-propanol:ethyl acetate:water; 30:5:15). D, Metabolism of XP developed on PEI plates. The assay conditions were identical to those described in C, but samples were deposited on PEI-cellulose plates and migrated in 0.8 m LiCl.
Figure 9.
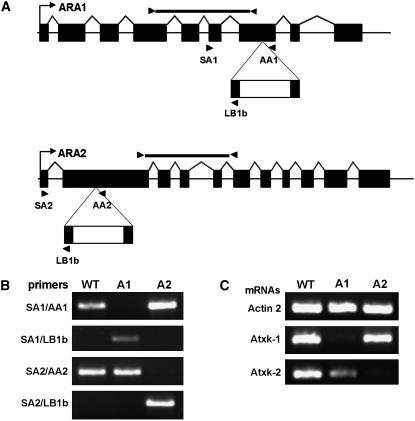
Genetic characterization of Arabidopsis knockout mutants in At-xk1 and At-xk2 alleles A, A scheme of the At-xk1∷T-DNA and At-xk2∷T-DNA loci. The gene structure shows exons (black rectangles), and 5′- and 3′-untranslated regions (white triangles). The pROK2 T-DNA is represented below, and primers used for genomic-PCR and RT-PCR analysis are indicated by arrowheads. B, Characterization of T-DNA insertion locus by PCR using the primer sets as indicated and DNA template isolated from wild type (Col-0; WT) and from mutants At-xk1∷T-DNA (A1) and At-xk2∷T-DNA (A2). C, Semiquantitative RT-PCR analysis of the actin 2 (At3g18780, control), Atxk-1, and Atxk-2 mRNAs in wild-type and mutant plants as described in “Materials and Methods.” The black bars in A represent the part of the mRNA analyzed by RT-PCR using the primer sets RTAtXK1F/RTAtXK1R and RTAtXK2F/RTAtXK2R.
Analysis of Insertional Arabidopsis Mutants
To further prove AtXK-2 to be responsible for DX phosphorylation in planta, we characterized Arabidopsis T-DNA insertional mutants. Mutants were identified in the database from the Salk collection (http://signal.salk.edu). Homozygous plants were selected by PCR, and sequence analysis of the junction fragments obtained with T-DNA and gene-specific primers identified the T-DNA insertion in exon 7 for At2g21370 and in exon 2 for At5g49650 (Fig. 9, A and B). RT-PCR analysis indicated that the mRNAs were not expressed in the corresponding homozygous mutant plants (Fig. 9C). No phenotype could be observed under standard culture conditions. Using the Arabidopsis microarray database Genevestigator (Zimmermann et al., 2004), we checked the expression pattern of the mRNAs transcripts of Atxk-1 and Atxk-2: Atxk-1 is expressed in all organs and tissues, though predominantly in leaves and pedicels. Atxk-2 is more ubiquitously expressed, with a slightly higher expression level in petals and stamens. Analysis on the eFP browser (http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi) confirmed these results and showed that Atxk-1 expression is enhanced by drought, genotoxic stress, or oxidative stress, while Atxk-2 expression is stimulated by osmotic stress.
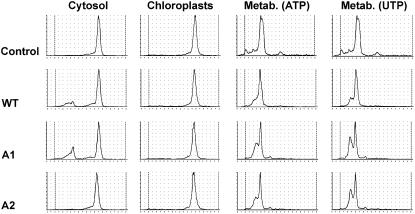
For close inspection of biochemical properties in vivo, biochemical characterizations were initiated. The presence of XK and DXK activities in cytosolic extracts and plastid fractions for each plant type was verified (Fig. 10). While in wild-type and Atxk-1∷T-DNA cytosolic plant extracts apparent activities could be detected, Atxk-2∷T-DNA plant extracts were not able to phosphorylate d-xylulose or DX (Fig. 10). None of the chloroplast fractions was able to phosphorylate the sugars, which confirmed the result presented in Figure 2. The tests were performed using ATP, GTP, CTP, or UTP as a phosphate donor and resulted each time in a negative enzyme assay. The results are consistent with the fact that XK is missing in plant chloroplasts and that AtXK-1, annotated as xylulose kinase, encodes a protein with different, yet unknown, enzyme activity. As a control, it was verified that the chloroplast protein extract was metabolically active using XP as substrate (Fig. 10), as was the Synechocystis extract.
Figure 10.
Biochemical characterization of the Arabidopsis knockout mutants in At-xk-1 and At-xk-2 alleles. XK activities were measured in cytosolic fractions and chloroplastidic fractions. Protein extracts from wild-type Col-0 plants (WT), Atxk-1∷T-DNA (A1), and Atxk-2∷T-DNA (A2) knockout mutants were incubated in the presence of d-[U-14C]Xyl and Xyl isomerase. The protein extracts were incubated for 4 h in the presence of Tris-HCl 50 mm, MgCl2 20 mm, KF 10 mm, NTP 20 mm, and 3.05 mm d-[U-14C]Xyl. After incubation, an aliquot (2 μL) of the reaction mixture was directly spotted on a silica plate, and the product DXP (or XP) was separated from the substrate DX (or X) by TLC as described in previous legends and analyzed with a radioactivity TLC scanner (Berthold). The catalytical capacity of this protein extract was checked by apparent metabolization (Metab.) of XP in the presence of 6 mm ATP or UTP.
Biochemical Phenotype and Chemical Complementation with DX of OC-Treated Plants
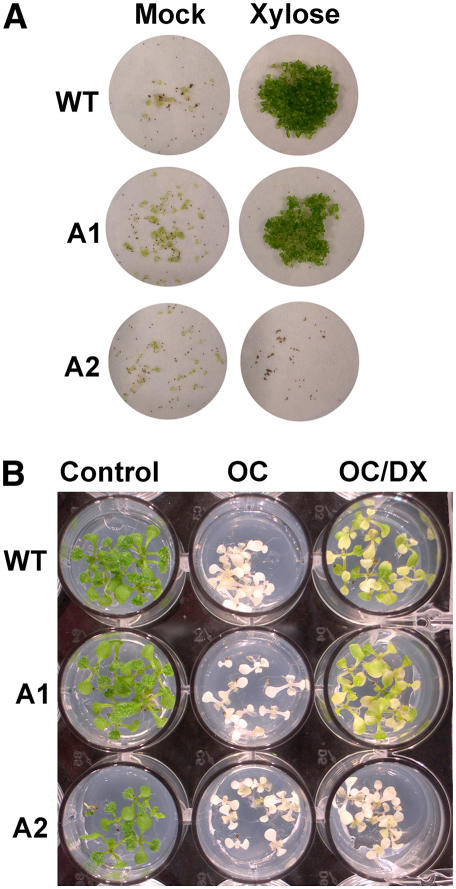
To further characterize the biochemical phenotype of mutant plants, we tested their capacity to metabolize exogenous sugar added to the culture medium. In vivo, Atxk-1 knockout mutants, as well as wild-type Columbia (Col)-0, were able to use Xyl as a carbohydrate source in dark culture conditions (Fig. 11A). In contrast to this, Atxk-2 knockout mutants did not germinate in the presence of Xyl, which was even found toxic as compared to seeds that were allowed to germinate without any sugar (Fig. 11A). To assess the role of AtXK-2 in the phosphorylation of DX in Arabidopsis, we tested the capacity of wild-type, Atxk-1∷T-DNA, and Atxk-2∷T-DNA plants to phosphorylate DX in planta. For this purpose, oxo-clomazone (OC; also referred to as keto-clomazone), a bleaching herbicide affecting DXS activity (Müller et al., 2000; Ferhatoglu and Barrett, 2006), was used to inhibit the MEP pathway and, thereby, the formation of β-carotene normally protecting photosynthetic reaction centers from photooxidation. We tested the capacity of OC-treated wild-type and mutant plants to phosphorylate DX into DXP, allowing after incorporation the bypass of the inhibition of DXS and, consequently, a reversion of the bleaching effect of OC (Fig. 11B). In the first series of experiments, we determined the minimal concentration of OC causing a bleached phenotype on Arabidopsis seedlings and the DX concentration needed to overcome, at least partially, the effect (data not shown). Reference concentrations were 0.2 μm OC and 2 mm DX. Under those conditions, a pale green complexion was recovered with wild-type and Atxk-1∷T-DNA plants (Fig. 11B), indicating a partial recovery of the biosynthesis of photosynthetic pigments derived from DXP via the MEP pathway. In contrast to this, the Atxk-2∷T-DNA plants remained albino (Fig. 11B) and AtXK-1 was unable to take over the function of AtXK-2 in the Atxk-2∷T-DNA knockout mutant. In summary, these investigations led us to conclude that AtXK-2 is the sole enzyme able to phosphorylate DX in planta.
Figure 11.
Chemical complementation of Arabidopsis knockout mutants in At-xk-1 and At-xk-2 alleles. A, Phenotype of wild-type Col-0 plants (WT), Atxk-1∷T-DNA (A1), and Atxk-2∷T-DNA (A2) knockout mutants growing on d-Xyl as a carbon source as compared to a mock experiment containing no carbon source. Seeds (about 25) were bleach sterilized, transferred into 100-mL Erlenmeyer flasks containing liquid Murashige and Skoog (20 mL) medium, and allowed to germinate in the dark for 3 weeks under constant shaking. After this period, the Erlenmeyer flasks were transferred into a culture room at 23°C, with 16-h-light and 8-h-darkness cycles for 2 further weeks. The content was suction filtered and documented by taking photographs on white filter paper. B, In vivo complementation by DX of wild-type Col-0 plants (WT), Atxk-1∷T-DNA (A1), and Atxk-2∷T-DNA (A2) knockout mutants treated with OC compared to untreated seedlings (Control) and seedlings that were germinated on OC. Seeds were bleach sterilized and allowed to germinate on solid Murashige and Skoog medium containing 2% Suc. Note that only in the wild-type plants and the Atxk-1∷T-DNA (A1) knockout mutant DX could partially overcome the bleaching effect of OC, a clear proof that absence of plastidial XK had no impact on greening, whereas the absence of cytosolic XK (A2) did not allow chemical complementation of OC inhibition by DX.
DISCUSSION
Plants incorporate at rather high efficiencies different carbohydrates providing sources of energy and carbon scaffolds. Along with other polyols, sugars that are taken up by the cells (Büttner and Sauer, 2000; Reinders et al., 2005) need to be activated by phosphorylation for their integration into metabolic pathways. DX and ME are known to be phosphorylated and used as precursors for the biosynthesis of isoprenoids synthesized via the MEP pathway (Rohmer, 1999). The uptake mechanism for DX is still unknown, whereas ME seems to be transported via the bacterial sorbitol phosphotransferase system (Testa et al., 2004). Nothing is known about the uptake system in plant cells, but Wolfertz et al. (2004) estimated the rates of deuterated DX incorporation into the MEP pathway from the labeling degree found in isoprenoid end products and speculated that the labeling is due to kinase activity. These observations stand for high DX incorporation/phosphorylation in plants. In a previous study, it was shown that, in plants, DX incorporation is sufficient to overcome the absence of active DXS (Estévez et al., 2000) and that it apparently stimulates the MEP pathway and the production of isoprenoid end products like isoprene (Wolfertz et al., 2004) or carotenoids (Lois et al., 2000).
Our studies demonstrated DXK activities in different plant protein extracts. When we used radiolabeled ME as substrate, no kinase activity could be detected independently of the source of plant extracts (Arabidopsis, tobacco cell suspensions, tobacco plants, pea, bean, radish, etc.; data not shown). These results corroborate our earlier observations on the toxicity of ME on tobacco BY-2 cells (Hemmerlin et al., 2003a). Two hypotheses can explain this observation: either this activity is catalyzed by a membrane protein, which we did not isolate in the crude soluble extract, or the activity simply does not exist. In plants, sorbitol transporters do not bear phosphotransferase activity (Gao et al., 2003) as do bacterial transporters (Testa et al., 2004). Therefore, it is reasonable to assume that plants are not able to phosphorylate ME, which might explain the rather toxic effect of an accumulation of this polyol. As a consequence, DX is the sole exogenous precursor that can be used for integration into the plastid isoprenoid biosynthetic pathway.
Phosphorylations of carbohydrates are known to be carried out by the sugar-kinase protein family. Comparisons of sugar kinase genes from various organisms indicated the existence of three evolutionarily independent gene families: the hexokinase-like family (with specificities for Glc, Fru, ribulose, xylulose, and Fuc), the ribokinase-like family (with specificities for Rib and Fru), and the galactokinase-like family (with specificity for Gal, N-acetylgalactosamine; Bork et al., 1993). The identification of a XK being able to phosphorylate DX in planta is a comparable result to what was observed in prokaryotic cells (Wungsintaweekul et al., 2001). Nevertheless, the identified protein was not identical to that predicted by the same research group after sequence homology analysis with the Arabidopsis genome.
So far, only low interest had been focused on plant XK. In a quite early study, XK activity was evidenced in pea, spinach, corn, and wheat protein extracts (Zahnley and Axelrod, 1964). XK (ATP: d-xylulose 5-phosphotransferase; EC 2.7.1.17) is predicted to be implicated in the ARACyc pathway (http://www.arabidopsis.org:1555/ARA/NEW-IMAGE?type=PATHWAY&object=XYLCAT-PWY&detail-level=3), and catalyzes the formation of xp (d-threo-2-pentulose 5-phosphate) from d-xylulose. XP represents a central metabolite within the oxidative pentose phosphate pathway that comprises three enzymatic reactions that convert Glc-6-phosphate into ribulose-5-phosphate and generates two molecules of NADPH. Therefore, this pathway plays a key function in the cell for the production of NADPH. It also provides intermediates for other biosynthetic pathways (Kruger and von Schaewen, 2003), for instance, for fatty acid biosynthesis, nitrate reduction, and the shikimate pathway (Emes and Neuhaus, 1997). In plants, it is compartmentalized within the cytosol and the plastids (Schnarrenberger et al., 1995). Both pathways are linked via a pentose phosphate translocator that is ubiquitously expressed in the various plant tissues (Eicks et al., 2002). In bacteria, as well as in plants, d-xylulose is produced from d-Xyl by the action of Xyl isomerase (EC 5.3.1.5). This substrate is obtained after degradation of the primary cell wall followed by transport within the cell (Sherson et al., 2003), a process activated in response to stress or during fruit ripening (Labavitch, 1981).
Compared to other sugar kinases like ribulokinase (Lee et al., 2001), XK shows stronger substrate specificities in S. cerevisiae (Richard et al., 2000) or in E. coli (Wungsintaweekul, 2001). Nevertheless, the enzyme accepts DX as a substrate. Interestingly, in yeast, an organism using exclusively MVA for isoprenoid biosynthesis, XK activity could be detected in total protein extracts (Tritsch et al., 2004) but DXK could not (this study). In other organisms, there was a clear correlation between XK and DXK activities. The question that still remains open is whether high concentrations of DX interfere with the general metabolism of the cell, especially whether the carbohydrate metabolism is affected. For instance, DX incorporation also stimulated XK activities in tobacco BY-2 cells, but not in Arabidopsis seedlings (data not shown). Furthermore, in Arabidopsis XK is encoded by a small gene family with two members (At2g21370 and At5g49650) coding for two enzymes that evolved independently and are targeted to different subcellular localizations, cytosolic versus plastidial, as it was established in this study. This is in accordance with the compartmentalization of the pentose phosphate pathways. Although both enzymes contain carbohydrate kinase signatures and are annotated as XK, the results in Figures 7 and 11 show that the plastidial enzyme is unable to overcome the lack of the cytosolic enzyme in planta and cannot functionally complement an E. coli knockout mutant. In addition, Xyl isomerase, which in bacteria is always associated with XK, in Arabidopsis is encoded by a single annotated gene (http://www.expasy.org/uniprot/Q9FKK7) with the prediction of a cytosolic protein. In fungi, which lack Xyl isomerase, the combined action of d-Xyl reductase (EC 1.1.1.21) and xylitol dehydrogenase (EC 1.1.1.9) is required to achieve conversion of Xyl into xylulose (Jackson and Nicholson, 2002). Whereas in Arabidopsis a gene that codes for a putative cytosolic aldose reductase (At5g01670) occurs, the corresponding orthologs of Xyl reductase and xylitol dehydrogenase could not be identified. Moreover, as chloroplasts derived by endosymbiosis from ancestral cyanobacteria (Curtis and Clegg, 1984), the presence of these genes was checked in Synechocystis. None of the aforementioned genes or Xyl isomerase could be identified (http://www.kazusa.or.jp/cyano/Synechocystis/index.html). In fact, there is no real need for a xk activity in the plastid. Although the substrate specificity of At-XK1 could not be identified, we clearly showed that this enzyme was not able to phosphorylate d-xylulose or DX in vivo and that the cytosolic AtXK-2 was solely responsible for this enzyme activity. Therefore, it is now reasonable to propose that Atxk-1 codes for a chloroplast-targeted protein with different functions. Alternative biochemical techniques have to be adopted to solve the functional problem.
Our results point to a clear sequence of events needed for DX incorporation into the MEP pathway. DX is phosphorylated within the cytosol, followed by translocation from the cytosol into the plastids. Plastids contain numerous metabolite transporters within their envelope (Ferro et al., 2003; Knappe et al., 2003; Weber et al., 2005). The possibility of DXP to be transported by the well-characterized xylulose 5-phosphate/phosphate translocater (Eicks et al., 2002) was already evidenced by Flügge and Gao (2005). This transporter accepts other sugar-phosphates, but under physiological conditions xylulose 5-phosphate and triose-phosphate correspond to those that are favored (Eicks et al., 2002). This transporter, acting as an antiporter exchanging sugar-phosphate against inorganic phosphate, accepts neither d-xylulose (Eicks et al., 2002) nor DX (Flügge and Gao, 2005). This is an additional argument in favor of DXP phosphorylation within the cytosol, prior to transport into the plastid and incorporation into the MEP pathway.
In conclusion, our work clarifies where phosphorylation of DX takes place, which was previously a matter of debate (Wungsintaweekul et al., 2001; Wolfertz et al., 2004).
MATERIALS AND METHODS
Chemical Materials
Ni2+ spin columns were purchased from Qiagen. d-Xylulose and d-Xyl were purchased from Fluka and the MitoTracker Red CMXRos probe from Molecular Probes Europe BV. DX was chemically synthesized as described by Hemmerlin et al. (2003a). OC was provided by Dr. Klaus Grossmann (BASF). Silica (silica 60 F254) and polyethyleneimine (PEI)-cellulose F plates, utilized for TLC, were purchased from Merck. The vectors pGEMT (Promega) and pET3a (Novagen) were used for transformation of Escherichia coli cells. PerkinElmer Life Sciences was the source of [2-C14]pyruvate (50 μCi, specific activity 10 mCi mmol−1), and [2-14C]deoxy-d-xylulose was enzymatically synthesized from [2-14C]pyruvate as described by Hemmerlin et al. (2003a). d-[U-14C]Xyl (82 mCi/mmol, 200 μCi/mL) was obtained from Amersham.
Growth Conditions for Bacterial and Yeast Strains
E. coli strain XL1-Blue was purchased from Stratagene, whereas BL21(DE3) and Rosetta-gami(DE3) pLacI were purchased from Novagen. The E. coli DY329 strain used for genetic engineering was obtained from Dr. Donald Court (National Cancer Institute). All strains were grown on LB broth medium (Gibco-BRL) supplemented with ampicillin (100 μg/mL) if needed, or on M9 minimum medium supplemented with 0.05% (w/v) yeast extract. Synechocystis sp. PCC 6803 was obtained from the Institut Pasteur and was grown in BG-11 medium (Sigma). Saccharomyces cerevisiae and Staphylococcus aureus strains were handled as described by Tritsch et al. (2004).
Plant Material and Culture Conditions
Radish (Raphanus sativus) seeds were germinated on tap water for 4 d. Seeds of the T-DNA insertion lines SALK_042149 and SALK_079018, generated at the Salk Institute Genome Analysis Laboratory (Alonso et al., 2003), were obtained from the Arabidopsis Biological Resource Center under the names N542149 and N519643, respectively. When not specifically indicated, wild-type Arabidopsis (Arabidopsis thaliana; ecotype Col-0) was used. Seeds were ethanol and bleach sterilized, and then transferred into liquid Murashige and Skoog medium, pH 5.7 (Duchefa). Cultures were grown under a 16-h-light/8-h-dark photoperiod under continuous shaking (100 rpm). Seedlings were recovered after 21 d and stored at −80°C. When grown on soil, Arabidopsis plants were transferred to growth chambers (at 21°C under a 16-h-light/8-h-dark photoperiod). For complementation experiments, seeds were surface sterilized and plated on agar-solidified Murashige and Skoog medium containing different chemicals as described for each experiment. Tobacco (Nicotiana tabacum) BY-2 cells (Nagata et al., 1992) were cultured as described in detail (Hemmerlin et al., 2003a), while tobacco cv xanthi cells were grown as described by Hemmerlin et al. (2003b).
Isolation of Total and Chloroplast Plant Protein Extracts
Several plant, bacterial, and yeast extracts were used to screen for DXK activity. The extraction of total proteins was performed as described by Tritsch et al. (2004). Cytosolic fractions were obtained after centrifugation at 100,000g for 1 h at 4°C. Chloroplasts from radish and Arabidopsis were purified using a Percoll gradient as described by Gerrits et al. (2001). For enzymatic assays, chloroplasts were lysed by hypotonic treatment with Tris-HCl (62.5 mm), pH 8.0, and MgCl2 (2 mm).
Partial Purification of an Enzyme with 1-Deoxy-d-Xylulokinase Activity from Arabidopsis
All procedures were conducted at 4°C unless noted otherwise. Frozen 3-week-old Arabidopsis seedlings (50 g) were ground in a mortar in presence of liquid nitrogen. The powder was suspended in 40 mL of ice-cold purification buffer (50 mm Tris-HCl buffer, pH 7.5, 5 mm β-mercaptoethanol) supplemented with 4% (w/v) polyvinylpyrrolidone (Sigma). Heat-labile proteins were precipitated by incubation at 50°C (20 min). The supernatant was cleared up by centrifugation at 17,000g, then mixed with PEG6000 to a final 20% (w/v) concentration. The homogenate was stirred for 30 min, then incubated for 2 h on ice. Precipitated proteins were removed by centrifugation at 17,000g for 20 min and the supernatant was loaded on a DEAE-Sepharose column (2.4 × 15 cm) equilibrated with the purification buffer (flow rate: 25 mL h−1). The column was carefully washed with the same buffer to eliminate unbound proteins and PEG. Proteins were eluted with a linear gradient of NaCl (400 mL, 0–0.4 m in the buffer). Fractions (5 mL) were collected and assayed for DXK activity. Active fractions were pooled and desalted by dialysis against purification buffer (3 × 1 L), followed by loading onto a Sepharose Q column (1 × 8 cm) equilibrated with the same buffer. After washing, bound proteins were eluted with a linear gradient (60 mL) of increasing concentration of NaCl (0–0.3 m) in the buffer. Fractions (1 mL) were collected, assayed for DXK activity, and analyzed by SDS-PAGE. Those with the highest activity were pooled and concentrated using ultrafiltration Centricon 30 concentrators (Millipore).
Protein Concentration Determination and SDS-PAGE
Protein concentration was estimated using the protocol of Bradford (1976), with the Bio-Rad protein assay reagent (Bio-Rad) and bovine serum albumin as a standard. Vertical SDS-PAGE was done according to Laemmli (1970) using a minigel system (Hoefer Scientific Instruments) and 12% (w/v) acrylamide/0.1% (w/v) SDS gel (0.75 mm) gels.
Identification by Mass Spectrometry and Database Searches
SDS-PAGE gels were scanned (GS-800; Bio-Rad) and analyzed with QuantityOne software (Bio-Rad). Proteins bands were excised and in-gel digested with trypsin according to published methods (Jeno et al., 1995), modified for use with a robotic digestion system (MassPREP; Micromass). MALDI-TOF-MS measurements were carried out on an UltraflexTOF/TOF (Bruker Daltonics). Samples were handled as described by Hwang et al. (2006). The resulting peptide mass fingerprints were compared to a local copy of the database Swissprot and TrEMBL using MASCOT (Perkins et al., 1999) search software. The parameters used in the search were as followed: peptide mass tolerance 50 ppm, one missed cleavage, carboxymethylated Cys, Met oxidation, and N-terminal acetylation.
Isolation of Arabidopsis cDNAs Coding for Putative Xylulose Kinases
Total RNA was isolated from seedlings (0.8 g) according to the protocol described by Chomczynski and Sacchi (1987). The final RNA pellet was dissolved in DEPC-treated water (100 μL). mRNAs were purified using the Dynabeads mRNA kit (Dynal A.S.) according to the manufacturer's protocol. Double-strand cDNA synthesis was carried out using 2 μg of mRNA, 1 μg of the oligonucleotide (dT)18, and the SuperScript system for cDNA synthesis (Life Technologies S.A.R.L.) according to the manufacturer's instructions. PCR amplification (50 μL) was achieved using one-twentieth of double-strand cDNA, 0.2 μm of the specific forward primer (AtXK1F, His6-AtXK1F, or His6-AtXK2F), the reverse primer (AtXK1R or AtXK2R), and the 2× High Fidelity PCR Master system (Roche Diagnostics). The sequences of the primers are listed in Table I. The reaction was set up for 30 cycles with an annealing temperature of 45°C, an elongation temperature of 72°C for 45 s, and a denaturation temperature of 94°C for 45 s. The PCR products were purified using the QIAEX II kit (Qiagen), cloned into pGEMT (Promega), and sequenced from the 5′ end of the cDNA under the conditions and with the reagents for PCR sequencing (T3 dye primers; Applied Biosystems).
Table I.
Oligonucleotides used in this study
The nucleotides in bold represent restriction sites used for further cloning. KO, Knockout.
| Name | Sequence | Use |
|---|---|---|
| (dT)18NotI | 5′-GACTAGTTCTAGATCGCGAGCGGCCGCCC(T)18-3′ | cDNA preparation |
| His6-AtXK1-F | 5′-GGGAATTCCATATGCACCATCATCATCATCATGATTTTGGTACTTCTG-3′ | E. coli protein expression |
| AtXK1-F | 5′-GGGAATTCCATATGGATTTTGGTACTTCTG-3′ | E. coli protein expression |
| AtXK1-R | 5′-CGCGGATCCGCGTCAGAAGTGGCCTAACTTCTC-3′ | E. coli protein expression |
| His6-AtXK2-F | 5′-GGGAATTCCATATGCACCATCATCATCATCATGCGGATCTCTCTCTTCC-3′ | E. coli protein expression |
| AtXK2-R | 5′-CGCGGATCCGCGTCAGAAGTGGCCTAACTTCTC-3′ | E. coli protein expression |
| PT-AtXK1GFPF | 5′-CATGCCATGGTGATTCTTCGTCAATTTCAG-3′ | GFP expression |
| PT-AtXK1GFPR | 5′-GCTCACCATGGTACCAAAATCCATACCCAG-3′ | GFP expression |
| TcAtXK1GFPFSacI | 5′-CGGCATGGACGAGCTCAGTGGCAATAAAGGAACGAACT-3′ | GFP expression |
| TcAtXK1GFPRXbaI | 5′-TCCTAGTCTAGATCACAAACCACTGTTCTGTTTTGCG-3′ | GFP expression |
| TcAtXK1GFP-MutRXbaI | 5′-TCCTAGTCTAGATCAGTTCTGTTTTGCGCCCTTTAGCGCA-3′ | GFP expression |
| TcAtXK1GFPFNcoI | 5′-CATGCCATGGGCAATAAAGGAACGAACT-3′ | GFP expression |
| TcAtXK1GFPRNcoI | 5′-ATGCCATGGCATGCAAACCACTGTTCTGTTTTGCG-3′ | GFP expression |
| AtXK2GFPFSacI | 5′-GACGAGCTCATGGCGGATCTCTCTCTTC-3′ | GFP expression |
| AtXK2GFPRXbaI | 5′-TCCTAGTCTAGATCAGAAGTGGCCTAACT-3′ | GFP expression |
| AtXK2GFPFNcoI | 5′-CATGCCATGGATGGCGGATCTCTCTCTTC-3′ | GFP expression |
| AtXK2GFPRNcoI | 5′-CATGCCATGGCATGGAAGTGACCTAACTTCTC-3′ | GFP expression |
| SA1 | 5′-GGCTTGACCAGGATTTCCAGATG-3′ | KO mutant analysis |
| AA1 | 5′-CTGGGAGCCAAGTTAGGATCAGC-3′ | KO mutant analysis |
| SA2 | 5′-CTCTCTTCCTCCCGATTCCCTCT-3′ | KO mutant analysis |
| AA2 | 5′-TAGGAGATTCCTTAACCGAAAAAGC-3′ | KO mutant analysis |
| LBb1 | 5′-GCGTGGACCGCTTGCTGCAACT-3′ | KO mutant analysis |
| RTAtXK1F | 5′-CTCTACAACCAGAGCTGCCC-3′ | RT-PCR |
| RTAtXK1R | 5′-TCCATACCTCGCATCATCAA-3′ | RT-PCR |
| RTAtXK2F | 5′-GATGCTGCTGGGATGAATTT-3′ | RT-PCR |
| RTAtXK2R | 5′-CATGACCCTCAAGACTGGGT-3′ | RT-PCR |
| Act2F2 | 5′-GCACCCTGTTCTTCTTACCG-3′ | RT-PCR |
| Act2R2 | 5′-GCTGGTCTTTGAGGTTTCCA-3′ | RT-PCR |
| CAT1 | 5′-GAGTCCGAATAAATACCTGTG-3′ | E. coli deletion mutant |
| CAT4 | 5′-CCGAATTTCTGCCATTCATCC-3′ | E. coli deletion mutant |
| XKCATF | 5′-CGGCTAACTGTGCAGTCCGTTGGCCCGGTTATCGGTAGCGATACCGGGCATTGAGTCCGAATAAATACCTGTG-3′ | E. coli deletion mutant |
| XKCATR | 5′-CATACTAAATATTAATTAATTGCTGAGATATATAGATGTGAATTATCCCCCACCCCCGAATTTCTGCCATTCATCC-3′ | E. coli deletion mutant |
| XF | 5′-GAAGATGGCGAGCTGGATAAACGC-3′ | E. coli deletion mutant |
| XR | 5′-GATAAACCAGCGCCCCGACAAC-3′ | E. coli deletion mutant |
d-DXK and d-XK Enzyme Assays
The kinetic parameters (Vmax and Km) of XK using either d-xylulose or DX as substrate were determined using a coupled spectrophotometric assay. The assays (500 μL) were carried out at 37°C in the assay buffer (Tris-HCl buffer, pH 7.5 [50 mm], containing MgCl2 [5 mm], KCl [10 mm], and DTE [2 mm]). Initial concentrations of ATP, phosphoenolpyruvate, and NADH were 2.5, 0.6, and 0.25 mm, respectively. The concentration of d-xylulose was increased from 0.15 to 0.8 mm for the E. coli enzyme and from 0.03 to 0.2 mm for the Arabidopsis enzyme. The concentration of DX was increased from 0.4 to 2 mm for the E. coli enzyme and from 0.3 to 1.2 mm for the Arabidopsis enzyme. After addition of pyruvate kinase (8 U) and lactate dehydrogenase (8 U), the mixture was preincubated for 5 min to follow unspecific formation of ADP. XK from E. coli (0.11 μg for d-xylulose and 2.2 μg for DX) or Arabidopsis (0.15 μg for d-xylulose and 0.6 μg for DX) was added and the reaction was monitored at 340 nm to follow the oxidation of NADH to NAD+.
The radiometric DXK assays were performed according to the method conceived to determine d-XK activity (Tritsch et al., 2004). The enzymatic reaction (25 μL) was carried out at 37°C in the assay buffer. The concentrations of ATP and DX were 20 mm and 3.5 mm, respectively. At given times, aliquots (2 μL) were withdrawn and loaded onto silica plates that were developed in (6:1:3, v/v/v) n-propyl alcohol:ethyl acetate:water and analyzed using an automatic TLC-linear analyzer (LB2820-1; Berthold) or a PhosphorImager (Molecular Dynamics). The nucleoside triphosphate specificity was analyzed using the radiometric assay. The concentration of ATP, GTP, CTP, or UTP was 4 mm. To determine the rate of XK activity, d-xylulose was obtained in situ from d-Xyl (3 mm) and [U-14C]d-Xyl (0.067 μCi, 33 μm) and the enzymatic reaction was initiated by addition of XK (0.03 μg for E. coli or 0.05 μg for Arabidopsis). Aliquots (2 μL) were withdrawn after 2, 4, and 6 min incubation to measure the amount of synthesized XP (Tritsch et al., 2004). To measure the rate of DXK activity, the concentrations of DX and [14C]DX were 3 mm and 0.5 mm. The enzymatic reaction was initiated by addition of XK of E. coli (0.22 μg) or Arabidopsis (0.15 μg). Aliquots (2 μL) were withdrawn after 3, 6, and 9 min incubation to determine the amount of DXP.
Transient Expression of GFP Fusion Proteins
At-xk1 and At-xk2 were 3′ and 5′ fused to GFP using the primers listed in Table I and transformed into tobacco BY-2 or xanthi cells. The open reading frame corresponding to the first 43 amino acids of AtXK-1 was modified by PCR using PT-AtXK1GFP-F and PT-AtXK1GFP-R (Table I) as primers and cDNAs as template as described in the preceding section. The amplified fragment was digested with NcoI and cloned in-frame into the NcoI site of the GFP expression vector (pGFP; Hemmerlin et al., 2006) to yield a fusion of the putative transit peptide with the N terminus of GFP (pPTA1-GFP). Two further constructs were made to obtain an N-terminal fusion protein. For this, the primer sets TcAtXK1GFPFNcoI/TcAtXK1GFPRNcoI and TcAtXK2GFPFNcoI/TcAtXK2GFPRNcoI were used to amplify the inserts and to clone them into the NcoI restriction site of pGFP. The resulting plasmids were named pTcAtxk-1-GFP and pAtxk-2-GFP. Finally, in the same way, constructs containing inserts coding for C-terminal GFP fusion proteins were made. The primer set TcAtXK1GFPFSacI/TcAtXK1GFPRXbaI, TcAtXK1GFPFSacI/TcAtXK1GFP-MutRXbaI, or AtXK2GFPFSacI/AtXK2GFPRXbaI was utilized to amplify a DNA fragment used to clone Atxk-1 or Atxk-2 in-frame at the 3′ site of GFP, after digestion, into the SacI and XbaI sites of pGFP. The resulting plasmids were named pGFP-TcAtxk-1, pGFP-TcAtxk-1-MutSGL, and pGFP-Atxk-2. To obtain the plasmid pPT5-RFP, the chloroplast-specific transit peptide PT5 (Hemmerlin et al., 2003b) was digested with NcoI and cloned into pRFP (Hemmerlin et al., 2006).
Tobacco suspension cells were used for transformation by tungsten particle shooting with an inflow gun containing the different plasmid constructs as indicated. After incubation (5–20 h at 23°C), transformed cells were examined using a Zeiss LSL510 confocal laser scanning microscope equipped with an inverted Zeiss Axiovert 10M microscope and a 63×, 1.2 numerical aperture water immersion objective. GFP was excited at 488 nm using an argon laser and emission spectra were recorded from 505 nm to 530 nm. Chlorophyll as well as RFP were excited at 543 nm using a helium laser and emission was recorded at wavelength higher than 585 nm. Images were handled with the Zeiss LSM Image Browser Version 2.50.0929 software and exported as TIFF files before processed for printing using Photoshop 5.0 (Adobe Systems).
Cloning of Kinase Genes into the Prokaryotic pET3-a Expression Vector, Protein Overexpression, and Purification
The genes encoding putative XK from Arabidopsis were digested with the endonucleases NdeI and BamHI, for which restriction sites were introduced by PCR amplification using the primers listed in Table I, then ligated into the corresponding cloning sites of the prokaryotic expression vector pET3-a (Novagen) prepared by the same way. This allowed us to obtain the plasmids designated as pET-(His)6-AtXK-1 and pET-(His)6-AtXK-2. The E. coli gene cloned into pET-3a expression vector was obtained as described by Tritsch et al. (2004).
E. coli BL21-DE3 or Rosetta-gami cells were transformed with pET-(His)6-Ec-XK, pET-(His)6-AtXK-1, and pET-(His)6-AtXK-2. Overnight cultures were 100-fold diluted into LB medium and grown at 37°C until the OD600 reached 0.3, at which time IPTG was added to a final concentration of 0.5 mm. After 12 h of incubation at 23°C, the cells were harvested. Purified protein used for enzyme characterization was obtained using the Ni2+ spin columns, according to the manufacturer's protocol. The enzymes were dialyzed overnight against Tris-HCl buffer (50 mm, pH 7.5) containing 5 mm β-mercaptoethanol. The degree of purification of the fractions was analyzed by SDS-PAGE.
Functional Complementation in E. coli Knockout Mutants
The xylB gene is part of an operon (xylAB operon: 3,725,940–3,728,922 min) containing two genes: xylA encoding Xyl isomerase, an enzyme catalyzing the conversion of d-Xyl into d-xylulose, and xylB, respectively (http://regulondb.ccg.unam.mx/index.html). To generate an E. coli strain deleted in d-XK, xylB was replaced by the chloramphenicol resistance cassette. For this, the cassette was amplified from pLysE (Novagen) using CAT1 and CAT4 as primer sets (Table I). The cassette was cloned into pGEMT (Promega), sequenced, and bacteria transformed with these plasmids were allowed to grow on selective LB medium. The E. coli DY329 strain was engineered as described by Yu et al. (2000) using the primers sets XKCATF and XKCATR, listed in Table I. Deletion of the gene was examined by isolation of genomic DNA and PCR amplification with XF and XR. It was verified that xylA (coding for Xyl isomerase), the first enzyme constituting the operon, remained functional. Total protein extracts isolated from the xk∷cat strain were subjected to a coupled XK enzyme assay in which Xyl isomerase was missing and a recombinant E. coli XK was used as described by Tritsch et al. (2004). The strain was transformed with pET, pET-DXKcoli, pET-At-XK1, or pET-At-XK2. The strains were cultured in LB medium (3 mL), washed three times with sterile water, diluted 100 times, and 10 μL was deposited on 1/5 sections of each petri dish containing LB (20 mL), LB (20 mL) supplemented with chloramphenicol (30 μg/mL), M9 medium (20 mL) supplemented with 0.2% Glc, or 20 mL M9 medium supplemented with 0.2% Xyl. The LB-petri dishes were incubated for 2 d at 30°C, whereas the M9 petri dishes were incubated for 5 d.
Characterization of the At-xk-1 and At-xk-2 Alleles
Both Arabidopsis knockout mutants were generated at the Salk Institute Genomic Analysis Laboratory collection (http://signal.salk.edu; Alonso et al., 2003). Atxk-1 (SALK_094661, N542149) and the Atxk-2 knockout mutants (SALK_079018, N519643) were obtained from the Nottingham Arabidopsis Stock Centre (http://Arabidopsis.info/). Using the primer sets SA1/AA1 and SA1/LBb1, homozygous plants Atxk-1∷T-DNA were selected. In the same way, using the SA2/AA2 and SA2/LBb1 primer pairs, Atxk-2∷T-DNA plants were selected. PCR amplifications were performed with Taq polymerase (Invitrogen) according to the manufacturer's instructions, using 250 pm of each sense (S) and antisense (A) primer. An initial denaturation step for 3 min at 94°C was followed by 30 cycles of 45 s at 94°C, 45 s at 52°C, and 45 s at 72°C. For RT-PCR experiments, 1 μg of total RNA was extracted with TRIzol reagent (Invitrogen), DNAse I treated, and finally reverse transcribed by the aid of SuperScript III reverse transcriptase (Invitrogen) using 500 ng of oligo(dT). A fraction (about one-twentieth) of the first-strand cDNAs was used as a template for PCR with gene-specific primers (in a volume of 20 μL with 1 unit of Taq polymerase, 250 μm each dNTP, and 250 pm of each primer [RTAtXK1F/RTAtXK1R or RTAtXK2F/RTAtXK2R]). An initial denaturation step for 5 min at 95°C was followed by 35 cycles of 45 s at 95°C, 45 s at 55°C, and 1 min at 72°C. Amplification of actin 2 (At3g18780) cDNA using primers Act2F2 and Act2R2 served as an internal control. In addition, for further control, primers were designed to amplify intron-containing DNA sequences, should any contaminating genomic DNA be present. PCR products were separated on 1% (w/v) agarose gels containing ethidium bromide and visualized by UV light.
Acknowledgments
Prof. Donald L. Court (National Cancer Institute, Frederick, MD) is acknowledged for providing the DY329 E. coli strain and the Salk Institute Genomic Analysis Laboratory for making available the insertional mutants. We thank Dr. Klaus Grossmann (BASF, Limburgerhof, Germany) for a generous gift of OC and Ms. Deborah Brandow (Novartis, Basel) for reading the English manuscript. We are indebted to Dr. Hubert Schaller (IBMP, Strasbourg, France) for helpful advice in handling Arabidopsis insertion mutants and Marta Ramel (IBMP) for taking care of the plants in the greenhouse. We thank the Centre National de la Recherche Scientifique, the Université Louis Pasteur, the Région Alsace, La Ligue, and the Association pour la Recherche sur le Cancer for the support of the Inter-Institute confocal microscopy equipment at IBMP Strasbourg.
This work was supported by the Centre National de la Recherche Scientifique, the Université Louis Pasteur, and the Agence Nationale de la Recherche (grant TERPENE Nb ANR–NT05–3_45695). In addition, M.R. is supported by the Institut Universitaire de France, and M.H. is the recipient of a doctoral fellowship from the Région Alsace.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Andréa Hemmerlin (andrea.hemmerlin@ibmp-ulp.u-strasbg.fr).
References
- Adam KP, Thiel R, Zapp J (1999) Incorporation of 1-[1-13C]deoxy-D-xylulose in chamomile sesquiterpenes. Arch Biochem Biophys 369: 127–132 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Arigoni D, Eisenreich W, Latzel C, Sagner S, Radykewicz T, Zenk MH, Bacher A (1999) Dimethylallyl pyrophosphate is not the committed precursor of isopentenyl pyrophosphate during terpenoid biosynthesis from 1-deoxyxylulose in higher plants. Proc Natl Acad Sci USA 96: 1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernart MW, Gerwick WH, Corcoran EE, Lee AY, Clardy J (1992) Laurencione, a heterocycle from the red alga Laurencia spectabilis. Phytochemistry 31: 1273–1276 [Google Scholar]
- Bork P, Sander C, Valencia A (1993) Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase and galactokinase families of sugar kinases. Protein Sci 2: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier F, d'Harlingue A, Suire C, Backhaus RA, Camara B (1998) Dedicated roles of plastid transketolases during the early onset of isoprenoid biogenesis in pepper fruits. Plant Physiol 117: 1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Broers STJ (1994) Über die frühen Stufen der Biosynthese von Isoprenoiden in Escherichia coli. PhD thesis. Dissertation No. 10978. Eidgenössische Technische Hochschule, Zurich
- Büttner M, Sauer N (2000) Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta 1465: 263–274 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Curtis SE, Clegg MT (1984) Molecular evolution of chloroplast DNA sequences. Mol Biol Evol 1: 291–301 [DOI] [PubMed] [Google Scholar]
- Dittrich P, Angyal SJ (1988) 2-C-Methyl-D-erythritol in leaves of Liriodendron tulipifera. Phytochemistry 27: 935 [Google Scholar]
- Duvold T, Calí P, Bravo J-M, Rohmer M (1997) Incorporation of 2-C-methyl-D-erythritol, a putative isoprenoid precursor in the mevalonate-independent pathway, into ubiquinone and menaquinone of Escherichia coli. Tetrahedron Lett 38: 6181–6184 [Google Scholar]
- Eicks M, Maurino V, Knappe S, Flügge UI, Fischer K (2002) The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol 128: 512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes MJ, Neuhaus HE (1997) Metabolism and transport in non-photosynthetic plastids. J Exp Bot 48: 1995–2005 [Google Scholar]
- Estévez JM, Cantero A, Romero C, Kawaide H, Jimenez LF, Kuzuyama T, Seto H, Kamiya Y, León P (2000) Analysis of the expression of Cla1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2C-methyl-D-erythritol 4-phosphate pathway in Arabidopsis. Plant Physiol 124: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhatoglu Y, Barrett M (2006) Studies of clomazone mode of action. Pestic Biochem Physiol 85: 7–14 [Google Scholar]
- Ferro M, Salvi D, Brugière S, Miras S, Kowalski S, Louwagie M, Garin J, Joyard J, Rolland N (2003) Proteomics of the chloroplast envelope membranes from Arabidopsis thaliana. Mol Cell Proteomics 2: 325–345 [DOI] [PubMed] [Google Scholar]
- Flügge UI, Gao W (2005) Transport of isoprenoid intermediates across chloroplast envelope membranes. Plant Biol 7: 91–97 [DOI] [PubMed] [Google Scholar]
- Fontana A, Kelly MT, Prasad JD, Anderson RJ (2001) Evidence for the biosynthesis of squalene via the methylerythritol phosphate pathway in a Streptomyces sp. obtained from a marine sediment. J Org Chem 66: 6202–6206 [DOI] [PubMed] [Google Scholar]
- Gao Z, Maurousset L, Lemoine R, Yoo S-D, van Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131: 1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits N, Turk SCHJ, van Dun KPM, Hulleman SHD, Visser RGF, Weisbeek PJ, Smeekens SCM (2001) Sucrose metabolism in plastids. Plant Physiol 125: 926–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner J-L, Jaun B, Arigoni D (1998) Biosynthesis of isoprenoids in Escherichia coli: the fate of the 3-H and 4-H atoms of 1-deoxy-D-xylulose. Chem Commun 1857–1858
- Hampel D, Mosandl A, Wüst M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66: 305–311 [DOI] [PubMed] [Google Scholar]
- Hemmerlin A, Hoeffler J-F, Meyer O, Tritsch D, Kagan I, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003. a) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco BY-2 cells. J Biol Chem 278: 26666–26676 [DOI] [PubMed] [Google Scholar]
- Hemmerlin A, Reents R, Mutterer J, Feldtrauer J-F, Waldmann H, Bach TJ (2006) Monitoring farnesol induced toxicity in tobacco BY-2 cells with a fluorescent analog. Arch Biochem Biophys 448: 93–103 [DOI] [PubMed] [Google Scholar]
- Hemmerlin A, Rivera SB, Erickson HK, Poulter CD (2003. b) Enzymes encoded by the farnesyl diphosphate synthase gene family in the Big Sagebrush Artemisia tridentata ssp. spiciformis. J Biol Chem 278: 32132–32140 [DOI] [PubMed] [Google Scholar]
- Hemmi H, Yamashita S, Nakayama T, Nishino T (2002) Novel sugar phosphotransferase system applicable to the efficient labeling of the compounds synthesized via the non-mevalonate pathway in Escherichia coli. J Biosci Bioeng 93: 515–518 [DOI] [PubMed] [Google Scholar]
- Hill RE, Sayer BG, Spenser JD (1989) Biosynthesis of vitamin B6: incorporation of D-1-deoxyxylulose. J Am Chem Soc 111: 1916–1917 [Google Scholar]
- Hoeffler J-F, Hemmerlin A, Grosdemange-Billiard C, Bach TJ, Rohmer M (2002) Isoprenoid biosynthesis in higher plants and Escherichia coli: on the branching in the methylerythritol phosphate pathway and the independent biosynthesis of isopentenyl diphosphate and dimethylallyl diphosphate. Biochem J 366: 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K-H, Carapito C, Böhmer S, Leize E, van Dorsselaer A, Bernhardt R (2006) Proteome analysis of Schizosaccharomyces pombe by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 6: 4115–4129 [DOI] [PubMed] [Google Scholar]
- Jackson S, Nicholson SW (2002) Xylose as a nectar sugar: from biochemistry to ecology. Comp Biochem Physiol 131: 613–620 [DOI] [PubMed] [Google Scholar]
- Jeno P, Mini T, Moes S, Hintermann E, Horst M (1995) Internal sequences from proteins digested in polyacrylamide gels. Anal Biochem 224: 75–82 [DOI] [PubMed] [Google Scholar]
- Kasahara H, Hanada A, Kuzuyama T, Tagagi M, Kamiya Y, Yamaguchi S (2002) Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J Biol Chem 277: 45188–45194 [DOI] [PubMed] [Google Scholar]
- Knappe S, Flügge UI, Fischer K (2003) Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol 131: 1178–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger NJ, von Schaewen A (2003) The oxidative pentose phosphate pathway: structure and organization. Curr Opin Plant Biol 6: 236–246 [DOI] [PubMed] [Google Scholar]
- Kuntz L, Tritsch D, Grosdemange-Billiard C, Hemmerlin A, Willem A, Bach TJ, Rohmer M (2005) Isoprenoid biosynthesis as target for antibacterial and antiparasitic drugs: phosphonohydroxamic acids as inhibitors of deoxyxylulose phosphate reducto-isomerase. Biochem J 386: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuyama T, Takahashi S, Seto H (1999) Construction and characterization of Escherichia coli disruptants defective in the yaeM gene. Biosci Biotechnol Biochem 63: 776–778 [DOI] [PubMed] [Google Scholar]
- Kuzuyama T, Takahashi S, Takagi M, Seto H (2000) Characterization of 1-deoxy-D-xylulose 5-phosphate reductoisomerase, an enzyme involved in isopentenyl diphosphate biosynthesis, and identification of its catalytic amino acid residues. J Biol Chem 275: 19928–19932 [DOI] [PubMed] [Google Scholar]
- Labavitch JM (1981) Cell wall turnover in plant development. Annu Rev Plant Physiol 32: 385–406 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lange BM, Wildung MR, McCaskill D, Croteau R (1998) A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA 95: 2100–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LV, Gerratana B, Cleland WW (2001) Substrate specificity and kinetic mechanism of Escherichia coli ribulokinase. Arch Biochem Biophys 396: 219–224 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Lois LM, Campos N, Rosa-Putra S, Danielsen K, Rohmer M, Boronat A (1998) Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of D-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxal biosynthesis. Proc Natl Acad Sci USA 95: 2105–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois LM, Rodríguez-Concepción M, Gallégo F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J 22: 503–513 [DOI] [PubMed] [Google Scholar]
- Luan F, Wüst M (2002) Differential incorporation of 1-deoxy-D-xylulose into (3S)-linalool and geraniol in grape berry and mesocarp. Phytochemistry 60: 451–459 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Feldmann KA, Herréra-Estrélla L, Rocha-Sosa M, León P (1996) CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J 9: 649–658 [DOI] [PubMed] [Google Scholar]
- Müller C, Schwender J, Zeidler J, Lichtenthaler HK (2000) Properties and inhibition of the first two enzymes of the non-mevalonate pathway of isoprenoid biosynthesis. Biochem Soc Trans 28: 792–793 [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “Hela” cell in the cell biology of higher plants. Int Rev Cytol 132: 1–30 [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Reinders A, Panshyshyn JA, Ward JM (2005) Analysis of transport activity of Arabidopsis sugar alcohol permease homolog AtPLT5. J Biol Chem 280: 1594–1602 [DOI] [PubMed] [Google Scholar]
- Richard P, Toivari MH, Penttilä M (2000) The role of xylulokinase in Saccharomyces cerevisiae xylulose catabolism. FEMS Microbiol Lett 190: 39–43 [DOI] [PubMed] [Google Scholar]
- Rohdich F, Kis K, Bacher A, Eisenreich W (2001) The non-mevalonate pathway of isoprenoids: genes, enzymes and intermediates. Curr Opin Chem Biol 5: 535–540 [DOI] [PubMed] [Google Scholar]
- Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16: 565–574 [DOI] [PubMed] [Google Scholar]
- Rosa-Putra S, Charon L, Danielsen K, Pale-Grosdemange C, Lois LM, Campos N, Boronat A, Rohmer M (1998. a) 5-Hydroxypentane-2,3-dione (laurencione), a bacterial metabolite of 1-deoxy-D-threo-pentulose. Tetrahedron Lett 39: 6185–6188 [Google Scholar]
- Rosa-Putra S, Lois LM, Campos N, Boronat A, Rohmer M (1998. b) Incorporation of [2,3-13C2] and [2,4-13C2]-D-1-deoxyxylulose into ubiquinone of Escherichia coli via the mevalonate-independent pathway for isoprenoid biosynthesis. Tetrahedron Lett 39: 23–26 [Google Scholar]
- Rosenfeld SA, Stevis PE, Ho NW (1984) Cloning and characterization of the xyl genes from Escherichia coli. Mol Gen Genet 194: 410–415 [DOI] [PubMed] [Google Scholar]
- Sagner S, Eisenreich W, Fellermeier M, Latzel C, Bacher A, Zenk MH (1998) Biosynthesis of 2-C-methyl-D-erythritol in plants by rearrangement of the terpenoid precursor, 1-deoxy-D-xylulose 5-phosphate. Tetrahedron Lett 39: 2091–2094 [Google Scholar]
- Schnarrenberger C, Flechner A, Martin W (1995) Enzymatic evidence for a complete oxidative pentose phosphate pathway in chloroplasts and an incomplete pathway in the cytosol of spinach leaves. Plant Physiol 108: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M (1994) Terpenbiosynthese in Ginkgo biloba: eine überraschende Geschichte. PhD thesis 10951. Eidgenössische Technische Hochschule, Zurich
- Schwender J, Zeidler J, Groner R, Müller C, Focke M, Braun S, Lichtenthaler FW, Lichtenthaler HK (1997) Incorporation of 1-deoxy-D-xylulose into isoprene and phytol by higher plants and algae. FEBS Lett 414: 129–134 [DOI] [PubMed] [Google Scholar]
- Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54: 525–531 [DOI] [PubMed] [Google Scholar]
- Spiteller D, Jux A, Piel J, Boland W (2002) Feeding of [5,5-2H2]-1-desoxy-D-xylulose and [4,4,6,6,6-2H5]-mevalolactone to a geosmin-producing Streptomyces sp. and Fossombronia pusilla. Phytochemistry 61: 827–834 [DOI] [PubMed] [Google Scholar]
- Sprenger GA, Schorken U, Wiegert T, Grolle S, de Graaf AA, Taylor SV, Begley TP, Bringer-Meyer S, Sahm H (1997) Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-D-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci USA 94: 12857–12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CA, Cornish RM, Poulter CD (2004) The sorbitol phosphotransferase system is responsible for transport of 2-C-methyl-D-erythritol into Salmonella enterica serovar typhimurium. J Bacteriol 186: 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Méndez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270: 1043–1049 [DOI] [PubMed] [Google Scholar]
- Tritsch D, Hemmerlin A, Rohmer M, Bach TJ (2004) A sensitive radiometric assay to measure D-xylulose kinase activity. J Biochem Biophys Methods 58: 75–83 [DOI] [PubMed] [Google Scholar]
- Weber APM, Schwacke R, Flügge U-I (2005) Solute transporters of the plastid envelope membrane. Annu Rev Plant Biol 56: 133–164 [DOI] [PubMed] [Google Scholar]
- Wolf E, Kennedy IA, Himmeldirk K, Spenser ID (1997) 5-Hydroxypentane-2,3-dione and 3-amino-1-hydroxypropan-2-one, putative precursors of vitamin B6. Can J Chem 75: 942–948 [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F (2004) Rapid regulation of the methylerythritol 4-phosphate pathway during isoprene synthesis. Plant Physiol 135: 1939–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wungsintaweekul J (2001) Enzymes of the alternative terpenoid pathway in Escherichia coli. PhD thesis. Technische Universität München, Munich
- Wungsintaweekul J, Herz S, Hecht S, Eisenreich W, Feicht R, Rohdich F, Bacher A, Zenk MH (2001) Phosphorylation of 1-deoxy-D-xylulose by D-xylulokinase of Escherichia coli. Eur J Biochem 268: 310–316 [DOI] [PubMed] [Google Scholar]
- Yokota A, Sasajima K (1984) Formation of 1-deoxy-D-threo-pentulose and 1-deoxy-L-threo-pentulose by cell-free extracts of microorganisms. Agric Biol Chem 48: 149–158 [Google Scholar]
- Yokota A, Sasajima K (1986) Formation of 1-deoxy-ketose by pyruvate dehydrogenase and acetoin dehydrogenase. Agric Biol Chem 50: 2517–2524 [Google Scholar]
- Yu D, Ellis HM, Lee E-C, Jenkins NA, Copeland NG, Court DL (2000) An efficient recombinant system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA 97: 5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahnley JC, Axelrod B (1964) D-Xylulokinase and D-ribulokinase in higher plants. Plant Physiol 40: 372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]