Abstract
The synergid cell of Torenia fournieri attracts pollen tubes by a diffusible but yet unknown chemical attractant. Here we investigated the species difference of the attractant using five closely related species in two genera, namely T. fournieri, Torenia baillonii, Torenia concolor, Lindernia (Vandellia) crustacea, and Lindernia micrantha. These five species have an exserted embryo sac, and ablation experiments confirmed that their synergid cells attracted the pollen tube. When ovules of T. fournieri and one of the other species were cultivated together with pollen tubes of each species, pollen tubes were significantly more attracted to synergid cells of the corresponding species. The attraction was not affected by the close proximity of embryo sacs of different species. This suggests that the attractant is a species-preferential molecule that is likely synthesized in the synergid cell. The calcium ion, long considered a potential attractant, could not serve as the sole attractant in these species, because elevation of the calcium ion concentration did not affect the observed attraction. In vivo crossing experiments also showed that the attraction of the pollen tube to the embryo sac was impaired when pollen tubes of different species arrived around the embryo sac, suggesting that the species preferentiality of the attractant may serve as a reproductive barrier in the final step of directional control of the pollen tube.
The chemistry of pollen tube guidance has been studied for more than a century. Many attempts have been made to identify the guidance cues that regulate the directional growth of pollen tubes in pistils. Several molecules potentially involved in pollen tube guidance have been identified, including the guidance cue. External calcium ions were first identified as a potential attractant in the snapdragon (Antirrhinum majus) pistil using classic in vitro tests (Mascarenhas and Machlis, 1962). Other small molecules, including sugars (Reger et al., 1992) and nitric oxide (Prado et al., 2004), are also reported to control directionality of the pollen tube by using in vitro tests. A 9.9-kD basic protein, chemocyanin, was isolated from lily (Lilium longiflorum) stigma proteins by biochemical fractionation using in vitro tests of its ability to attract pollen tubes (Kim et al., 2003). A single homolog of chemocyanin may also be involved in pollen tube guidance in Arabidopsis (Arabidopsis thaliana), according to in vivo experiments (Dong et al., 2005). A gradient of the concentration of water in lipids (triacylglycerides) on the stigmas of tobacco (Nicotiana tabacum) and Arabidopsis was proposed to be critical for the initial directional growth of the pollen tube entering the stigma (Wolters-Arts et al., 1998). Transmitting tissue-specific protein in tobacco (Cheung et al., 1995; Wu et al., 1995, 2000) and stigma/stylar Cys-rich adhesin and pectin in lily (Mollet et al., 2000; Park and Lord, 2003) were identified in style tissue and shown to be necessary for the growth of the pollen tube in the style. An appropriate concentration of γ-aminobutyric acid (GABA) concentrated toward the nucellus tissue surrounding the embryo sac was proposed to aid the pollen tube in navigating toward the ovule (Palanivelu et al., 2003). In the maize (Zea mays) embryo sac (female gametophyte), ZmEA1 is expressed in both the egg cell and the synergid cells, and its product appears to be secreted into the micropyle, which was shown to be required for pollen tube guidance (Márton et al., 2005). Guidance by these molecules allows pollen tubes to grow directionally toward the target embryo sacs. However, both in vivo and in vitro confirmation that these molecules are the true attractants governing pollen tube guidance are required.
The two synergid cells to either side of the egg cell are the most plausible emitters of chemoattractants and are involved in navigating the final growth of the pollen tube toward the embryo sac. In Torenia fournieri, pollen tubes are directly attracted to the exserted embryo sac in vitro (Higashiyama et al., 1998). Laser ablation experiments identified the source of the diffusible signal as the two synergid cells (Higashiyama et al., 2001). Once attracted, pollen tubes of T. fournieri never leave the embryo sac and form narrow coils on the surface of the micropylar end of the embryo sac before entering the sac, suggesting that the pollen tube is trapped at the highest concentration of the chemoattractant. In Arabidopsis, the target embryo sac itself guides the final steps of the pollen tube into the ovary (Hülskamp et al., 1995; Ray et al., 1997; Shimizu and Okada, 2000), and use of the mutant line myb98, which is defective in synergid cell development, revealed that the last guidance toward the micropyle is provided by the synergid cell (Kasahara et al., 2005).
Two molecules have been reported to be candidates for pollen tube attractants that are derived from the synergid cells. One is the external calcium ion. In vitro test results suggest that calcium is an attractant derived from the pistil, in particular the ovule (Mascarenhas and Machlis, 1962, 1964; Reger et al., 1992). Using various histochemical methods including calcium antimonate precipitation, proton-induced x-ray emission, and fluorescent calcium probes, high concentrations of calcium have been observed in synergid cells and the neighboring extracellular matrices in various species (Jensen, 1965; Chaubal and Reger, 1990, 1992a, 1992b, 1993; Huang and Russell, 1992; Tirlapur et al., 1993; Tian and Russell, 1997), including T. fournieri (Kristóf et al., 1999). The second potential attractant is ZmEA1 (Márton et al., 2005). The ZmEA1 gene was originally identified from a maize egg cell cDNA library and was shown to be expressed more abundantly in the synergid cell. ZmEA1 protein fused with green fluorescent protein appeared to be secreted toward the extracellular matrix of the nucellus at the micropyle, and some antisense RNA and RNAi lines showed reduced fertility because of a defect in pollen tube guidance at the micropyle. ZmEA1 is a member of a large family of EA1-like genes found in flowering plants (Gray-Mitsumune and Matton, 2006). ZmEA1 is a good candidate for a synergid cell-derived chemoattractant, but ability of purified ZmEA1 to attract the pollen tube has not yet been demonstrated.
Species difference can provide insight into the chemical properties of the chemoattractant, which might be a more complex compound such as ZmEA1 rather than a low-Mr compound such as the calcium ion. Species difference could also function as a reproductive barrier at the pollen tube guidance step. To determine if the attractant derived from the synergid cell is a species-specific molecule, an in vitro system using the exserted embryo sac seems indispensable, because it enables the exclusion of other guidance steps. When the exserted embryo sac is used, the basal end of the synergid cell is directly exposed to the medium and the attraction signal spreads into the medium from the synergid cell; pollen tubes do not need to grow on the surface of the surrounding tissues of the embryo sac, which may also contribute to pollen tube guidance. By mixing ovules of two species, one can critically examine whether pollen tubes sense the attraction signal of a different species under conditions in which they can surely respond to the attraction signal of their own species. In addition, the condition of the synergid cell is easily observed in the exserted embryo sac, but the attraction can only be examined in embryo sacs with complete synergid cells (the synergid is a fragile cell; Higashiyama et al., 1998). Ablation experiments using UV lasers are also possible in the exserted embryo sac, as shown in T. fournieri (Higashiyama et al., 2001), and can be used to confirm the role of the synergid cell in pollen tube attraction.
In this study, to characterize the properties of the chemoattractant derived from the synergid cell, we investigated the species difference of the attractant using an in vitro T. fournieri system and four closely related species that possess an exserted embryo sac. The attractant from the synergid cell appeared to be a species-preferential molecule and may have a role in the reproductive barrier. Finally, it was suggested that the calcium ion was not solely the synergid cell-derived attractant in T. fournieri.
RESULTS
Scrophulariaceae Species with an Exserted Embryo Sac Can Be Fertilized in Vitro in the Same Manner as T. fournieri
To determine if the attractant derived from the synergid cell was a species-specific molecule, we first surveyed several plant species with exserted embryo sacs. As reviewed by Maheshwari (1950), some flowering plant species exhibit exserted embryo sacs, including some species of Philadelphus, Thesium, Galium, Utricularia, Lindernia (Vandellia), and Torenia. This study required plants with ovaries with a large number of ovules that are suitable for cultivation. Because Galium species possess only a few ovules per ovary, and excision is difficult because the ovules are embedded in the ovarian tissue, these species were unsuitable. Philadelphus species, such as Philadelphus satsumi, possess a large number of ovules with an exserted embryo sac, and their flowers are readily available because of their popularity as ornamental plants. However, Philadelphus ovules tended to brown and inhibit growth of pollen tubes in culture. Utricularia (Utricularia spp.), an aquatic vermivorous plant, was not used because its flowers are difficult to obtain under laboratory conditions.
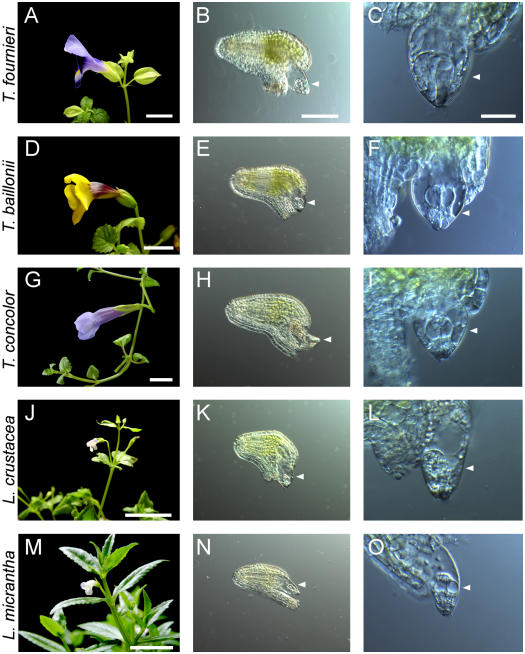
The Torenia and Lindernia genera of the Scrophulariaceae contain several species with an exserted embryo sac. In plants of these genera, up to several hundred ovules were obtained from individual ovaries. These plants easily grew and bloomed in growth chambers; their pollen tubes and ovules were able to be cultivated in the same manner as those of T. fournieri. Torenia plants and flowers were larger than those of Lindernia, and the Torenia genus provided the most easily usable exserted embryo sacs. All of the Torenia species tested possessed an exserted embryo sac, but Lindernia showed variations; among the four tested species, Lindernia crustacea (Vandellia crustacea) and Lindernia micrantha (Vandellia angustifolia) possessed an exserted embryo sac, but Lindernia setulosa (Vandellia setulosa) and Lindernia antipoda (Vandellia anagallis) possessed a normal embryo sac enclosed by the integument. Therefore, we chose five species in two genera for subsequent analyses: T. fournieri, Torenia baillonii, Torenia concolor, L. crustacea, and L. micrantha. Flowers and ovules of these five species are shown in Figure 1. All five species exhibit an exserted embryo sac of the Polygonum type that protrudes from the micropyle of the ovule.
Figure 1.
The five Scrophulariaceae species used in this study with exserted embryo sacs. Flowers (A, D, G, J, and M), ovules (B, E, H, K, and N), and exserted embryo sacs (C, F, I, L, and O) of T. fournieri (A–C), T. baillonii (D–F), T. concolor (G–I), L. crustacea (J–L), and L. micrantha (M–O) are shown. Arrowheads indicate an exserted embryo sac. Bars in A, D, G, J, and M indicate 1 cm; the bar in B indicates 100 μm for B, E, H, K, and N; and the bar in C indicates 30 μm for C, F, I, L, and O.
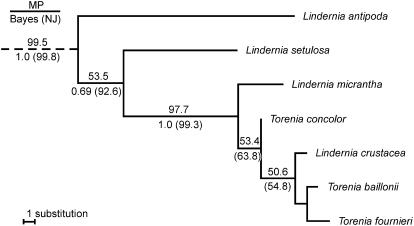
Figure 2 presents the results of a phylogenetic analysis using the large subunit of Rubisco (rbcL) nucleotide sequences from three Torenia and four Lindernia species, as well as 32 other species in the Scrophulariaceae (Wolfe and dePamphilis, 1998). The genes from the Torenia and Lindernia species formed a monophyletic group in the Scrophulariaceae with high bootstrap values: 99.5% for maximum parsimony (MP) analysis and 99.8% for neighbor-joining (NJ) analysis and high posterior probability (1.0 for Bayes' theorem). Within this monophyletic group, the five species with exserted embryo sacs subsequently used in our analyses constituted a clade with high bootstrap values (97.7% for MP and 99.3% for NJ) and high posterior probability (1.0 for Bayes' theorem). These results suggest that the five species are closely related. Among the five species, T. baillonii appeared closest to T. fournieri, while L. micrantha appeared most divergent.
Figure 2.
Phylogenetic reconstruction based on MP analysis of rbcL nucleotide sequences. From an analysis of 41 Scrophulariaceae species, only the branch that contained Torenia and Lindernia is shown. L. antipoda and L. setulosa, which do not produce exserted embryo sacs, are also included. Bootstrap values in percentages for the MP and NJ methods and the posterior probability (for Bayes' theorem) are indicated.
Confirmation of the Synergid Cell Origin of the Attractant in the Studied Species
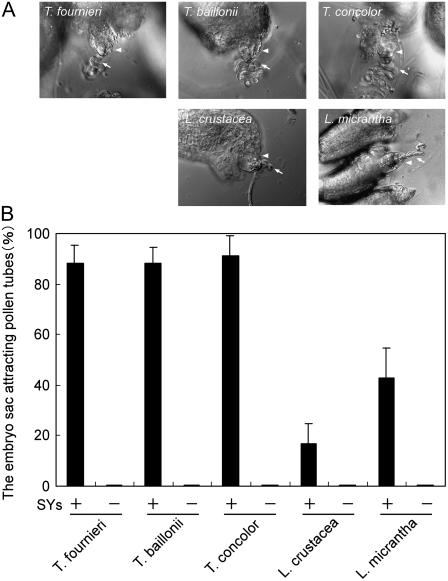
Before investigating the species difference, we confirmed that the pollen tube attraction occurred in vitro in all plant species and that the source of the attraction signal was the synergid cell, as in T. fournieri (Fig. 3). Ovules and a pollinated style of each species were cultivated in a medium previously used for T. fournieri (Higashiyama et al., 1998, 2001). In proportion to the size of the pistil, both the number of ovules and the length and number of pollen tubes increased on this medium. The number of ovules in an ovary was 300 to 500 in T. fournieri and T. baillonii, more than 500 in T. concolor, about 150 in L. crustacea, and about 300 in L. micrantha. After overnight growth in culture, attraction of the pollen tube to the embryo sac was observed in all species (Fig. 3A); there were multiple pollen tubes around ovules, and some of them grew toward the micropylar end of the embryo sac to form a narrow coil, as described for T. fournieri (Higashiyama et al., 1998). Discharge of the pollen tube contents and onset of seed development were observed as frequently as in T. fournieri. When pollen tubes of these species were germinated on the medium, no attraction of pollen tubes was observed (data not shown), as also shown for T. fournieri (Higashiyama et al., 1998), probably because the pollen tubes failed to acquire the response capability.
Figure 3.
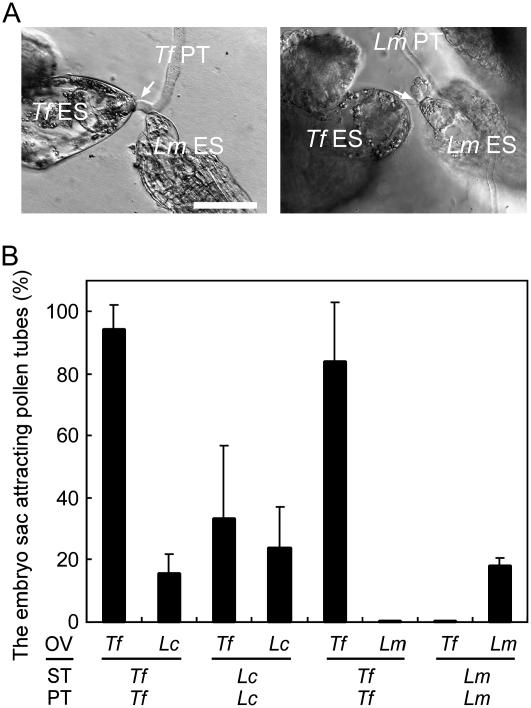
Pollen tube attraction by synergid cell of each plant species in vitro. A, DIC images of pollen tube attraction. The species of both the ovule and pollen tube are indicated at the top left of each segment. Arrowheads indicate an exserted embryo sac, and arrows indicate attracted pollen tubes. B, Frequencies of pollen tube attraction are shown for complete embryo sacs (SYs+) and embryo sacs with two ablated synergid cells (SYs−). Each column indicates the mean value with the sd of three replications. Bar = 50 μm.
The ratios of the complete embryo sac (possessing the egg cell, two synergid cells, and the central cell) attracting pollen tubes are shown in Figure 3B. Most complete embryo sacs of the Torenia species attracted pollen tubes (88.4% ± 6.9% in T. fournieri [n = 141], 88.0% ± 6.5% in T. baillonii [n = 161], and 91.2% ± 8.1% in T. concolor [n = 159]); the percentages refer to the ratio of embryo sacs attracting pollen tubes out of all complete embryo sacs with sd of three replications, and n refers to total number of complete embryo sacs counted. Considering the distance of attraction, a few hundred micrometers at most (Higashiyama et al., 2003), ovules that had pollen tubes in the vicinity (approximately 100 μm) were included in calculating the ratio of pollen tube attraction. The complete embryo sacs of Lindernia species also attracted pollen tubes, but at lower frequencies than Torenia embryo sacs (16.7% ± 8.0% in L. crustacea [n = 132] and 42.8% ± 11.7% in L. micrantha [n = 120]). This result was due to limitations to the numbers and lengths of pollen tubes in Lindernia; pollen tubes could not cover the entire area of the ovules, and there were no pollen tubes in the vicinity of many ovules. It has been proposed that the attraction of the T. fournieri pollen tube by the synergid cell is effective within a few hundred micrometers in this medium (Higashiyama, 2002).
We next confirmed the contribution of the synergid cell to the attraction. When the synergid cells on either side of the egg cell were ablated using a UV laser, the attraction was completely halted in all species (0%; n = 62 for T. fournieri, n = 72 for T. baillonii, n = 66 for T. concolor, n = 60 for L. crustacea, and n = 60 for Lindernia angustifolia; Fig. 3B). We confirmed that in all tested species, the synergid cell attracts the pollen tube, as previously reported for T. fournieri (Higashiyama et al., 2001).
Species Preferentiality of the Synergid Cell-Derived Pollen Tube Attractant
To examine the species difference of the attractants derived from the synergid cells, we cultivated T. fournieri ovules with ovules of other plant species (Fig. 4). Pollen tubes of each plant species were then grown. Ovules of T. fournieri and the other species could be distinguished by appearance (size, shape, and color of chloroplasts in the integument), as shown in Figure 1. However, it was impossible to discriminate among pollen tubes of different species. Therefore, ovules were mixed together and pollen tubes of each plant species were grown in separate experiments. Mixing of ovules allowed us to check that pollen tubes were able to respond to the attractant of the same species.
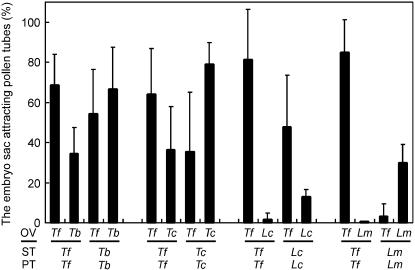
Figure 4.
Species preferentiality of the pollen tube attraction signal derived from the synergid cell. Each column indicates the mean value with the sd of three replications. OV, Ovule; PT, pollen tube; ST, style; Tf, T. fournieri; Tb, T. baillonii; Tc, T. concolor; Lc, L. crustacea; Lm, L. micrantha.
Figure 4 shows the results of pollen tube attraction in the presence of ovules of two different species in a culture. When T. fournieri and T. baillonii ovules were cultivated together in the same dish, T. fournieri pollen tubes tended to grow toward T. fournieri embryo sacs (68.6% ± 15.4%; n = 102 [total no. of embryo sacs counted]), although the tubes also grew toward T. baillonii embryo sacs (34.5% ± 12.9%; n = 132). Similarly, T. baillonii pollen tubes tended to grow toward both T. baillonii embryo sacs (66.8% ± 20.6%; n = 200) and T. fournieri embryo sacs (54.3% ± 22.0%; n = 112). The difference of the attraction was significant (χ2 test; P < 0.01). A similar tendency was observed when T. concolor was used in place of T. baillonii; T. fournieri pollen tubes were attracted by T. fournieri embryo sacs at 64.2% ± 22.7% (n = 96) and by T. concolor embryo sacs at 36.4% ± 21.6% (n = 113), and T. concolor pollen tubes were attracted by T. concolor embryo sacs at 79.1% ± 10.8% (n = 90) and by those of T. fournieri at 35.4% ± 29.8% (n = 146; χ2 test; P < 0.01). When L. crustacea was used, few T. fournieri pollen tubes grew toward L. crustacea embryo sacs (1.7% ± 3.4%; n = 134). In contrast, L. crustacea pollen tubes grew toward the embryo sacs of both T. fournieri (47.7% ± 25.8%; n = 96) and L. crustacea (13.1% ± 3.6%; n = 101); interestingly, L. crustacea pollen tubes tended to grow toward the embryo sacs of T. fournieri (t test; P < 0.05). In the most divergent combination, T. fournieri and L. micrantha, pollen tubes of each species grew primarily toward embryo sacs of the same species; T. fournieri pollen tubes were specifically attracted to T. fournieri embryo sacs at 85.0% ± 16.3% (n = 159; L. micrantha; 0% [n = 154]), and L. micrantha pollen tubes were attracted to their own embryo sacs at 30.0% ± 9.1% (n = 110) and to those of T. fournieri at 3.1% ± 6.3% (n = 90). These results indicate that the attraction signals of these plant species differ.
To determine whether different concentrations of the same attractant are responsible for the species-preferential attraction responses, we used T. fournieri and Lindernia ovules to examine whether intraspecies attraction is affected by the presence of ovules of a different species (Fig. 5). If the attractant was a species-preferential difference in concentration and not a species-preferential molecule, the resulting higher concentration of attractant should mask or erase the signal conveyed by a lower concentration of attractant. At the start of cultivation, a micromanipulator was used to move about 10 ovules of both species (10 each of Torenia and Lindernia ovules) to face the embryo sacs toward sacs of the other species. As the distance between the micropylar ends of these embryo sacs (filiform apparatus of synergid cells) was within 30 μm, they were always in the range of attraction of the T. fournieri synergid cells (Higashiyama et al., 2003; R. Inatsugi, A. Nakano, and T. Higashiyama, unpublished data). The resulting frequency of attraction was similar to that without manipulation of ovules, and the attraction was not impaired by the presence of embryo sacs of another species (Fig. 5; n = 30 in total for each column). Thus, the attractant derived from the synergid cell, at least in T. fournieri and closely related species, is likely a species-preferential molecule.
Figure 5.
Species preferentiality assay following micromanipulation of ovules to place embryo sacs of different genera opposite each other. A, DIC images of T. fournieri (left) and L. micrantha (right) pollen tube attraction in the presence of a heterogeneous embryo sac. Arrows indicate attracted pollen tubes. B, Frequency of pollen tube attraction following micromanipulation of ovules to place embryo sacs of different genera opposite each other. Ten sets of ovules were used in each experiment, and each column indicates the mean value with the sd of three replications. ES, Embryo sac; OV, ovule; PT, pollen tube; ST, style; Tf, T. fournieri; Lc, Lindernia crustacea; Lm, L. micrantha. Bar = 50 μm.
Contribution of the Stigma and Style Tissues to Species Preferentiality
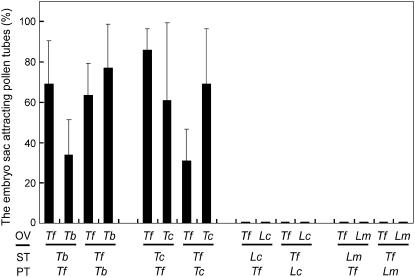
Pollen tubes used in attraction studies with Torenia require a period of tube elongation within the style to gain their competence to respond to the synergid chemical signal (Higashiyama et al., 1998). To determine whether species preferentiality was involved in acquiring this competence, we next changed species of the style tissue (Fig. 6). The growth of the pollen tube decreased with increasing divergence between the pollen species and the style species. However, in all combinations tested, pollen grains germinated on the stigma and began to grow in the stylar canal of heterogeneous species. When T. fournieri pollen grains were applied to T. baillonii stigmas, emergent T. fournieri pollen tubes still tended to grow toward T. fournieri embryo sacs (69.0% ± 21.6%; n = 82) rather than to T. baillonii embryo sacs (33.9% ± 17.5%; n = 104). In contrast, T. baillonii pollen tubes that grew through T. fournieri styles tended to grow toward T. baillonii embryo sacs (77.2% ± 21.5%; n = 101) rather than to T. fournieri embryo sacs (63.4% ± 15.8%; n = 79). The tendency of attraction was significant (χ2 test; P < 0.01). A similar pattern was observed when T. concolor was used instead of T. baillonii (χ2 test; P < 0.01). These results indicate that pollen tube detection of the synergid cell attractant is conferred by pollen species, not by sporophytic factors found in the style on which the pollen tube grows.
Figure 6.
Species preferentiality of pollen tube attraction with stigma and style tissues of different species. Each column indicates the mean value with the sd of three replications. Lindernia pollen tubes did not come out of the T. fournieri styles, and Lindernia stigma/style tissues poorly supported growth of Torenia pollen tubes semi-in vitro. OV, Ovule; PT, pollen tube; ST, style; Tf, T. fournieri; Tb, T. baillonii; Tc, T. concolor; Lc, L. crustacea; Lm, L. micrantha.
When Lindernia species were used, Lindernia pollen tubes did not emerge from the cut ends of T. fournieri styles, as described later; however, T. fournieri pollen tubes did emerge from Lindernia styles, but the number of pollen tubes decreased (Fig. 6). The Torenia pollen tubes growing through Lindernia styles showed impaired growth, as they were considerably shorter than tubes grown in Torenia styles. These T. fournieri pollen tubes were not attracted to the synergid cells of either T. fournieri or the Lindernia species. They appeared to fail to acquire the capability to respond to the attraction signal because of the heterogeneous conditions of the stigma and style.
Evaluation of Potential Low-Mr Attractants
The species preferentiality of the pollen tube attractant is inconsistent with the classical hypothesis of calcium ion as the attractant. The low-molecular mass external calcium ion has been thought to be the pollen tube attractant derived from the ovule, or more precisely, the synergid cell, and was thought to form a concentration gradient in the pistil. To examine whether calcium ions are the attractant derived from the T. fournieri synergid cell, we increased the concentration of calcium ions in the medium. If an appropriate calcium ion concentration gradient had already been established by the synergid cell in the medium, the additional elevation of the calcium ion concentration in the medium should disturb the signal.
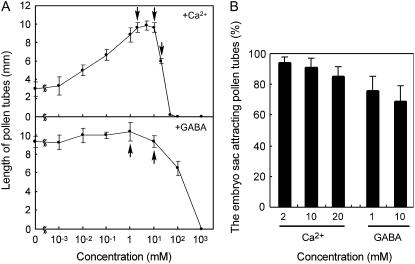
Figure 7A shows the relation between the calcium ion concentration (top) and the GABA concentration (bottom) in the medium and length of pollen tubes growing semi-in vitro. The effects of GABA, a potential chemoattractant derived from ovular sporophytic tissues (Palanivelu et al., 2003), were examined in the presence of 2 mm calcium ions. Calcium ions are generally essential to pollen tube growth. In T. fournieri, calcium ions promoted pollen tube growth and were most effective at 2 mm, which was the concentration of the in vitro T. fournieri system. At calcium concentrations greater than 10 mm, pollen tube growth was considerably impaired. GABA also promoted the growth of T. fournieri pollen tubes, as reported for Arabidopsis (Palanivelu et al., 2003), but at GABA concentrations greater than 10 mm, the growth of T. fournieri pollen tubes was impaired.
Figure 7.
Pollen tube attraction in the presence of high concentrations of Ca2+ and GABA in the medium. A, Relationship between the concentrations of Ca2+ and GABA and pollen tube length. GABA was tested in the presence of 2 mm Ca2+. Arrows indicate concentrations of Ca2+ or GABA tested in B. B, Frequencies of pollen tube attraction in the presence of high concentrations of Ca2+ and GABA. The mean value with the sd of three replications is shown.
Figure 7B shows the effects of increasing the calcium ion and GABA concentrations in the medium on the percentage of embryo sacs attracting pollen tubes. Even when the calcium ion and GABA concentrations were increased to 20 and 10 mm, respectively, T. fournieri pollen tubes were still attracted by the synergid cell, although pollen tube growth began to be impaired. These results indicate that calcium ions and GABA cannot be the sole attractants of pollen tubes derived from T. fournieri synergid cells.
In Vivo Crossing Analysis
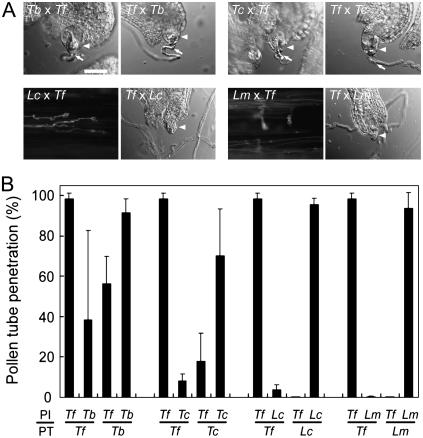
Finally, we examined the possibility that the species preferentiality of the attractant serves as a reproductive barrier in vivo. In all crosses tested, pollen grains were germinated on heterogeneous stigma and pollen tubes began to grow (Fig. 8). Figure 8B shows frequencies of penetration of the embryo sac by the pollen tube in each cross. In most combinations, except for the pollination of Lindernia pollen on Torenia pistils, pollen tubes reached the ovary locules where exserted embryo sacs were present (Fig. 8A, except Lc × Tf and Lm × Tf). Pollen tubes of heterologous species arrived at the ovary in the same time period as tubes of the same species but showed a decreased ratio of penetration of the embryo sac, as shown in Figure 8B. This raises the possibility that pollen tube guidance in the ovary was impaired, suggesting that the species preferentiality of the attraction signal from the synergid cell acts as a reproductive barrier. At a considerable time after pollination, such as a few days later, most embryo sacs of Torenia had received pollen tubes by interspecific crosses, and some sacs of Lindernia had received tubes by intergenic crosses using T. fournieri (data not shown). Fertilization may have been delayed in these crossings.
Figure 8.
In vivo crossing analysis. A, DIC images show ovules with or without pollen tube penetration, and aniline-blue-stained images show pollen tubes whose growth has stopped in the style (Lc × Tf; Lm × Tf). The ovules and pollen tubes were excised and observed at 1 d after pollination, as described previously (Higashiyama et al., 1997). Labels indicate the species of pollen (former) and pistil (latter) used in each cross. Arrowheads indicate an exserted embryo sac, and arrows indicate pollen tubes attracted to the sacs. B, Frequencies of penetration of the embryo sac by the pollen tube at 1 d after pollination. Each column indicates the mean value with the sd of three replications. PI, Pistil; PT, pollen tube; Tf, T. fournieri; Tb, T. baillonii; Tc, T. concolor; Lc, L. crustacea; Lm, L. micrantha. Bar = 50 μm.
Because no Lindernia pollen tubes were observed in Torenia ovaries, we stained pollen tubes in the Torenia style with aniline blue and found that the pollen tubes stopped growing within the style after a period of normal growth (Fig. 8A, Lc × Tf and Lm × Tf). The tubes stopped growing at a particular place and formed narrow coils or zigzag growth patterns, and finally their growth appeared to be arrested. Interestingly, the tubes of L. crustacea and L. micrantha grew straight within the length of their own pistils, growing straight for 6.2 ± 0.3 mm (no. of stylar canals observed, n = 6) and 6.8 ± 0.3 mm (n = 6), respectively, whereas the length of pistils of L. crustacea was 5.3 ± 0.2 mm (n = 10) and that of L. micrantha was 6.3 ± 0.2 mm (n = 10).
DISCUSSION
In this study, we demonstrated that the attraction signal from the T. fournieri synergid cell and closely related species is strongly species preferential. Species preferentiality and specificity in the last phases of guidance has also been implied in interspecific and intergenic crosses using Arabidopsis (Shimizu and Okada, 2000; Shimizu, 2002; Palanivelu and Preuss, 2006). However, directional growth of pollen tubes in the pistil is governed by complex multistep controls from both the female sporophyte and gametophyte (Higashiyama et al., 2003; Johnson and Lord, 2006). Thus, it is difficult to evaluate the species difference at specific guidance steps; for example, impaired guidance in the ovary locule may be due to species preferentiality in not only the guidance cue but also the capacitation of the pollen tube (Higashiyama et al., 2003). Thus, we mixed ovules of different species and examined which species were targeted by pollen tubes. The frequency of same-species pollen tube attraction could be checked to confirm that pollen tubes are primed to respond to the signal in the medium. Laser ablation experiments confirmed that the attraction signal in the medium is derived from the synergid cells in both T. fournieri and other species (Fig. 3). These results show convincingly that the attraction signal from the synergid cell is species preferential. Specificity occurred at the genus level but did not occur at the species level of Torenia. It might be possible to speculate that at the species level of Torenia, an attractant molecule could be sensed by a receptor of other plant species but could not match it. Divergence in functional similarity of the attractant closely paralleled that of phylogenetic distance.
In the most divergent species combination, T. fournieri and L. micrantha, the attraction signal from the synergid cell of each species did not interfere with the attraction of the other species (Fig. 5). The attraction was also not disturbed in the T. fournieri and L. crustacea combination. Thus, we excluded the possibility that these species use the same attractant but at different concentration ranges. Each species likely uses a different molecule, and these molecules likely diverged rapidly during evolution. These attractants may be molecules synthesized in the synergid cell, like the ZmEA1 protein in maize (Márton et al., 2005). Analysis of genes specifically expressed in the synergid cell will provide insight into the molecular mechanism of chemoattraction, including the synthesis and secretion of attractants. For example, the MYB98 gene of Arabidopsis is specifically expressed in the synergid cell and governs pollen tube guidance and the formation of the filiform apparatus, which appears to be important for secretion of the chemoattractant (Kasahara et al., 2005).
It should also be noted that we do not exclude the possibility that Torenia and Lindernia species use attractants at different concentration ranges. For example, L. crustacea pollen tubes were more attracted to T. fournieri embryo sacs rather than to L. crustacea embryo sacs (Fig. 4). Frequency of pollen tube attraction in vitro depends on quantity of the source of the attractant, probably due to effective distance of attraction; embryo sacs with two synergid cells could attract more pollen tubes in vitro than that with one synergid cell (Higashiyama et al., 2001). Because Torenia has larger ovules and synergid cells than does Lindernia (Figs. 1 and 5), it might be possible that Torenia synergid cells secret larger amount of attractants than Lindernia synergid cells to attract more pollen tubes.
We showed that, in T. fournieri, external calcium ions could not serve as the sole synergid cell-derived attractant (Fig. 7). Although calcium has been detected in the synergid cell and the neighboring extracellular matrix, as described above (Jensen, 1965; Chaubal and Reger, 1990, 1992a, 1992b, 1993; Huang and Russell, 1992; Tirlapur et al., 1993; Tian and Russell, 1997; Kristóf et al., 1999), Ca2+ may play a primary role in pollen tube discharge, gamete fusion, or both, but not in species-related pollen tube attraction. In an Arabidopsis mutant in which a plasma membrane Ca2+ pump of the pollen tube, ACA9, is disrupted, the pollen tube arrives at the embryo sac but cannot discharge its contents to the receptive synergid cell and shows overgrowth in the embryo sac (Schiøtt et al., 2004). ACA9 appears to be involved in intercell communication between the synergid cell and the pollen tube that triggers pollen tube discharge, along with the FERONIA and SIRENE genes that are expressed in the synergid cell (Huck et al., 2003; Rotman et al., 2003). The role of Ca2+in gamete fusion has been suggested in a maize in vitro fertilization system; an isolated sperm cell can fuse autonomously with an isolated egg cell in the presence of a high concentration (5 mm) of calcium (Faure et al., 1994).
GABA has also been proposed to have a role in pollen tube guidance at the ovule of Arabidopsis, as a sporophytic guidance cue (Palanivelu et al., 2003). GABA slightly promoted the growth of Torenia pollen tubes in vitro at approximately 1 mm, as in Arabidopsis. However, we observed no change in pollen tube guidance when the GABA concentration in the medium was elevated, although our results do not exclude the possibility that GABA is a sporophytic guidance cue from the ovule. It should also be noted that in Torenia, the embryo sac is directly exposed to the placenta, and guidance of the funiculus does not appear to occur.
The stigma and style tissues contribute to capacitation of the pollen tube (Higashiyama et al., 1998; Palanivelu and Preuss, 2006). Even when the stigma and style species were changed in the Torenia genus, the species preferentiality was not altered (Fig. 6). The species preferentiality of the attraction signal from the synergid cell appeared to depend on the genotype of the pollen tube and the synergid cell (or the ovule). Arabidopsis pollen tubes acquire competence when passed through the stigma and style of Arabidopsis arenosa, Olimarabidopsis pumila, Capsella rubella, and Sysimbrium irio, suggesting that the ability of the stigma and style tissues to promote pollen tube competence is highly conserved (Palanivelu and Preuss, 2006). Torenia pollen tubes, however, failed to be capacitated by Lindernia stigma/style tissue. The capacitation signal from stigma/style tissue might be species preferential, and these species might be diverged enough where this capacitation fails, although it should be considered that Lindernia stigma/style tissue did not support normal growth of Torenia pollen tubes in the medium.
In most in vivo crossing combinations, the arrival of the pollen tube at the embryo sac was delayed or failed, although the pollen tubes appeared to enter the ovary normally (Fig. 8). The species preferentiality of the synergid cell attractant may be involved in this defect, possibly functioning as a reproductive barrier. Delayed pollen tube arrival may result in out competition by pollen tubes of the same species. Many pre- and postfertilization reproductive barriers exist in flowering plants (Shimizu, 2002). Severe species preferentiality of the attractant derived from the synergid cell is likely to be one of these strong barriers. Lindernia pollen tubes, on the other hand, ceased growth at a particular place in the T. fournieri stylar canal after growing slightly longer than the length of own pistils. A mechanism to stop the growth of the pollen tube autonomously may exist based on the tube length. It is also interesting that L. crustacea pollen tubes cannot enter the ovary due to this mechanism, a kind of reproductive barrier, although they have an ability to respond to the synergid cell attractant of T. fournieri (Fig. 4).
CONCLUSION
The attraction signal from the synergid cells in Torenia and closely related Lindernia species was shown to be strongly species preferential. It was shown that external calcium ions could not be the sole attractant derived from the T. fournieri synergid cell. The species preferentiality of the attractant signal may also function as a reproductive barrier in the final step of guidance. Because the attractant is likely to be a molecule that rapidly diverged during evolution, molecules synthesized in the synergid cell, such as proteins and peptides, may be candidates for this attractant. Thus, analysis of genes specifically expressed in the synergid cell will provide insight into the molecular mechanisms of pollen tube attraction.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Plants of the five species used in the study, Torenia fournieri, Torenia baillonii, Torenia concolor, Lindernia crustacea, and Lindernia micrantha, were grown in a regulated chamber at 25°C with a 16-h photoperiod (approximately 150 μmol m−2 s−1). Lindernia setulosa and Lindernia antipoda were grown in the same condition and used for phylogenetic analysis. For each experiment, ovules with placenta were excised from flowers with freshly opened stigmas using a stereomicroscope. Except for T. fournieri, these plants were automatically self pollinating; thus, they were emasculated before flowering by removing the sympetalous petals from which the stamens emerged. The pistils maturated normally and were pollinated with pollen grains from other flowers. None of these plants exhibited self incompatibility.
Phylogenetic Analysis
Total DNAs were isolated from the seven species of Torenia and Lindernia. rbcL was PCR amplified using RH1 (5′) and 1352R (3′) primers, as reported in Wolfe and dePamphilis (1997). PCR products were directly sequenced using RH1, 1352R, and a forward primer (5′-CTACGTCTGGAAGACCTGCGAATCC-3′) at nucleotide positions 371 to 395 on the sequence for T. fournieri (AF026842). The rbcL sequences of other 32 species of Scrophulariaceae (Wolfe and dePamphilis, 1998) were obtained from GenBank. Tobacco (Nicotiana tabacum) and Nicotiana debneyi (Solanaceae) were used as the outgroup of this study as described by Wolfe and dePamphilis (1998).
The rbcL sequences were aligned using ClustalX (Thompson et al., 1997), and nucleotide positions 213 to 1,533 on the full coding sequence of rbcL for tobacco were used to obtain the best alignment. Unweighted most parsimonious analyses of rbcL genes were performed, including bootstrapping (Felsenstein, 1985) based on 1,000 replications of full heuristic searches (with the tree bisection-reconnection branch-swapping algorithm), using PAUP 4.0b10. Distance matrix was calculated by applying the Jukes-Cantor method (Jukes and Cantor, 1969) in PAUP 4.0b10. NJ trees (Saitou and Nei, 1987) were constructed using PAUP 4.0b10, including bootstrap analyses (Felsenstein, 1985) based on 1,000 replications. Bayesian analyses were performed using MrBayes 3.1 (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003). A general time reversible model with a proportion of invariable sites and a γ-shaped distribution of rates across sites (GTR + I + G) model were deduced with hierarchical likelihood ratio tests using Mrmodeltest 2.1 (program distributed by J. Nylander, Evolutionary Biology Centre, Uppsala University). The Bayesian analysis of rbcL genes was initiated with a random starting tree and ran two runs with four chains of Markov chain Monte Carlo (MCMC) iterations simultaneously for 1,500,000 generations, keeping one tree every 100 generations. The first quarter generations (5,000 trees) were discarded as burn in, and the remaining trees were used to calculate a 50% majority rule tree and to determine the posterior probabilities for the individual branches. The sd between two MCMC runs was below 0.01 for the MCMC iteration, indicating convergence.
Culture Conditions for in Vitro Crossing
For in vitro crosses, ovules of one or two species were excised from placenta in modified Nitsch's medium (Higashiyama et al., 1998) containing 13% (w/v) polyethylene glycol 4000, 1% (w/v) Suc, and 1.5% ultra-low-gelling-temperature agarose (agarose type IX-A; Sigma; Higashiyama and Inatsugi, 2006) and cocultivated with a hand-pollinated method described previously for T. fournieri (Higashiyama et al., 1998). When pollen tubes of Torenia species were used with Torenia styles, the ovules were cultivated in an area of 6 × 6 mm2 on a coverslip in glass-bottom dish so that the tubes covered the entire area of the ovules. In contrast, when pollen tubes of L. crustacea or L. micrantha species were used with Lindernia styles, the ovules were cultivated in areas of 2 × 2 mm2 and 3 × 3 mm2, respectively. When ovules of two different species were mixed, the numbers of ovules of each species were adjusted to approximately equal numbers and cultivated at 30°C overnight.
Microscopy
Cultures, excised ovules, and pollen tubes on the stylar canals were observed under an inverted microscope equipped with differential interference contrast (DIC) and an epifluorescence system (IX71, Olympus). Pollen tubes on the stylar canals were stained with aniline blue and observed as described (Higashiyama et al., 1997). All photographs were taken using a 3-CCD camera (C7780, Hamamatsu Photonics) attached to the microscope.
Laser Ablation
A Nd:YAG laser (355 nm; Sigma Koki) was used for laser ablation of cells as described (Higashiyama et al., 2001). The laser beam was focused at the edge of the targeted synergid cells of ovules scattered in the medium. More than 20 ovules were treated in each experiment using one dish.
Micromanipulation of Ovules
For micromanipulation of ovules, water-saturated silicone oil (KF-96-100CS, Shin-Etsu Chemical) was layered on the medium to prevent dehydration during the subsequent micromanipulation. Ovules were thrust using a glass needle produced by a glass needle puller (PC-10, Narishige) and moved using a manipulator (MMN-1, MMO-202 N, MMO-220A, Narishige) attached to the inverted microscope. About 20 ovules (each 10 ovules) were manipulated in each experiment using one dish.
Analysis of the Roles of Ca2+ and GABA
For the analysis of Ca2+, culture medium was prepared as described above but without calcium and agarose. Volumes of 500 μL of the liquid medium containing various concentrations of calcium were prepared in 1.5-ml tubes. Three styles, just after pollination, were cut to 10-mm lengths and placed in each tube so that only the cut end was immersed in the medium. After cultivation for 16 h at 30°C, the lengths of the pollen tubes grown in the medium after passing the style were measured using stereomicroscopy. The relationship between the calcium concentration and the pollen tube length was examined to determine the optimal concentration of Ca2+ for pollen tube growth, and this concentration was included in the medium used in the in vitro crossing experiment to examine the effect of Ca2+ on pollen tube attraction.
For the GABA analysis, 2 mm Ca2+ was added to the medium, and the relationship between the GABA (Wako Pure Chemical Industries) concentration and the pollen tube length was examined as for calcium. The optimal concentration of GABA for pollen tube growth was then included in the medium for the in vitro crossing experiment to examine the effect of GABA on pollen tube attraction.
In Vivo Crossing
Pollen grains of each species were applied to Torenia pistils and emasculated pistils of the same species. At 1 d after pollination, ovules (and pollen tubes) were excised from the ovaries and observed in 0.12 m sorbitol. When pollen tubes were not observed in an ovary, pollen tubes on the stylar canal were observed following aniline blue staining, as described previously (Higashiyama et al., 1997).
The rbcL sequence data from this article can be found in the DDBJ/EMBL/GenBank data libraries under accession numbers as follows: T. fournieri, AB259804; T. baillonii, AB259805; T. concolor, AB259806; L. crustacea, AB259807; L. micrantha, AB259808; L. setulosa, AB259809; and L. antipoda, AB259810.
Acknowledgments
We thank Mr. Shinei Kato (Tsuruoka, Yamagata) and Dr. Hirokazu Tsukaya (University of Tokyo; National Institute for Basic Biology, Okazaki) for providing Lindernia plant materials.
This work was supported by the Japan Science and Technology Agency (core research for evolutional science and technology award to T.H.), by the Program for the Promotion of Basic Research Activities for Innovative Biosciences (to T.K. and T.H.), and by the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant-in-aid for scientific research on priority areas no. 17027006 and grant-in-aid for exploratory research no. 17657022 to T.H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Tetsuya Higashiyama (higashi@biol.s.u-tokyo.ac.jp).
References
- Chaubal R, Reger BJ (1990) Relatively high calcium is localized in synergid cells of wheat ovaries. Sex Plant Reprod 3: 98–102 [Google Scholar]
- Chaubal R, Reger BJ (1992. a) Calcium in the synergid cells and other regions of pearl millet ovaries. Sex Plant Reprod 5: 34–46 [Google Scholar]
- Chaubal R, Reger BJ (1992. b) The dynamics of calcium distribution in the synergid cells of wheat after pollination. Sex Plant Reprod 5: 206–213 [Google Scholar]
- Chaubal R, Reger BJ (1993) Prepollination degeneration in mature synergids of pearl millet: an examination using antimonite fixation to localize calcium. Sex Plant Reprod 6: 225–238 [Google Scholar]
- Cheung AY, Wang H, Wu HM (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]
- Dong J, Kim S, Lord EM (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure JE, Digonnet C, Dumas C (1994) An in vitro system for adhesion and fusion of maize gametes. Science 263: 1598–1600 [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1985) Confidence limits on phylogenies: an approach using bootstrap. Evolution Int J Org Evolution 38: 16–24 [DOI] [PubMed] [Google Scholar]
- Gray-Mitsumune M, Matton DP (2006) The egg apparatus 1 gene from maize is a member of a large gene family found in both monocots and dicots. Planta 223: 618–625 [DOI] [PubMed] [Google Scholar]
- Higashiyama T (2002) The synergid cell: attractor and acceptor of the pollen tube for double fertilization. J Plant Res 115: 149–160 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Inatsugi R (2006) Comparative analysis of biological models used in the study of pollen tube growth. Plant Cell Monogr 3: 265–286 [Google Scholar]
- Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1997) Kinetics of double fertilization in Torenia fournieri based on direct observations of the naked embryo sac. Planta 203: 101–110 [Google Scholar]
- Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1998) Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10: 2019–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Kuroiwa H, Kuroiwa T (2003) Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol 6: 36–41 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293: 1480–1483 [DOI] [PubMed] [Google Scholar]
- Huang BQ, Russell SD (1992) Synergid degeneration in Nicotiana: a quantitative, fluorochromatic and chlorotetracycline study. Sex Plant Reprod 5: 151–155 [Google Scholar]
- Huck N, Moore JM, Federer M, Grossniklaus U (2003) The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130: 2149–2159 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogeny. Biometrics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Schneitz K, Pruitt RE (1995) Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell 7: 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen WA (1965) The ultrastructure and histochemistry of the synergids of cotton. Am J Bot 52: 238–256 [PubMed] [Google Scholar]
- Johnson MA, Lord E (2006) Extracellular guidance cues and intracellular signaling pathways that direct pollen tube growth. Plant Cell Monogr 3: 223–242 [Google Scholar]
- Jukes TH, Cantor CR (1969) Evolution of protein molecules. In HN Munro, ed, Mammalian Protein Metabolism, Vol 3. Academic Press, New York, pp 21–132
- Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) Myb98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17: 2981–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100: 16125–16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristóf Z, Tímár O, Imre K (1999) Changes of calcium distribution in ovules of Torenia fournieri during pollination and fertilization. Protoplasma 208: 149–155 [Google Scholar]
- Maheshwari P (1950) An Introduction to the Embryology of Angiosperms. McGraw-Hill, New York
- Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307: 573–576 [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP, Machlis L (1962) Chemotropic response of Antirrhinum majus pollen to calcium. Nature 196: 292–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP, Machlis L (1964) Chemotropic response of pollen of Antirrhinum majus to calcium. Plant Physiol 39: 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Park SY, Nothnagel EA, Lord EM (2000) A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12: 1737–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114: 47–59 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Lord EM (2003) Expression studies of SCA in lily and confirmation of its role in pollen tube adhesion. Plant Mol Biol 51: 183–189 [DOI] [PubMed] [Google Scholar]
- Prado AM, Porterfield DM, Feijó JA (2004) Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131: 2707–2714 [DOI] [PubMed] [Google Scholar]
- Ray SM, Park SS, Ray A (1997) Pollen tube guidance by the female gametophyte. Development 124: 2489–2498 [DOI] [PubMed] [Google Scholar]
- Reger BJ, Chaubal R, Pressey R (1992) Chemotropic responses by pearl millet pollen tubes. Sex Plant Reprod 5: 47–56 [Google Scholar]
- Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE (2003) Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol 13: 432–436 [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Schiøtt M, Romanowsky SM, Bækgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu KK (2002) Ecology meets molecular genetics in Arabidopsis. Popul Ecol 44: 221–233 [Google Scholar]
- Shimizu KK, Okada K (2000) Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 127: 4511–4518 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian HQ, Russell SD (1997) Calcium distribution in fertilized and unfertilized ovules and embryo sacs of Nicotiana tabacum L. Planta 202: 93–105 [Google Scholar]
- Tirlapur UK, Van Went JL, Cresti M (1993) Visualization of membrane calcium and calmodulin in embryo sacs in situ and isolated from Petunia hybrida L. and Nicotiana tabacum L. Ann Bot (Lond) 71: 161–167 [Google Scholar]
- Wolfe AD, dePamphilis CW (1997) Alternate paths of evolution for the photosynthetic gene rbcL in four nonphotosynthetic species of Orobanche. Plant Mol Biol 33: 965–977 [DOI] [PubMed] [Google Scholar]
- Wolfe AD, dePamphilis CW (1998) The effect of relaxed functional constraints on the photosynthetic gene rbcL in photosynthetic and nonphotosynthetic parasitic plants. Mol Biol Evol 15: 1243–1258 [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392: 818–821 [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang H, Cheung AY (1995) A pollen-tube growth-stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82: 395–403 [DOI] [PubMed] [Google Scholar]
- Wu HM, Wong E, Ogdahl J, Cheung AY (2000) A pollen tube growth-promoting arabinogalactan protein from Nicotiana alata is similar to the tobacco TTS protein. Plant J 22: 165–176 [DOI] [PubMed] [Google Scholar]