Abstract
In this investigation, multichannel laser scanning confocal microscopy and quantitative image analysis were used to determine whether interference with the function of a pericyte component, the NG2 (Nerve/Glial antigen 2), inhibits neovascularization and tumor growth in uveal melanoma xenografts. Orthotopic human uveal melanoma (OCM-1A) xenografts were induced in NG2 knockout and wild type mice, which were immunosuppressed with cyclosporin A. Endothelial cells were identified, using a cocktail of antibodies against endoglin (CD105), PECAM-1 (CD31), and VEGF receptor-2 (flk-1). Pericytes were identified by labeling with antibodies against the PDGF β-receptor and NG2 proteoglycan. The mean microvascular density (MVD) in NG2 knockout eyes was therefore 43.7% lower than wild type controls (n= 18 eyes, p < 0.05). The mean tumor end-volume in knockout and wild type mice was 0.1873 mm3 and 3.6262 mm3 respectively (n=18, p< 0.0001). Inhibition of pericytes through a key pericyte component, NG2 proteoglycan, decreases neovascularization and tumor end-volume, rendering pericyte and NG2 proteoglycan as potential cellular and molecular therapeutic targets in uveal melanoma.
Keywords: angiogenesis, uvea, melanoma, microcirculation, model, neovascularization, NG2, pericyte, proteoglycan
INTRODUCTION
Neovascularization in uveal melanoma is considered as a rate-limiting step for metastasis. The walls of neovascular capillaries are composed of two principal cell types: vascular endothelial cells (EC) and pericytes (Rouget cells) (Folkman, 1971) (Rouget, 1879). Pathological neovascularization occurs by angiogenesis and vasculogenesis. Angiogenesis occurs by sprouting from preexisting blood vessels (McDonald and Choyke, 2003). In vasculogenesis, blood vessels develop from bone marrow-derived pericyte (Ozerdem et al., 2005) (Rajantie et al., 2004) and endothelial precursors (Rafii et al., 2002). Whereas blood vessel endothelial cells in cancer have been studied extensively, much less is known about pericytes. In the current study, we analyze the functional properties of NG2 proteoglycan on pericytes, and the contribution of pericytes in neovascularization of uveal melanoma.
In this investigation, multichannel laser scanning confocal microscopy and quantitative image analysis were used to determine whether interference with the function of a pericyte component, the NG2 proteoglycan, inhibits neovascularization and tumor growth in uveal melanoma xenografts.
METHODS
All animal studies were performed in accordance with the ARVO Statement for the Use of Animals in Research. NG2 null mice (Grako et al., 1999) (Ozerdem and Stallcup, 2004) were generated via a conventional homologous recombination approach (Mansour et al., 1988) (Capecchi, 1989). The mice were back-crossed onto a C57BL/6 genetic background, and NG2+/− heterozygotes were mated to establish separate NG2 knockout (NG2−/−) and wild type (NG2+/+) colonies (Ozerdem, 2006) (Ozerdem and Hargens, 2005). In the NG2 knockout mouse, proliferation of both pericytes and endothelial cells are significantly reduced, and the pericyte:endothelial cell ratio falls to 0.24 from the wild type value of 0.86. Similarly, bFGF-induced angiogenesis is reduced more than four-fold in the NG2 knockout cornea compared to that seen in the wild type (Ozerdem and Stallcup, 2004). Inhibition of pericyte-NG2 in the NG2 knockout mouse inhibits neovascularization, lymphangiogenesis, and tumor growth in the prostate cancer (Ozerdem, 2006). Inhibition of pericyte-NG2 normalizes the interstitial fluid pressure in orthothopic B16F1 skin melanoma grafts in the NG2 knockout mouse (Ozerdem and Hargens, 2005).
The OCM-1A human uveal melanoma cell line (Kan-Mitchell et al., 1989) (Ozerdem et al., 2002a) was used to induce orthotopic uveal melanoma xenografts. Beginning three days prior to surgery, 60 mg/kg cyclosporin A (Sandimmune, Novartis, East Hanover, NJ) was administered daily via intraperitoneal injections. We used a similar microsurgical technique described previously for inducing uveal melanoma xenografts (Hu et al., 1994) (Grossniklaus et al., 1995) (Yang et al., 2004) with several modifications: A five microliter suspension of 5x105 OCM-1A cells was injected into the suprachoroidal space 1.5 mm posterior to the corneoscleral limbus. The injection was performed using a custom-made 32 gauge, 3/8 inch needle with a bevel angle of 12 degrees, attached to a no.701 syringe. Arrows in Figure 1A indicate the route (tunnel) of microinjection. The mice were enucleated 2 weeks after surgery.
FIGURE 1.
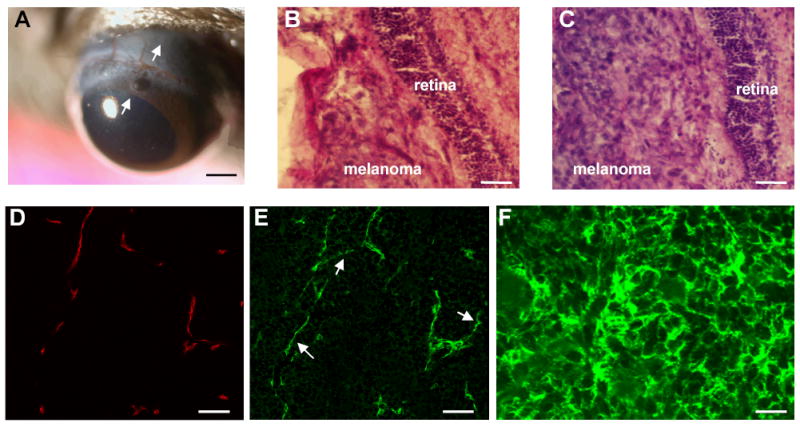
OCM-1A cells were inoculated into suprachoroidal spaces of cyclosporin A-immmunosuppressed mice. Arrows indicate the direction of the tangential tunnel prepared through the corneoscleral junction and the sclera. Two weeks following surgery, anterior segments, lens, and vitreous look clear, with a patchy area of iris atrophy near the limbus (A). Both NG2 knockout (B) and wild type (C) eyes developed tumors in 2 weeks in the choroid between the sclera and retina. Frozen sections of OCM-1A tumor grafts grown in NG2 knockout and wild type mice were double-immunostained and imaged with a scanning laser confocal microscope. Blood vessel endothelium was identified using a cocktail of antibodies against endoglin (CD105), PECAM-1 (CD31) and VEGF receptor-2 (flk-1) (D), and pericyte was identified using PDGF β-receptor antibodies (E). The histologic section shown in figures 1D and E is from a wild type mouse and double-immunostained for endothelium markers (CD105+CD31+flk-1) in 1D, and a pericyte marker PDGF β-receptor in 1E. Confocal microscopy revealed extensive investment of endothelium with pericytes (D and E). Pericytes were easily identified in neovascular walls, and were also found to be forming segments of vessels not lined with endothelium (Figure E, arrows). Figure 1F shows OCM-1A tumor cells with NG2 expression in an NG2 knockout mouse. OCM-1A tumor cells showed NG2 expression (F). Scale bars indicate 750μm in (A), 100 μm in (B and C), 75 μm in (D and E), 30μm in (F).
Immunohistochemistry, Confocal Microscopic Imaging, and Image Analysis
Tissues were fixed in 4% paraformaldehyde for 6 hours, cryoprotected in 20% sucrose overnight, and frozen in optimal cutting temperature (O.C.T.) embedding compound (Miles, Inc., Elkhardt, IN). Cryostat sections (40 μm) were air-dried onto Superfrost slides (Fisher Scientific, Pittsburgh, PA). Blood vessel endothelial cells (EC) were identified using a cocktail of rat antibodies against mouse endoglin (CD105), PECAM-1 (CD31), and VEGF receptor-2 (flk-1) (Pharmingen, San Diego, CA) (Chang et al., 2000) (Ozerdem and Stallcup, 2003) (Ozerdem and Stallcup, 2004), a strategy that was utilized previously to maximize labeling of all blood vessel endothelial cells.
Pericytes were identified by labeling with rabbit PDGF β-receptor antibodies, and rabbit or guinea pig antibodies against the NG2 proteoglycan(Ozerdem, 2006) (Ozerdem et al., 2005) (Ozerdem and Stallcup, 2003) (Ozerdem, 2004; Ozerdem and Stallcup, 2004) . We used the frozen sections of mouse retinal neovasculature as a positive control for vascular immunohistochemistry. Rabbit polyclonal glial fibrillary acidic protein (GFAP) (Accurate Chemical, Westbury, NY) and PDGF a-receptor antibodies (a kind gift from Dr. William B. Stallcup) were used to identify glia. We used the frozen sections of mouse optic nerve as a positive control for glial and oligodendroglial precursor cells, respectively. Slides incubated with non-specific immunoglobulin served as a negative control.
The immunostained sections (3 sections per eye from 18 eyes) were analyzed with a Fluoview 1000 (Olympus, Melville, NY) multi-channel laser scanning confocal microscope for microvascular density (MVD). The Volocity image analysis software (Openlab-Improvision Inc, Lexington, MA) was used for quantification of MVD in pixels in sections immunostained with endoglin (CD105), PECAM-1 (CD31) and VEGF receptor-2 (flk-1). The average equatorial radius (R) of each tumor in hematoxylin and eosin-stained histological slides was measured by using the Volocity image analysis software. Then the volume of each tumor was estimated by using the formula 4/3 x π x R3.
RESULTS
On dilated indirect ophthalmoscopic examination, the tumor mass was identified at the peripheral fundus. The visual axis was intact, and the anterior chamber, cornea, lens, and vitreous were clear (Figure 1A). Both NG2 knockout (Figure 1B) and wild type (Figure 1C) eyes developed tumors in uvea. Immunostaining for NG2 and PDGF β-receptors revealed NG2-negative and PDGF β-receptor -positive pericytes in NG2 knockout mice and NG2-positive and PDGF β-receptor positive pericytes in the wild type mice. PDGF β-receptor and [endoglin (CD105), PECAM-1 (CD31), and VEGF receptor-2 (flk-1)] double-immunostaining revealed investment of endothelium with pericytes (Figure 1D and E). OCM-1A tumor cells showed NG2 expression, both in NG2 knockout (Figure 1F) and wild type mice. The mean vascular density was 153.3 pixels in NG2 knockout eyes (standard error of mean +/− 22.7) and 272.3 pixels in wild type eyes (standard error of mean +/− 38.7). The mean microvascular density (MVD) in NG2 knockout eyes was therefore 43.7% lower than wild type controls and this difference was statistically different (n= 18 eyes, p=0.0166, Mann-Whitney U test). We have not encountered any cells within uveal melanoma showing glial or oligodendroglial differentiation by using GFAP or PDGF-a receptor immunohistochemistry. The mean tumor end-volume in the knockout and wild type mice was 0.1873 mm3 and 3.6262 mm3 respectively (n=18, p< 0.0001 Mann Whitney U test).
DISCUSSION
The use of the xenograft model and cyclosporin administration for immunosupression may somewhat confound the interpretation of the results in the context of tumor–host interactions and neovascular cell recruitment. The cyclosporin inhibits angiogenesis by inhibiting migration of vascular precursors. This effect is mediated through the inhibition of cyclooxygenase (Cox)-2, the transcription of which is modulated by the nuclear factor of activated T cells (NFAT) activation (Hernandez et al., 2001). Therefore, immunosuppression of the mice in order to prevent rejection of the human tumor xenograft, although necessary, presents a significant problem. However, four observations presented in our study seem especially noteworthy.
First, pericytes contribute to the neovascular vessels of uveal melanoma extensively. To our knowledge, there are at least two reports on the presence of alpha-smooth muscle actin-positive pericytes in uveal melanoma (Makitie et al., 1999) (Clarijs et al., 2005). One of the traditional markers for pericyte identification has been the expression of alpha-smooth muscle actin (ASMA). However, a growing body of evidence suggests that ASMA as a late marker for differentiated pericytes may be poorly expressed in nascent microvasculature (McDonald and Choyke, 2003) . Since only a small fraction (1–10%) of nascent pericytes can be identified on the basis of ASMA expression (Balabanov and Dore-Duffy, 1998) (Ozerdem et al., 2005) (Song et al., 2005), we used NG2 and PDGF β-receptor immunohistochemistry to identify pericytes.
The current investigation represents the first report on therapeutic targeting of pericytes in uveal melanoma. This establishes pericytes as potential cellular targets in uveal melanoma, in addition to other cell types. Presence of NG2-negative and PDGF β-receptor-positive pericytes in uveal melanoma in NG2 knockout mice also suggests that neovascular pericytes in uveal melanoma derive from the host tissue, but not from the grafted tumor.
Second, genetic inhibition of pericyte-NG2 proteoglycan decreases microvascular density in uveal melanoma. NG2, a membrane-spanning chondroitin sulfate proteoglycan associated with mitotically active, nascent pericytes exhibits several properties which suggest that it is a functional player in neovascularization (Ozerdem, 2004) (Ozerdem and Stallcup, 2004) (Ozerdem and Stallcup, 2003) (Ozerdem et al., 2002b) (Ozerdem et al., 2001). NG2 appears to serve as a co-receptor for both bFGF (basic fibroblast growth factor) and PDGF AA (platelet-derived growth factor AA isoform) (Goretzki et al., 1999) (Grako and Stallcup, 1995). Pericyte-NG2 chondroitin sulfate proteoglycan binds to extracellular matrix components such as type VI, type V, and type II collagen, tenascin, and laminin in the extracellular matrix (Tillet et al., 1997) (Burg et al., 1996). Biochemical data also demonstrate the involvement of both galectin-3 and α3β1 integrin in the EC response to pericyte-NG2, and show that NG2, galectin-3, and α3β1 form a complex on the cell surface, promoting cell motility (Fukushi et al., 2004). Recent investigations have revealed decreased neovascularization following intrinsic (NG2 knockout mice) or extrinsic (hydron polymer pellets containing NG2 neutralizing antibody) targeting of NG2 proteoglycan in retinal ischemia (retinopathy of prematurity), neurofibromatosis type 1 (NF1), in bFGF-induced neovascularization in cornea, and prostate cancer (Ozerdem, 2004; Ozerdem and Stallcup, 2004) (Ozerdem, 2006) . Intrinsic inhibition of pericyte-NG2 has been shown to inhibit interstitial fluid hypertension within orthothopic B16F1 skin melanoma grafts (Ozerdem and Hargens, 2005). The current investigation reveals that pericytes and NG2 proteoglycan may also serve as potential cellular and molecular anti-neovascular therapeutic targets in uveal melanoma. Further studies are warranted to elucidate the role of pericyte-NG2 proteoglycan in interstitial fluid pressure in uveal melanoma and its liver metastases.
Third, the reduction of the microvascular density leads to a significant inhibition of uveal melanoma growth. These results suggest a 95% reduction of the tumor end-volume at the time of sacrifice in NG2 knockout mice (i.e., 2 weeks after tumor inoculation).
Fourth, expression of NG2 proteoglycan on both pericytes and uveal melanoma cells render this proteoglycan as a double target. Therapeutic strategies targeting multiple cell types such as pericytes, endothelial cells, and tumor cells maximize the efficacy of the anti-tumor treatment (Bergers et al., 2003) (Ozerdem, 2004; Ozerdem et al., 2005) (Ozerdem, 2006). The current study expands our understanding of the neovascularization process in uveal melanoma xenografts and establishes a role for pericytes and NG2 proteoglycan as potential therapeutic targets.
FIGURE 2.
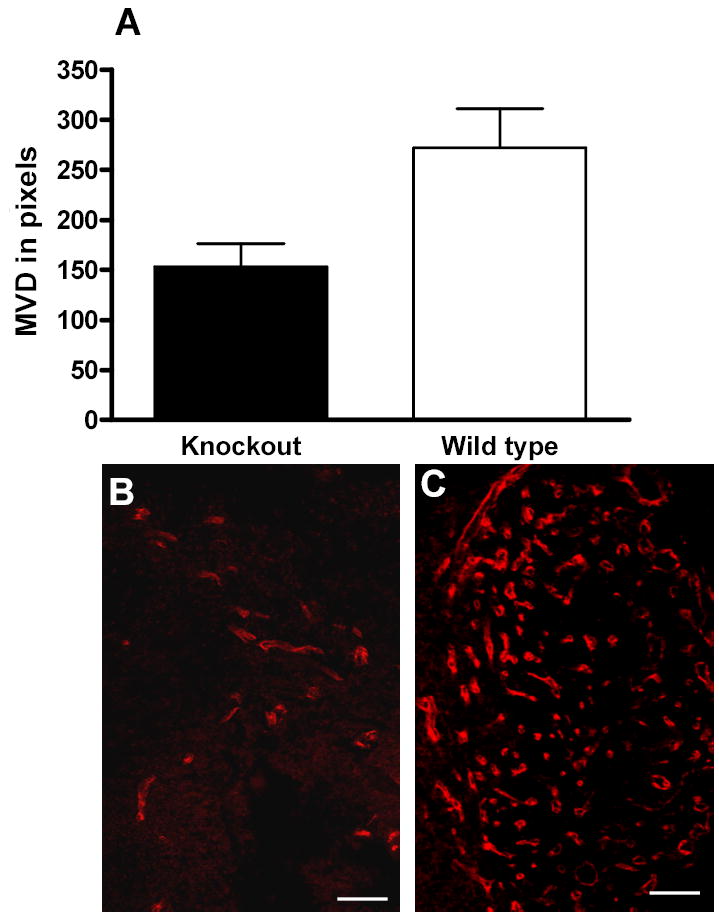
Microvascular density was quantified in pixels using Volocity image analysis software on 54 frozen sections which were sampled from 18 eyes, and immunostained for endoglin (CD105), PECAM-1 (CD31), and VEGF receptor-2 (flk-1). Mean microvascular density (MVD) in NG2 knockout eyes was 43.7% lower than wild type controls and this difference was statistically significant (n= 18 eyes, p=0.0166, Mann-Whitney U test) (A). MVD in uveal melanoma in a knockout eye is depicted in (B), while the tumor in a control eye (wild type) is shown in (C). Scale bars indicate 75 μm in (B and C).
Acknowledgments
Our laboratory has been supported by grants from NIH RO3 HD044783, University of California Tobacco-Related Disease Research Program (TRDRP 13IT-0067), and the U.S. Department of Defense Prostate Cancer Research Program PC020822.
Footnotes
Presented in part in molecular basis of ocular disease paper session in ARVO 2005 Annual Meeting, Ft Lauderdale, FL.
References
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarijs R, van Dijk M, Ruiter DJ, de Waal RM. Functional and morphologic analysis of the fluid-conducting meshwork in xenografted cutaneous and primary uveal melanoma. Invest Ophthalmol Vis Sci. 2005;46:3013–3020. doi: 10.1167/iovs.04-0876. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet- derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112:905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp Cell Res. 1995;221:231–240. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Barron BC, Wilson MW. Murine model of anterior and posterior ocular melanoma. Curr Eye Res. 1995;14:399–404. doi: 10.3109/02713689508999938. [DOI] [PubMed] [Google Scholar]
- Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LK, Huh K, Gragoudas ES, Young LH. Establishment of pigmented choroidal melanomas in a rabbit model. Retina. 1994;14:264–269. doi: 10.1097/00006982-199414030-00014. [DOI] [PubMed] [Google Scholar]
- Kan-Mitchell J, Mitchell MS, Rao N, Liggett PE. Characterization of uveal melanoma cell lines that grow as xenografts in rabbit eyes. Invest Ophthalmol Vis Sci. 1989;30:829–834. [PubMed] [Google Scholar]
- Makitie T, Summanen P, Tarkkanen A, Kivela T. Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 1999;40:2471–2480. [PubMed] [Google Scholar]
- Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- Ozerdem U. Targeting neovascular pericytes in neurofibromatosis type 1. Angiogenesis. 2004;7:307–311. doi: 10.1007/s10456-004-6643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U. Targeting of pericytes diminishes neovascularization and lymphangiogenesis in prostate cancer. Prostate. 2006;66:294–304. doi: 10.1002/pros.20346. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46:3502–3506. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Hargens AR. A simple method for measuring interstitial fluid pressure in cancer tissues. Microvasc Res. 2005;70:116–120. doi: 10.1016/j.mvr.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Mach-Hofacre B, Varki N, Folberg R, Mueller AJ, Ochabski R, Pham T, Appelt K, Freeman WR. The effect of prinomastat (AG3340), a synthetic inhibitor of matrix metalloproteinases, on uveal melanoma rabbit model. Curr Eye Res. 2002a;24:86–91. doi: 10.1076/ceyr.24.2.86.8159. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002b;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget CMB. Sur la contractilité capillaires sanguins. Comptes rendus de l'Académie des. Sciences. 1879;88:916–918. [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillet E, Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- Yang H, Dithmar S, Grossniklaus HE. Interferon alpha 2b decreases hepatic micrometastasis in a murine model of ocular melanoma by activation of intrinsic hepatic natural killer cells. Invest Ophthalmol Vis Sci. 2004;45:2056–2064. doi: 10.1167/iovs.03-1331. [DOI] [PubMed] [Google Scholar]


