Abstract
Regulation of the cytosolic acetyl-coenzyme A carboxylase (ACCase) gene promoter from common bean (Phaseolus vulgaris) was studied in transgenic Arabidopsis (Arabidopsis thaliana) plants using a β-glucuronidase (GUS) reporter gene fusion (PvACCase∷GUS). Under normal growth conditions, GUS was expressed in hydathodes, stipules, trichome bases, flowers, pollen, and embryos. In roots, expression was observed in the tip, elongation zone, hypocotyl-root transition zone, and lateral root primordia. The PvACCase promoter was induced by wounding, Pseudomonas syringae infection, hydrogen peroxide, jasmonic acid (JA), ethylene, or auxin treatment. Analysis of PvACCase∷GUS expression in JA and ethylene mutants (coronatine insensitive1-1 [coi1-1], ethylene resistant1-1 [etr1-1], coi1-1/etr1-1) suggests that neither JA nor ethylene perception participates in the activation of this gene in response to wounding, although each of these independent signaling pathways is sufficient for pathogen or hydrogen peroxide-induced PvACCase gene expression. We propose a model involving different pathways of PvACCase gene activation in response to stress.
Acetyl-CoA carboxylase (ACCase; EC 6.4.1.2) catalyzes the ATP-dependent formation of malonyl-CoA from acetyl-CoA and bicarbonate. ACCase and its product, malonyl-CoA, play a key role in both primary and secondary metabolism. In plastids, malonyl-CoA is essential for fatty acid synthesis (Harwood, 1988); cytosolic malonyl-CoA is required for flavonoid, stilbene, and very long-chain fatty acid (VLCFA) synthesis (Ebel and Hahlbrock, 1977; Schröder et al., 1988; Roesler et al., 1994), as well as for the malonylation of 1-aminocyclopropane-1-carboxylic acid (ACC) and d-amino acids (Liu et al., 1983). Two physically distinct heteromeric and homodimeric forms are found in flowering plants. Most species have both forms with heteromeric enzymes in plastids and the homodimeric enzyme in the cytosol. In grasses, the homodimeric form is found in both the plastid and cytosol (Konishi and Sasaki, 1994; Konishi et al., 1996) and no heteromeric enzyme exists. Plastids of Brassica napus (Schulte et al., 1997) contain both a homodimeric and the expected heteromeric isoform. This could also be true for Arabidopsis (Arabidopsis thaliana) because one of the two predicted homodimeric ACCase genes (ACC2) encodes a putative chloroplastic transit peptide.
A recent study has shown that the cytosolic form of ACCase (ACC1) is essential for the survival of Arabidopsis. In this context, Baud et al. (2003) showed that acc1 mutants displayed defects in embryo development and in VLCFA synthesis. Mutants in the GURKE gene, which encodes ACC1, exhibit embryo defects and its cotyledons are absent or highly reduced (Kajiwara et al., 2004). Nonetheless, these phenotypes were reduced by an exogenous supply of malonate, suggesting that cytosolic ACCase is essential for normal embryo development (Baud et al., 2004).
A second key role for cytosolic ACCase is the synthesis of flavonoids. Flavonoids can act as sunscreens against harmful UV-B irradiation, thereby preventing damage to photosynthetic organs (Lois and Buchanan, 1994; Landry et al., 1995). Indeed, only the cytosolic form of ACCase, but not the chloroplastic isoform, is induced by UV-B irradiation. This response results in higher malonyl-CoA concentrations for flavonoid synthesis in the cytosol (Konishi et al., 1996). Flavonoids have also been implicated as endogenous negative regulators of polar auxin transport (Brown et al., 2001) and are important phytoalexins in legumes (Dixon and Pavia, 1995). In addition, it has been shown that flavonoids can provide protection by acting as scavengers of reactive oxygen species (ROS; Yamasaki et al., 1997).
A common bean (Phaseolus vulgaris) cytosolic ACCase (PvACCase) partial cDNA clone was previously reported (García-Ponce and Rocha-Sosa, 2000). mRNA accumulation of the first enzymes of flavonoid biosynthesis PvACCase and chalcone synthase (ChS) was induced by methyl jasmonate (MeJA), yeast (Saccharomyces cerevisiae) elicitor, or Pseudomonas syringae pv tabaci treatment (García-Ponce and Rocha-Sosa, 2000). Collectively, these data suggest coordinate regulation of these two early steps in the flavonoid pathway. Selective inhibitor treatments led to the conclusion that ethylene and oxylipins were necessary for the induction of the PvACCase gene in response to P. syringae pv tabaci (García-Ponce, 2000; García-Ponce and Rocha-Sosa, 2000).
Oxylipins are oxidation products derived from fatty acids and they have both signaling and antimicrobial activity. The best-studied oxylipin is jasmonic acid (JA). JA synthesis is induced in plants by wounding or pathogen attack, leading to the induction of a battery of defense genes (Devoto et al., 2005). The precursor of JA, 12-oxophytodienoic acid (OPDA), is induced after wounding or elicitor treatment (Parchmann et al., 1997; Stintzi et al., 2001). In Arabidopsis, OPDA can activate wound-induced gene expression in the absence of JA (Taki et al., 2005). In parsley (Petroselinum crispum), soybean (Glycine max), and bean, either OPDA or JA promotes the synthesis of flavonoids and the expression of their biosynthetic genes (Franceschi and Grimes, 1991; Dittrich et al., 1992; García-Ponce and Rocha-Sosa, 2000). In addition to oxylipins, ethylene has also been implicated in the activation of systemic plant defenses in response to pathogens (Penninckx et al., 1998) and wounding (O'Donnell et al., 1996). In Arabidopsis, ethylene also provokes an increase in flavonoid accumulation (Buer et al., 2006) and in carrot (Daucus carota) induces genes for flavonoid synthesis (Ecker and Davis, 1987). The JA and ethylene signaling pathways are induced concomitantly in Arabidopsis to activate defense responses after infection with a necrotrophic pathogen (Penninckx et al., 1998), but, after wounding, an antagonistic interaction between JA and ethylene was proposed for the local responses in Arabidopsis leaves (Rojo et al., 1999). In contrast, in tomato (Lycopersicon esculentum), ethylene potentiates JA action in response to wounding (O'Donnell et al., 1996). Thus, plant responses to pathogens and wounding are complex and participation of JA and ethylene varies.
Despite the importance of the cytosolic ACCase enzyme in plants and its documented increase after pathogen attack, to date, to our knowledge, there is no analysis of the tissue-specific expression of the ACCase genes and its regulation by developmental and environmental cues. To explore these facets of ACCase regulation, we analyzed expression in common bean and we generated transgenic Arabidopsis plants expressing the β-glucuronidase (GUS) reporter gene under the control of the common bean ACCase gene promoter. The patterns of PvACCase gene expression under normal growth or stress conditions were analyzed. To determine the hierarchy between JA and ethylene signals, an analysis of PvACCase gene promoter activity in JA (coronatine insensitive1-1 [coi1-1]) and ethylene (ethylene resistant1-1 [etr1-1]) perception mutants was also performed.
RESULTS
PvACCase mRNA Accumulation Is Induced by Wounding and Ethylene in Common Bean
Previously, we analyzed the PvACCase mRNA accumulation pattern in response to pathogen infection and MeJA or elicitor treatment in cell cultures and leaves from common bean. As mentioned above, in addition to being induced by MeJA in cell cultures and leaves, ACCase mRNA accumulated after yeast elicitor or P. syringae pv tabaci treatment. Inhibitors of the octadecanoid pathway severely reduced ACCase mRNA and protein accumulation induced by the yeast elicitor or P. syringae pv tabaci, indicating that jasmonates or a precursor mediate ACCase induction after pathogen infection (García-Ponce and Rocha-Sosa, 2000). Because JA induces genes that respond to wounding, we have now tested whether wounding affects PvACCase mRNA levels in bean leaves. A gene-specific probe for the 3′-untranslated region of the PvACCase gene was used in this expression analysis. PvACCase mRNA is detected 3 h after wounding and its level remained almost constant 12 h after wounding (Fig. 1A). Because ethylene has also been implicated in response to wounding or pathogen infection (O'Donnell et al., 1996; Penninckx et al., 1998), we treated common bean plants with ethephon (an ethylene-releasing agent) to determinate whether PvACCase expression is induced by ethylene. PvACCase mRNA was detected 1 h after ethephon addition, reached a peak at 6 h, and decreased afterward (Fig. 1B). Considered together with our previous data, these results suggest that a JA- and ethylene-responsive signaling pathway is necessary for the stress response of the PvACCase gene. In Arabidopsis, a JA/ethylene-mediated signaling pathway in response to wounding or pathogen attack has been described (Penninckx et al., 1998; Rojo et al., 1999). To analyze the role of JA and ethylene in the stress response of the PvACCase gene more deeply, we constructed Arabidopsis plants expressing the GUS reporter gene under the control of the PvACCase gene promoter.
Figure 1.
PvACCase mRNA expression is induced by wounding and ethylene in common bean plants. A, PvACCase mRNA accumulation in response to wounding. Leaves of 13-d-old plants were wounded with dialysis closure clips for the indicated times. B, Kinetics of PvACCase transcript accumulation following ethephon treatment. Plants were grown for 23 d in a hydroponic system and treated with 100 μm ethephon. Total RNA (30 μg/lane) was analyzed by hybridizing with a gene-specific probe for the 3′-untranslated region of PvACCase gene (AF007803). rRNA stained with ethidium bromide is shown as a loading control (bottom). A representative experiment of at least three independent experiments is shown.
Cloning and Analysis of the Common Bean PvACCase Gene Promoter Region
Genomic libraries were prepared from common bean DNA using the Universal Genome Walker kit (see “Materials and Methods”). Four PvACCase gene promoter fragments (406, 486, 786, and 2,716 bp upstream from the putative ATG start codon; data not shown) were sequenced and analyzed for regulatory motifs and promoter elements. At high stringency, matches to cis-elements previously identified as mediators of pathogen responses were found in PvACCase gene promoters (Fig. 2). For example, the W1-box, a cis-element that binds WRKY transcription factors (Eulgem et al., 1999), was found 288 bp upstream of the ATG start codon. The G-box (−1,092) and H-box (−1,048) involved in tissue-specific expression activation of the bean chs15 gene promoter (Faktor et al., 1997) were also found (Fig. 2). Other elements identified by promoter scanning using the PlantCARE database (Lescot et al., 2002; http://bioinformatics.psb.ugent.be/webtools/plantcare/html) include the ethylene response elements (−2,609 and −2,426), the TGA box (−1,292), and the CGTCA motifs (−1,289, −968, and −834), which are involved in auxin and MeJA responsiveness, respectively. Most of these elements are conserved in sequence, but not in position in the Arabidopsis and the soybean cytosolic ACCase gene promoters (Fig. 2).
Figure 2.
Schematic comparison of the cytosolic ACCase promoters from common bean, soybean, and Arabidopsis. The location of various putative cis-elements of interest was identified using the PLANTCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html). The numbers indicate the PvACCase gene promoter fragments used in detection analysis. Pv, Phaseolus vulgaris; Gm, Glycine max; and At, Arabidopsis.
Because the four DNA fragments from the putative control region of the PvACCase gene represent a deletion series from the 5′ end of the presumptive promoter, they were each fused transcriptionally to the GUS reporter gene and used to transform Arabidopsis ecotype Columbia-0 (Col-0). Homozygous T3 plants were analyzed from three independent lines per construct. Only the construct containing 2.7 kb upstream of the ATG start codon (PvACCase∷GUS) was able to support detectable GUS activity (data not shown). Therefore, the minimal promoter is >786 bp long, surprisingly large for a plant gene; the motifs conserved in soybean and Arabidopsis extend to −900 and −2,500 bp, respectively.
Organ-Specific Expression of PvACCase∷GUS Gene Fusion
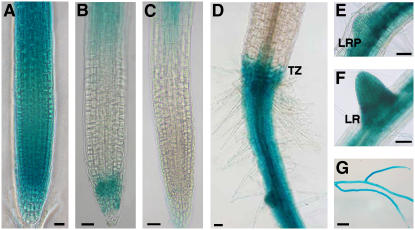
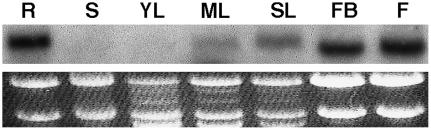
Tissue-specific expression of PvACCase∷GUS was monitored by histochemical staining. GUS activity was observed in hydathodes of young and adult leaves, stipules, stamens, stigma, pollen, siliques, embryos, and the base of some trichomes near the hydathodes (Fig. 3). In Figure 4, the expression pattern in roots of 3-, 5-, and 7-d-old seedlings is shown. At 3 d, GUS activity was observed in the whole root (Fig. 4A). At 5 and 7 d, GUS activity was detected only from the hypocotyl-root transition zone until the elongation zone. At the root tip, staining was noticed in 5-d-old seedlings, but was absent in 7-d-old seedlings (Fig. 4, B and C). Nonetheless, GUS activity was also detected at the sites of lateral root formation in 7-d-old seedlings (Fig. 4, E and F). By 14 d, secondary roots had developed and their GUS expression pattern was the same as that of the primary root, with staining in the elongation zone and root tip (Fig. 4). Overall, this pattern of expression (high in roots and flowers, low in leaves) was in agreement with the organ-specific accumulation of PvACCase mRNA in common bean plants (Fig. 5).
Figure 3.
Expression pattern of PvACCase∷GUS in transgenic Arabidopsis. A, Ten-day-old seedling. B, Close-up view of cotyledons showing GUS expression in stipules (s) and hydathodes (hy). C, Detail of the stipules. D, Detail of GUS expression at the base of trichomes (t). E, GUS expression in rosette leaf hydathodes. F, Close-up view of a hydathode. G, Flower. H, Embryo. I, Pollen. Photographs are representative of at least 10 independent experiments. Scale bars = 500 μm (A, B, E, and G); 50 μm (C, D, F, H, and I).
Figure 4.
Root expression pattern of PvACCase gene promoter. A, GUS staining in a 3-d-old root. B, GUS staining in the root tip and elongation zone of a 5-d-old root. C, GUS staining in the elongation zone of a 7-d-old root. D, Five-day-old seedling showing GUS activity in the hypocotyl-root transition zone (TZ). E, GUS expression in a lateral root primordium (LRP) of a 7-d-old seedling. F, Close-up view of a developing lateral root (LR) of a 7-d-old seedling. G, Lateral roots of a 14-d-old seedling. Photographs are representative of at least 10 independent experiments. Scale bars = 25 μm (A–C); 50 μm (D–F); 500 μm (G).
Figure 5.
PvACCase mRNA accumulation in organs of common bean. Total RNA (30 μg/lane) of several organs of bean plants was used for RNA-blot analysis. R, Root; S, stem; YL, young leaf of 10-d-old plants; ML, mature leaf of 23-d-old plants; SL, senescent leaf of 57-d-old plants; FB, floral bud; F, flower. rRNA staining with ethidium bromide is shown as a loading control. A representative experiment of at least three independent experiments is shown.
To confirm the results of the GUS analysis, whole-mount in situ hybridization was performed to localize ACCase mRNA. Gene-specific probes for Arabidopsis ACC1 and PvACCase genes were expressed in root tissue of 3-d-old seedlings in the elongation zone and root tip, as well as in the lateral root primordia (Fig. 6). No signal was observed when the AtACC1 and PvACCase sense probes were used. In addition, we also observed accumulation of AtACC1 mRNA in stipules and hydathodes (data not shown). These observations confirm that GUS accumulation from the PvACCase∷GUS transgene reflects the in vivo distribution of transcripts from both the PvACCase gene and the endogenous ACC1 gene.
Figure 6.
Whole-mount mRNA expression patterns in common bean and Arabidopsis roots. A, Whole-mount 3-d-old roots of common bean were hybridized with sense and antisense PvACCase probes (top). Whole-mount common bean lateral roots hybridized with sense and antisense PvACCase-specific probes (bottom). B, Whole-mount 3-d-old roots of Arabidopsis hybridized with a sense and antisense AtACC1-specific probe as indicated. A representative experiment of at least three independent experiments is shown. Scale bars = 250 μm (A); 25 μm (B).
PvACCase Response to Exogenous Auxin Application
Auxins are inducers of lateral root formation (Boerjan et al., 1995; Casimiro et al., 2001) because exogenous application of indole acetic acid (IAA) stimulates lateral root production (Evans et al., 1994). It is noteworthy that PvACCase∷GUS transgene expression during development has a strong correlation to the tissues where auxins are accumulated in Arabidopsis. For example, several studies have shown high auxin concentrations in Arabidopsis hydathodes, stipules, and root tips; furthermore, flavonoids colocalize with zones of auxin accumulation (Murphy et al., 2000; Peer et al., 2001; Aloni et al., 2003). To determine auxin responsiveness of the PvACCase gene promoter, 8-d-old roots were treated with IAA. At 6 h, the expression pattern was unaffected; however, at 24 and 48 h, GUS expression was more strongly activated in the initiating lateral roots following treatment with IAA (Supplemental Fig. S1). Based on these data, we suggest that the PvACCase gene promoter responds to a developmental signal (lateral root formation) induced by auxins and not directly to treatment with this phytohormone. Simultaneously, we analyzed the auxin effect on promoter induction in aerial tissues on PvACCase∷GUS 8-d-old seedlings. As illustrated in Figure 8, auxin treatment resulted in a significant induction of the PvACCase∷GUS gene promoter in leaves.
Figure 8.
Effect of hormones and oxidative stress on PvACCase expression. GUS expression in 8-d-old seedlings of Col-0, coi1-1, etr1-1, and coi1-1/etr1-1 plants that express the PvACCase∷GUS gene. Untreated (C) or 6 h after treatment with 100 μm MeJA, 200 μm ACC, 1 μm IAA, or 1 mm H2O2 as indicated above each photograph. The figures are representative of at least five independent experiments. Scale bar = 0.5 mm.
Stress Activation
Wounding induces expression of Arabidopsis genes required in flavonoid metabolism (i.e. ChS and Phe ammonia lyase; Reymond et al., 2000). We also demonstrated that wounding induced PvACCase mRNA accumulation in common bean leaves (Fig. 1). To assay wounding responsiveness of the PvACCase gene promoter, 5- to 6-week-old leaves from PvACCase∷GUS plants were mechanically wounded with dissection forceps and analyzed for GUS activity. Within 6 h, strong local staining was evident surrounding the wounded zone, whereas no GUS activity was observed in the middle of the unwounded leaf (Fig. 7A). Flavonoid phytoalexins have frequently been proposed to participate in plant response to pathogen attack and the corresponding enzymes involved are induced by pathogens (Bell et al., 1984; Lawton and Lamb, 1987; García-Ponce and Rocha-Sosa, 2000). We infected PvACCase∷GUS plants with P. syringae pv tomato/AvrRpm1 (Pst), a pathogen known to establish an incompatible interaction with Arabidopsis. There is strong PvACCase∷GUS induction by the pathogen, whereas the control mock-treated leaves showed a weak response, probably resulting from manipulation (Fig. 7B). This result is consistent with the role of flavonoids in plant defense and indicated that the 2.7-kb promoter fragment is sufficient for this response.
Figure 7.
Stress effects on PvACCase gene promoter activity. GUS expression in 5- to 6-week-old rosette leaves of Col-0, coi1-1, etr1-1, and coi1-1/etr1-1 plants that express the PvACCase∷GUS gene 6 h after wounding with forceps (A) and 6 h after spraying with 10 mm MgCl2 as a control and 6 h after spraying with P. syringae pv tomato/AvrRPM1 (B). Figures are representative of at least five independent experiments. Scale bar = 1 mm.
Previously, we showed that JA and ethylene induce PvACCase mRNA accumulation in common bean (García-Ponce and Rocha-Sosa, 2000; Fig. 1B); therefore, we decided to analyze PvACCase gene promoter responsiveness to these stimuli. Seedlings were treated with MeJA or the ethylene biosynthesis precursor, ACC, and tested for GUS activity. After both treatments, there was a significant increase in PvACCase∷GUS expression compared to the untreated control (Fig. 8).
Flavonoids are also scavengers of ROS, which are intermediary of the defense responses in plant-pathogen interactions (Yamasaki et al., 1997; Dat et al., 2000); therefore, PvACCase participation in oxidative stress was also assayed. When PvACCase∷GUS seedlings were treated with hydrogen peroxide (H2O2), stronger promoter expression was detected in leaves (Fig. 8).
PvACCase∷GUS Expression in Hormone Perception Mutants
To elucidate cross talk between different defense signaling pathways, we generated crosses between JA (coi1-1) or ethylene (etr1-1) perception mutants and our PvACCase∷GUS transgenic line. The coi1-1 mutation is recessive and leads to male sterility and insensitivity to coronatine and MeJA (Feys et al., 1994). We used several lines that were homozygous for kanamycin resistance (selection marker to the PvACCase gene promoter∷GUS cassette) and segregated 3:1 for sensitivity/insensitivity to MeJA when grown on Murashige and Skoog medium containing MeJA. Accordingly, 25% of the seedlings should be homozygous for coi1-1 and homozygous for PvACCase∷GUS. The etr1-1 mutation is dominant due to a mutation within the ethylene-binding site and shows ethylene insensitivity and Glc hypersensitivity (Chang et al., 1993; Zhou et al., 1998). Homozygous lines from both crosses were used for analysis.
Exogenous MeJA induced GUS expression in the etr1-1 background, but, as expected, GUS expression was undetectable in the coi1-1 background as a result of JA insensitivity (Fig. 8). On the other hand, PvACCase∷GUS was induced when ACC was applied to the coi1-1 mutant, but no GUS activity was detected in the etr1-1 background (Fig. 8). Taken together, our results suggest that PvACCase∷GUS expression was activated through both ethylene and JA signaling pathways.
Pathogen attack and wounding both induced substantial GUS expression in the coi1-1 and etr1-1 mutant backgrounds. As in the PvACCase∷GUS wild-type line, wounding caused local staining surrounding the wound site (Fig. 7, A and B). These results indicate that JA and ethylene signaling pathways can each act independently to regulate PvACCase induction in response to these stimuli or that an entirely different signaling pathway is used. To distinguish experimentally between these two hypotheses, a double mutant, coi1-1/etr1-1, bearing the PvACCase∷GUS gene promoter was generated and challenged as described above. GUS expression was absent after pathogen infection, MeJA, or ACC treatments (Figs. 7B and 8). These observations confirm the hypothesis that PvACCase∷GUS activation can be mediated by at least two independent signaling pathways. Surprisingly, we detected the same expression pattern during the wound response in coi1-1/etr1-1 plants as in wild-type plants, indicating the participation of at least one signaling pathway independent of both JA and ethylene (Fig. 7A).
Treatment with IAA induced GUS expression in the coi1-1 mutant to a level similar to that observed in wild-type transgenic plants. In etr1-1 plants, however, IAA treatment did not induce GUS expression (Fig. 8). This result suggests that IAA activation of PvACCase expression most likely reflects ethylene accumulation induced by auxins because this auxin response is blocked in the etr1-1 background. As expected, in the coi1-1/etr1-1 double mutant, IAA was unable to induce PvACCase∷GUS expression (Fig. 8).
The impact of H2O2 on PvACCase∷GUS expression was also examined in coi1-1 and etr1-1 plants. As shown in Figure 8, GUS expression was induced by H2O2 in both mutants. In the coi1-1/etr1-1 double mutant, GUS activity was not detected after H2O2 treatment (Fig. 8). These results indicate that H2O2 induction of the PvACCase promoter can utilize either the ethylene- or JA-responsive signaling pathways, but not the hormone-independent pathway. The induction of marker genes responsive to different stresses and chemicals was examined by reverse transcription-PCR analysis as treatment controls. We selected the following genes for expression analysis: the JA- and ethylene-induced marker gene PDF1.2 (Penninckx et al., 1998), which corresponds to a plant defensin; the auxin-responsive gene IAA19 (Goda et al., 2004); the oxidative stress-responsive gene GST6 (Wagner et al., 2002); and the ethylene-inducible gene Hel1 (Potter et al., 1993), encoding a hevein-like protein with antifungal activity (Supplemental Fig. S2). Collectively, the treatment regimes indicate that there are at least three independent routes by which biotic and abiotic challenges can activate PvACCase gene expression (Fig. 9).
Figure 9.
Scheme of the activation of the PvACCase gene in response to biotic and abiotic stress. Pathogen- or ROS-induced PvACCase gene promoter activation can utilize either the JA (COI1)- or the ethylene (ETR1)-responsive signaling pathways. In the model, we position ROS downstream of the pathogen in agreement with the knowledge that ROS are generated during the hypersensitive response caused by an incompatible plant-pathogen interaction (Dat et al., 2000). Flavonoids produced in response to pathogens act as ROS scavengers, limiting the damage caused by this stress condition. IAA-induced PvACCase gene promoter activation is mediated through the capacity of auxins to promote ethylene synthesis. Wounding induces PvACCase gene activation by a signaling pathway independent of both JA and ethylene.
DISCUSSION
In plant cells, the cytosolic pool of malonyl-CoA generated by ACCase is required to support the biosynthesis of many secondary phytochemicals important for plant development, growth, and protection. These phytochemicals include VLCFA, flavonoids, and stilbenes (Ebel and Hahlbrock, 1977; Schröder et al., 1988; Roesler et al., 1994). Although the role of ACCase in flavonoid biosynthesis is well established (Ebel and Hahlbrock, 1977), little is known about the endogenous factors regulating its expression or its tissue and organ distribution in bean or Arabidopsis. We have previously demonstrated that, in bean cell cultures and leaves, PvACCase mRNA accumulates in response to elicitor or pathogen infection. Pathogen-induced PvACCase protein and mRNA accumulation was reduced by the application of inhibitors of oxylipin biosynthesis, suggesting a role for oxylipins as mediators in the induction of the PvACCase gene in response to pathogen infection (García-Ponce and Rocha-Sosa, 2000). Similar experiments with inhibitors of ethylene action indicated that ethylene was also required for pathogen-induced PvACCase mRNA accumulation (García-Ponce, 2000). To gain further understanding of PvACCase gene regulation in response to stress and to clarify the roles of JA and ethylene as response mediators, PvACCase∷GUS expression was analyzed in Arabidopsis transgenic wild-type and in JA or ethylene perception mutant plants; this approach circumvents the use of inhibitors and thus avoids secondary inhibitor effects.
The PvACCase control region contains several cis-elements that have been proven to control responses to wounding, pathogen infection, and hormones (Fig. 2). These elements are conserved in soybean and Arabidopsis cytosolic ACCase genes. Among the motifs are a G-box and an H-box separated by 38 bp. Promoters of genes in the phenylpropanoid pathway contain these regulatory elements and, in this context, they are necessary for flower- and root-specific expression (Faktor et al., 1997). Interestingly, we observed that PvACCase promoter induction was abolished in constructs that do not contain the G-box and H-box motifs (data not shown). In addition, adjacent G- and H-boxes in the promoter of the common bean chs15 gene are bound by transcription factor G/HBF-1. This protein is phosphorylated upon elicitor treatment (Dröge-Laser et al., 1997). The presence of these motifs in the PvACCase promoter region suggests coordinated regulation with other genes for phenylpropanoid synthesis. A W1-box was also found at −288 bp upstream to the ATG start codon in the PvACCase gene promoter. The W-box motif has been previously shown to bind WRKY transcription factors (Eulgem et al., 1999). WRKY factors play a key role in regulating the pathogen-induced defense program (Yang et al., 1999) and they also seem to be involved in other specific processes, such as trichome development and secondary metabolite biosynthesis (Walker et al., 1999).
Mutants in the ACC1 gene encoding the cytosolic ACCase in Arabidopsis are lethal in embryo development. Because all known mutants in flavonoid synthesis have normal embryos, the absence of VLCFA and their derivatives must be the cause of embryo arrest in acc1 plants (Baud et al., 2003). Interestingly, we detected PvACCase gene promoter-directed GUS activity in the Arabidopsis embryo (Fig. 3G), suggesting a key role of the PvACCase in common bean embryo development too.
Flavonoids are present in growing and maturing tissues of Arabidopsis, including siliques, inflorescence stems, flowers, stigma, pollen, and cauline and rosette leaves (Peer et al., 2001). The heterologous bean promoter programmed PvACCase∷GUS expression in the same tissues. To our knowledge, our work is the first to report the expression of a cytosolic ACCase gene in the hydathodes, stipules, and at the base of some trichomes of cauline and rosette leaves (Fig. 3). Hydathodes are connected to the vascular tissue and these glands lose water at the leaf tip and in the lobes. These glands are also sites of pathogen entry: The necrotrophic bacteria Xanthomonas campestris pv campestris invades Arabidopsis primarily through the hydathodes. Given the relatively fast induction of the superoxide dismutase gene in bacteria in the hydathodes, the existence of hydathode-localized defense reactions has been suggested (Hugouvieux et al., 1998). Indeed, induced expression of defense-related genes, such as ChS, has been reported (Aloni et al., 2003). Because hydathodes lack structural barriers against pathogen entry, expression of the PvACCase∷GUS gene fusion in these regions may indicate that gene products (i.e. flavonoids) contribute to defense, probably by acting as phytoalexins or scavengers of ROS.
The PvACCase promoter was also active in stipules and some trichomes (Fig. 3). Hydathodes and stipules are the primary sites of free IAA production in a leaf blade and trichomes are secondary sites (Aloni et al., 2003). In roots, we detected GUS expression in the root tip, elongation zone, lateral root primordia, and the hypocotyl-root transition zone (Fig. 4). Amazingly, the same root regions with GUS activity accumulate both flavonoids and auxins (Murphy and Taiz, 1999; Murphy et al., 2000; Peer et al., 2001; Ljung et al., 2005). In this context, it should be stressed that, in Arabidopsis roots, ChS and chalcone isomerase (Saslowsky and Winkel-Shirley, 2001) are located in sites where the PvACCase gene promoter is active (Fig. 4) and PvACCase and ACC1 mRNAs are present (Fig. 6). It has been suggested that flavonoids act as autocrine effectors that retain auxins in the cells in which they are synthesized (or accumulated), altering PIN-FORMED expression and localization and, consequently, polar auxin transport (Peer et al., 2004). To test the possibility that auxin regulates PvACCase gene expression, plants expressing the PvACCase∷GUS fusion were treated with IAA, resulting in GUS activity in leaf tissues (Fig. 8). In etr1-1 plants, no IAA induction was observed (Fig. 8), suggesting that IAA action is mediated through its capacity to induce ethylene synthesis (McKeon et al., 1995). Consequently, it is possible that the induction of the PvACCase promoter responds to a developmental signal and not directly to IAA.
PvACCase is induced by pathogen infection in common bean; a full induction requires oxylipins and ethylene (García-Ponce, 2000; García-Ponce and Rocha-Sosa, 2000). Now, we first demonstrate that PvACCase mRNA accumulates after wounding and ethylene treatment, reaching a peak at 3 and 6 h, respectively (Fig. 1). Histochemical analysis of transgenic plants expressing the PvACCase∷GUS fusion gene showed that the PvACCase promoter is induced upon wounding or pathogen infection of Arabidopsis (Fig. 7, A and B); incubation of seedlings with MeJA or ACC also resulted in the induction of GUS activity (Fig. 8). Collectively, these results implicate both hormones mediate wounding and pathogen responses. To determinate the number and properties of the signal transduction pathways, we analyzed GUS expression in two signal perception mutants. Wounded plants in both the coi1-1 and etr1-1 background showed GUS staining, as did the coi1-1/etr1-1 double mutants (Fig. 7A). Therefore, one signaling pathway is independent of both JA and ethylene. This notion is consistent with recent studies showing that OPDA induces several genes via a COI1-independent signaling pathway during the wound response (Taki et al., 2005).
In contrast to wounding, the PvACCase gene promoter was activated by Pst infection in wild-type and coi1-1 or etr1-1 plants; however, in the double mutant, Pst was unable to activate this promoter (Fig. 7B); therefore, JA and ethylene act independently to program the response of PvACCase to pathogens.
ROS are produced quickly and have an important role in triggering responses of plants to biotic and abiotic stresses. Flavonoids are scavengers of ROS in conjunction with peroxidase and are essential for the survival of uninfected tissue. The PvACCase gene promoter was activated in response to H2O2 in wild-type and coi1-1 or etr1-1 plants; in the double mutant, however, this promoter was inactive (Fig. 8). In line with this, it is possible that, in pathogen infection response, ROS act upstream of JA and ethylene for induction of PvACCase and that either COI1 or ETR1 signaling pathways are necessary for this activation.
Results described here provide data on PvACCase tissue expression during normal plant development in sites of flavonoid and auxin accumulation. In conclusion, PvACCase promoter activity was also induced under various stress conditions where metabolic alterations were triggered. We propose that the PvACCase gene is regulated by at least three independent routes in biotic and abiotic stress response (Fig. 9).
MATERIALS AND METHODS
Plant Material
Common bean (Phaseolus vulgaris L cv Negro Jamapa) was grown etiolated in trays with wet paper towels for 10 d. These plants were used for genomic DNA extraction and library construction. Arabidopsis (Arabidopsis thaliana ecotype Col-0) was the genetic background for all mutant and transgenic plants used in this work. The coi1-1 mutants were kindly provided by John G. Turner (University of East Anglia, UK), whereas the etr1-1 mutant was obtained from the Arabidopsis Biological Resource Center at Ohio State University.
RNA-Blot Analysis
RNA was prepared as described by Logemann et al. (1987). RNA-blot hybridization analysis was performed with 30 μg of total RNA per lane. Hybridization was done in 0.3 m sodium phosphate, pH 7.2, 7% SDS, and 1 mm EDTA at 65°C with the gene-specific probe for 3′-untranslated region of the PvACCase gene (AF007803). Blots were washed at low stringency with 1 m sodium phosphate, pH 7.2, 0.67% SDS.
Promoter Cloning
Genomic libraries were prepared from bean using the Universal Genome Walker kit (CLONTECH). DNA was digested with seven blunt-ended enzymes. The genomic fragments were then ligated to specific adapters provided in the kit, resulting in seven libraries. Based on the common bean ACCase gene sequence (GenBank accession no. DQ355997), two gene-specific primers were designed: GSP1, 5′-GTCTCATTGGCCCAGCTCCTAACACTACGT-3′ and GSP2, 5′-GAACTTGACTGCTGCCATTCCATTGTTTGC-3′. These primers facilitated amplification of the PvACCase 5′ regions as detailed in the manual (CLONTECH). Four fragments resulting from PCR reactions varied in length in the 5′ promoter region, but were identical in the regions of overlap and were sequenced; 5′ sequences upstream of the PvACCase gene were analyzed for potential regulatory motifs and promoter-like elements. The HindACC primer was designed to amplify the promoter region and to create a HindIII restriction site at the 5′ end of the amplified fragment (the HindIII site is indicated by the underlined bases): 5′-TTGAAGCTTTGAAAAACATGACACTTGT-3′. Another primer, BamACC, was designed to create a BamHI site at the 3′ end of the amplified fragment (the BamHI site is indicated by the underlined bases): 5′-TACATAGGAGACATACAGAAGGATCCCAAT-3′. These primers were used to amplify the promoter region. The resulting fragment was digested with HindIII and BamHI, column purified, and cloned into the respective sites in the pBin121 (CLONTECH) plant transformation vector. The resulting plasmid (ACCase-pBin121) was used to transform Arabidopsis.
Plant Transformation and Crossing
Arabidopsis was transformed using the floral-dip method (Clough and Bent, 1998) and subsequently grown to obtain seeds. T2 seeds from transgenic lines exhibiting 3:1 segregation for kanamycin resistance were selected and T3-independent homozygous lines exhibiting 100% kanamycin resistance were recovered. Two independent homozygous transgenic lines (PvACCase∷GUS) were crossed with mutant lines coi1-1 and etr1-1. F1 progeny were allowed to self pollinate and F2 seeds were harvested. F3 seeds from the cross PvACCase∷GUS/coi1-1 (coi1-1) were maintained heterozygous for coi1-1 because homozygotes are male sterile. Homozygous coi1-1 F3 plants were obtained from double selection with 50 μm JA and 50 μg/μL kanamycin. Homozygous PvACCase∷GUS/etr1-1 (etr1-1) F4 plants were obtained from double selection with 4% Glc and 50 μg/μL kanamycin. The coi1-1/etr1-1 double-mutant line was constructed by crossing between coi1-1 and etr1-1 mutant lines carrying PvACCase∷GUS. The double mutants were selected on the basis of their insensitivity to MeJA and resistance to ethylene. Derived homozygous lines from these crosses were used for all treatments.
Histochemical GUS Assays
GUS activity was detected by histochemical staining following the procedure of Jefferson (1987). Fresh and treated tissues were immersed in GUS staining buffer (100 mm sodium phosphate, pH 7.0, 10 mm EDTA, 0.5 mm Triton X-100, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, 2 mm 5-bromo-4-chloro-3-indolyl-β-d-GlcUA substrate predissolved in 0.5% N-dimethylformamide), and incubated for approximately 16 h at 37°C. The staining solution was removed and the samples were cleared in 80% ethanol. Roots were stained and cleared as described by Malamy and Benfey (1997).
Whole-Mount in Situ Hybridization
AtACC1 (At1g36160) and PvACCase (AF007803) sense and antisense probes corresponding to the coding region and the 3′-untranslated region, respectively, were in vitro synthesized and digoxigenin labeled (Roche Diagnostics). Short riboprobes (an average length of 150 bp) were produced by alkaline hydrolysis of longer transcripts. Whole-mount in situ hybridization was performed as described by Friml et al. (2003). Roots were observed using an Eclipse E600 stereoscopic microscope and an optic microscope equipped with a digital camera E995 (Nikon).
Plant Treatments
Leaves of 13-d-old bean plants were wounded perpendicularly on the central vein with dialysis closure clips and incubated for the indicated times leaving it on. Plants to be treated with ethylene were grown in a standard hydroponic system for 23 d and incubated with ethephon for the indicated times. Ethephon was dissolved in 0.5 m sodium phosphate, pH 7.2, and used at a final 100 μm concentration. Immediately after harvest, all plant material was immersed in liquid nitrogen prior to RNA extraction.
To improve the visualization of the effect of mechanical wounding, the middle of rosette leaves of 5- to 6-week-old Arabidopsis plants was crushed once or twice, with a dissection forceps and incubated for 6 h. Infection with Pseudomonas syringae was performed by spraying bacteria on the adaxial side of rosette leaves of 5- to 6-week-old plants using suspension of the strain DC300/avr RPM1 at 1 × 108 cfu/mL in 10 mm MgCl2, 0.02% (v/v) Silwet L-77. Treated plants were incubated for 6 h in an airtight transparent plastic box in a lighted growth chamber. For chemical treatments, seedlings were grown over sterilized nylon filters in a vertical orientation for 8 d and then transferred from standard Murashige and Skoog medium to fresh Murashige and Skoog medium containing standard concentrations reported for gene expression analysis. The concentrations used were 100 μm MeJA (Oñate-Sánchez and Singh, 2002); 200 μm ACC (He et al., 2004); 1 mm H2O2 (Wagner et al., 2002); and 1 μm IAA (Goda et al., 2004). After chemical treatment, plants were incubated for 6 h in a growth chamber. With this procedure, we avoided wounding the seedling during transfer. Control seedlings were incubated under the same conditions without any treatment.
Sequence data have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession number DQ355997.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of auxin application on PvACCase∷GUS expression in roots.
Supplemental Figure S2. RT-PCR analysis of marker gene expression.
Supplementary Material
Acknowledgments
We thank Dr. Virginia Walbot, Dr. Gladys Cassab, Dr. Helena Porta, Dr. Arturo Guevara, and Dr. José A. Rocha for critical reading and helpful comments on the manuscript.
This work was supported by Consejo Nacional de Ciencia y Tecnología (grant no. 28052N) and Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México (grants nos. IN212103 and IX215304). R.E.F.-B. was a recipient of Consejo Nacional de Ciencia y Tecnológia and Dirección General de Estudios de Posgrado-Universidad Nacional Autónoma de México fellowships.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mario Rocha-Sosa (rocha@ibt.unam.mx).
The online version of this article contains Web-only data.
References
- Aloni R, Schwalm K, Langhans M, Ulrich CI (2003) Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216: 841–853 [DOI] [PubMed] [Google Scholar]
- Baud S, Bellec Y, Miquel M, Bellini C, Caboche M, Lepiniec L, Faure JD, Rochat C (2004) gurke and pasticcino3 mutants affected in embryo development are impaired in acetyl-CoA carboxylase. EMBO Rep 5: 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Guyon V, Kronenberger J, Wuilleme S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl-CoA carboxylase I is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Bell JN, Dixon RA, Bailey JA, Rowell PM, Lamb CJ (1984) Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci USA 81: 3384–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis thaliana. Plant Physiol 140: 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beekma T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene response gene ETR1: similarity of product to 2-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57: 779–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto A, Ellis C, Magusin A, Chang H-S, Chilcott C, Zhu T, Turner JG (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58: 497–513 [DOI] [PubMed] [Google Scholar]
- Dittrich H, Kutchan TM, Zenk MH (1992) The jasmonate precursor 12-oxo-phytodienoic acid induces phytoalexin synthesis in Petroselinum crispum cell cultures. FEBS Lett 309: 33–36 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Pavia NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7: 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge-Laser W, Kaiser A, Lindsay WP, Halkier BA, Loake GJ, Doerner P, Dixon RA, Lamb C (1997) Rapid stimulation of a soybean protein-serine kinase that phosphorylates a novel bZIP DNA-binding protein, G/HBF-1, during the induction of early transcription-dependent defenses. EMBO J 16: 726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J, Hahlbrock K (1977) Enzymes of flavone and flavonol-glycoside biosynthesis: coordinated and selective induction in cell-suspension cultures of Petroselinum hortense. Eur J Biochem 75: 201–209 [DOI] [PubMed] [Google Scholar]
- Ecker JR, Davis RW (1987) Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA 84: 5202–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrook K, Somssich IE (1999) Early nuclear events in plant defense: rapid gene activation by WRKY transcription factors. EMBO J 18: 4689–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Ishikawa H, Estelle MA (1994) Responses of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxin response mutants. Planta 194: 215–222 [Google Scholar]
- Faktor O, Loake G, Dixon RA, Lamb CJ (1997) The G-box and H-box in a 39 bp region of a French bean chalcone synthase promoter constitute a tissue-specific regulatory element. Plant J 11: 1105–1113 [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Grimes HD (1991) Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci USA 88: 6745–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Benková E, Mayer U, Palme K, Muster G (2003) Automated whole mount localisation techniques for plant seedlings. Plant J 34: 115–124 [DOI] [PubMed] [Google Scholar]
- García-Ponce B (2000) Caracterización molecular de la respuesta a estrés de la acetil CoA carboxilasa de frijol (Phaseolus vulgaris L.). PhD thesis. Instituto de Biotecnología-Universidad Nacional Autónoma de México, Cuernavaca, Morelos, México
- García-Ponce B, Rocha-Sosa M (2000) The octadecanoid pathway is required for pathogen-induced multifunctional acetyl-CoA carboxylase accumulation in common bean (Phaseolus vulgaris L.). Plant Sci 157: 181–190 [DOI] [PubMed] [Google Scholar]
- Goda H, Sawa S, Asami T, Fujioka S, Shimada Y, Yoshida S (2004) Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 134: 1555–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood JL (1988) Fatty acid metabolism. Annu Rev Plant Physiol Plant Mol Biol 39: 101–138 [Google Scholar]
- He X, Zhang Z, Yan D, Zhang J, Chen S (2004) A salt responsive receptor-like kinase gene regulated by the ethylene signaling pathway encodes a plasma membrane serine/threonine kinase. Theor Appl Genet 109: 377–383 [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Barber CE, Daniels MJ (1998) Entry of Xanthomonas campestris pv campestris into hydathodes of Arabidopsis thaliana leaves: a system for studying early infection events in bacterial pathogenesis. Mol Plant Microbe Interact 11: 537–543 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 [Google Scholar]
- Kajiwara T, Furutani M, Hibara K, Tasaka M (2004) The GURKE gene encoding an acetyl-CoA carboxylase is required for partitioning the embryo apex into three subregions in Arabidopsis. Plant Cell Physiol 45: 1122–1128 [DOI] [PubMed] [Google Scholar]
- Konishi T, Sasaki Y (1994) Compartmentation of two forms of acetyl-CoA carboxylase in plants and the origin of their tolerance towards herbicides. Proc Natl Acad Sci USA 91: 3598–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Shinohara K, Yamada K, Sasaki Y (1996) Acetyl CoA carboxylase in higher plants: most plants other than gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol 37: 117–122 [DOI] [PubMed] [Google Scholar]
- Landry LG, Chapple CCS, Last RL (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109: 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MA, Lamb CJ (1987) Transcriptional activation of plant defense genes by fungal elicitor, wounding and infection. Mol Cell Biol 7: 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30: 325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hoffman NE, Yang SF (1983) Relationship between the malonation of 1-aminocyclopropane-1-carboxylic acid and d-amino acids in mung-bean hypocotyls. Planta 158: 437–441 [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 [DOI] [PubMed] [Google Scholar]
- Lois R, Buchanan BB (1994) Severe sensitivity to ultraviolet radiation in an Arabidopsis mutant deficient in flavonoid accumulation. Planta 194: 504–509 [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- McKeon T, Fernández-Maculet JC, Yang SF (1995) Biosynthesis and metabolism of ethylene. In PJ Davis, ed, Plant Hormones and Their Role in Plant Growth and Development. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 118–139
- Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211: 315–324 [DOI] [PubMed] [Google Scholar]
- Murphy AS, Taiz L (1999) Naphthyphthalamic acid is enzymatically hydrolyzed at the hypocotyl-root transition zone and other tissues of Arabidopsis thaliana seedlings. Plant Physiol Biochem 37: 413–430 [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917 [DOI] [PubMed] [Google Scholar]
- Oñate-Sánchez L, Singh KB (2002) Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiol 128: 1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchmann S, Gundlanch H, Mueller MJ (1997) Induction of 12-oxo-phytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiol 115: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS (2001) Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol 126: 536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S, Uknes S, Lawton K, Winter AM, Chandler D, DiMaio J, Novitzky R, Ward E, Ryals J (1993) Regulation of a Hevein-like gene in Arabidopsis. Mol Plant Microbe Interact 6: 680–685 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler KR, Shorrosh BS, Ohlrogge JB (1994) Structure and expression of an Arabidopsis acetyl-coenzyme A carboxylase gene. Plant Physiol 105: 611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Leon J, Sanchez-Serrano JJ (1999) Cross-talk between wound signaling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J 20: 135–142 [DOI] [PubMed] [Google Scholar]
- Saslowsky D, Winkel-Shirley B (2001) Localization of flavonoid enzymes in Arabidopsis roots. Plant J 27: 37–48 [DOI] [PubMed] [Google Scholar]
- Schröder G, Brown JWS, Schröder J (1988) Molecular analysis of revestarol synthase: cDNA genomic clones and relationship with chalcone synthase. Eur J Biochem 172: 161–169 [DOI] [PubMed] [Google Scholar]
- Schulte W, Topfer R, Stracke R, Schell J, Martini N (1997) Multi-functional acetyl-CoA carboxylase from Brassica napus is encoded by a multi-gene family: indication for plastidic localization of at least one isoform. Proc Natl Acad Sci USA 94: 3465–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, et al (2005) 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139: 1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Edwards R, Dixon DP, Mauch F (2002) Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol Biol 49: 515–532 [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA 1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Chen Ch, Wang Z, Fan B, Chen Z (1999) A pathogen- and salicylic acid-induced WRKY DNA-binding activity recognizes the elicitor response element of the tobacco class I chitinase gene promoter. Plant J 18: 141–149 [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.