Abstract
We have recently adapted a reinstatement model, commonly used to study relapse to drugs of abuse, to study the role of stress and anxiety in relapse to palatable food seeking [20]. We found that the anxiogenic drug yohimbine, as well as pellet-priming, reinstate food seeking in food restricted rats previously trained to lever press for palatable food pellets (25% fat, 48% carbohydrate). Here, we studied the generality of the effect of yohimbine and pellet-priming on reinstatement of food seeking by using three distinct pellet types: non-sucrose carbohydrate (NSC) (5.5% fat, 60% carbohydrate, 4.5% fiber), fiber (0% fat, 0% carbohydrate, 91% fiber) and sucrose (0% fat, 91% carbohydrate, 4% fiber). Rats were placed on a restricted diet (75–80% of daily standard food) and for 9–12 intermittent training days (9 h/d, every other day) lever-pressed for the food pellets under a fixed-ratio 1 (20-sec timeout) reinforcement schedule. Subsequently, the rats were given 9–10 daily extinction sessions during which lever-presses were not reinforced, and were then injected with yohimbine (0, 0.5, 1.0, 2.0 mg/kg, i.p.) or given a single food pellet to induce reinstatement of food seeking. Yohimbine reinstated food seeking previously reinforced by NSC and sucrose pellets, but had a minimal effect on food-seeking in rats previously trained to lever press for fiber pellets. Pellet-priming produced a greater degree of reinstatement of lever pressing in rats previously trained on NSC pellets than in rats trained on fiber or sucrose pellets. Results suggest that the magnitude of the effect of yohimbine and pellet-priming on reinstatement of food seeking depends in part on the composition of the food pellets used during training.
Keywords: Carbohydrate, Fiber, Palatable food, Stress, Sucrose, Relapse, Reinstatement, Yohimbine
1. INTRODUCTION
A major problem in the dietary treatment of obesity is the high rates of relapse to maladaptive eating habits [33]. This relapse is commonly triggered by stress and anxiety-like states [10,23]. Surprisingly, most preclinical studies have focused on the effect of stress on ongoing feeding behavior [11,21,32], with little attention to the effect of stress on relapse to excessive eating habits.
Recently, we adapted the rat reinstatement model that has widely been used to study relapse to drugs of abuse [34,36,38], to study stress-induced relapse to palatable food-seeking [20]. Briefly, rats were placed on a restricted diet (75–80% of their regular food) and were trained to lever press for food pellets (25% fat, 48% carbohydrate) for 9 h/day every other day. The choice of this training schedule was based on previous non-operant food consumption studies which have shown that rats placed on a restricted diet and given intermittent access to palatable food develop binge-like eating behavior [7,9]. Interestingly, these rats also become hypersensitive to the effects of stress on palatable food intake [21,22], a finding that is reminiscent of the facilitatory effects of stress on drug-seeking and drug-taking behaviors [15,31]. In our previous study, following pellet self-administration training, rats were subjected to daily extinction sessions, during which lever presses were not reinforced with a pellet. Reinstatement of extinguished lever responding was then induced by non-contingent exposure to a food pellet at the beginning of the session, a condition known to reinstate extinguished food responding [13], or by exposure to an anxiogenic (stress-provoking) drug, yohimbine.
Yohimbine, an α-2 adrenoceptor antagonist, is a commonly used pharmacological stressor that increases brain noradrenaline cell firing [2] and release [1]. The drug is known to produce stress and anxiety-like states in both humans and non-humans [4–6,12,24,25]. Recent studies using the reinstatement model have shown that yohimbine reinstates cocaine seeking in monkeys [28], as well as alcohol and methamphetamine seeking in rats [18,27,37]. Furthermore, yohimbine-induced reinstatement of drug seeking has been reported to be more robust than intermittent foot-shock that is commonly used as a stressor to study mechanisms of stress-induced relapse to drug seeking [3,26,30,35].
In the present study, we examined the generality of yohimbine- and pellet-priming-induced reinstatement of food-seeking behavior across three distinct pellet types: non-sucrose carbohydrate pellets (NSC) (5.5% fat, 60% carbohydrate, 4.5% fiber, 3.64 kcal/g), sucrose pellets (0% fat, 91% carbohydrate, 4% fiber, 3.64 kcal/g) and fiber pellets (0% fat, 0% carbohydrate, 91% fiber, 0 kcal/g). In addition, because in our previous study we found that rats placed on a restricted diet (75 to 80% of their regular food) and given intermittent access to palatable food exhibited a progressive escalation of food seeking during the timeout period when lever presses were not reinforced, we also examined the generality of this phenomenon in the present study by evaluating whether it occurs across the different pellet types.
2. MATERIALS AND METHODS
Subjects and apparatus
Male Long-Evans rats (N=28, Charles River, Raleigh, NC; 300–360 g) were housed in self-administration chambers for the duration of the experiment under a reverse 12-h:12-h light-dark cycle (lights off at 9:30 am). Rats were kept on a restricted diet of 20 g/d (about 75–80% of their regular daily Purina Rat Chow). The body weights of rats were taken daily 1–2 h prior to the start of the dark cycle. The procedures followed the “Principles of laboratory animal care” (NIH publication no. 85–23). Experiments were conducted in standard self-administration chambers (Med Associates, Georgia, VT). Each chamber had two levers 9 cm above the floor, but only one lever (“active,” retractable lever) activated the pellet dispenser, which delivered either NSC pellets containing 5.5% fat, 60 % carbohydrate, 4.5 % fiber, sucrose pellets with 0% fat, 91% carbohydrate, 4% fiber, or fiber pellets with 0% fat, 0% carbohydrates, 92% fiber (Bioserv, Frenchtown, NJ).
Drugs
Yohimbine HCl (RBI) was dissolved in distilled water and injected in a volume of 0.5 ml/kg. The yohimbine doses used (0.5, 1, 2 mg/kg, i.p.) were based on previous work [27,37].
Procedures
The experiments included 3 phases: pellet self-administration training (18–24 d), extinction (9–10 d) and reinstatement tests (5 d).
Self-administration training
Rats were given one to two 6-h daily sessions of “autoshaping” during which pellets were administered non-contingently every 5 min into a receptacle located near the active lever. Pellet delivery was accompanied by a 5-sec tone-light cue. Subsequently, over a period of 18–24 d (9–12 training sessions), the rats were trained to self-administer pellets every other day for 9-h/d (three 3-h sessions separated by 1 h) on a fixed-ratio-1 (20-sec timeout) schedule. At the start of each session, the houselight was turned on and the active lever was extended. Following each pellet delivery, the tone-light cue was turned on for 5 sec. Presses on a second, non-retractable inactive lever were recorded but had no programmed response. At the end of each session, the houselight was turned off and the active lever retracted. During the training days, rat chow (20 g) was given immediately after the first 3-h daily session, which started at 10 am; during the off days, the 20 g rat chow was given at the start of the dark cycle. Due to experimenter error, on training days 2 and 9 in the NSC group rats were fed after the 2nd 3-h training session; the training data for these days was estimated from the preceding and subsequent days.
Extinction phase
After training, the rats were given 9–10 daily extinction sessions. The experimental conditions were identical to those in training, except that active lever presses did not lead to pellet delivery. The rats were given daily three 3-h sessions (separated by 1 h). Subsequently, 3-h extinction sessions for 3–4 d were given, during which the rats were given daily injections of the yohimbine vehicle (sterile water) to habituate them to the injection procedure. During the extinction phase, rat chow (20 g) was given immediately after the first 3-h session.
Tests for reinstatemen
For each of the three experiments, we tested the effect of different doses of yohimbine (0, 0.5, 1 and 2 mg/kg, i.p.) on the reinstatement of food-seeking in four test sessions. Yohimbine or its vehicle was injected 30–45 min before the test sessions. This 3-h reinstatement test was conducted under extinction conditions. Rat chow (20 g) was given immediately after the session, which started at 30 min into the dark cycle. Subsequently, the rats were given an additional extinction session, 1 d following which we examined the effect of food-pellet type on pellet-induced reinstatement. Rats were re-exposed to a single pellet of the same type which they had trained for during food self-administration sessions, which was delivered non-contingently at the beginning of this 3-h test session. This reinstatement test was also conducted under extinction conditions.
Data analyses
Data analyses were conducted using two- or three-way mixed factorial ANOVAs. For the analysis of the yohimbine data, we used a two-way mixed factorial design, with a within-subjects factor of yohimbine dose (0, 0.5, 1 and 2.0 mg/kg, i.p.) and a between-subjects factor of pellet type (sucrose, fiber, or NSC pellets). For the analysis of the pellet priming data, we used a two-way mixed factorial design, with a within-subjects factor of pellet priming (priming, no priming) and a between-subjects factor of pellet type (sucrose, fiber, or NSC pellets). The statistical program used was Statview for Windows Version 5.0.1 (SAS Institute Inc., Cary, NC). Fisher’s PLSD post-hoc tests followed significant results (α=0.05).
3. RESULTS
Self-administration training and extinction
During the training phase, the rats were given 9-h access to the food pellets every other day. For pellets containing 5.5% fat, 60 % carbohydrate and 4.5% fiber (NSC pellets) (n=10), and 0% fat, 91% carbohydrate, 4% fiber (Sucrose pellets) (n=10), respectively, the rats gained weight when the pellets were available (on an average 12 g/d for the sucrose pellet group, and 13 g/d for the NSC pellet group), and lost weight when they were not (on an average losing 7 g/d for the sucrose pellet group, 6 g/d for the NSC group) (Fig. 1b and 1c). However, using pellets containing 0% fat, 0% carbohydrates, and 92% fiber (Fiber pellets), the rats (n=8) did not exhibit significant weight fluctuations (on an average gaining 3 g/d when pellets were available and losing 2 g/d when pellets were not available) (Fig. 1a). From the first to the last training day, the sucrose pellet group gained 63±6 g, the NSC pellet group gained 86±4 g, while the fiber pellet group gained only 7±9 g (Mean±SEM). A 3-way mixed factorial ANOVA using pellet availability and session day as the nested within-subject factors, food-pellet type as the between-subject factor, and body weight as the dependent measure revealed a significant two-way interaction between the factors of pellet type and pellet availability (F2,25=43.2, P < 0.01) and a significant three-way interaction between the factors of pellet type, pellet availability, and session day (F16,200 = 21.6, P < 0.01). The weight fluctuations in the NSC and sucrose pellet groups during self-administration training were not observed during the extinction and reinstatement phases when the pellets were not available.
Figure 1.
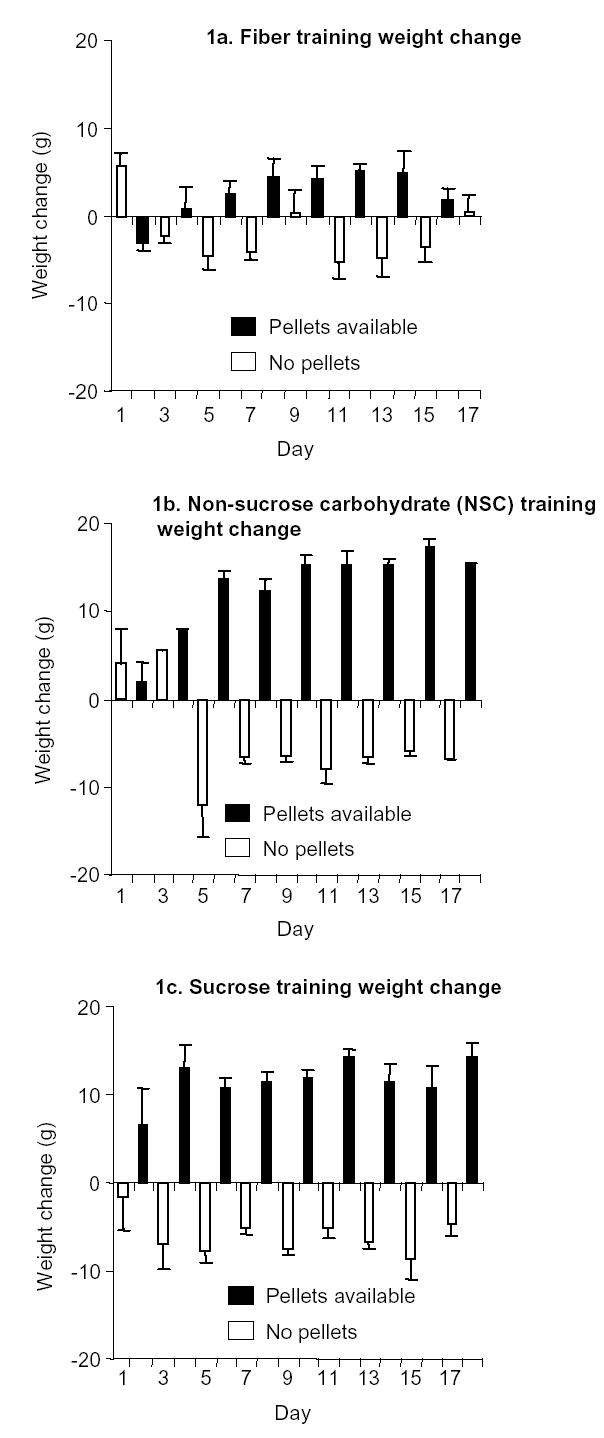
Daily weight fluctuations of rats during training. Rats were trained to self-administer food pellets for 9 h/day, every other day. Rats gained weight on the day pellets were made available, and lost weight on the subsequent day. (a) Daily weight fluctuations of rats self-administering fiber pellets: Mean (± SEM) daily weight fluctuations of rats allowed to self-administer fiber pellets (black) alternating with days of forced abstinence (white) (n = 8). (b) Daily weight fluctuations of rats self-administering non-sucrose carbohydrate (NSC) pellets: Mean (± SEM) daily weight fluctuations of rats allowed to self-administer NSC pellets (black) alternating with days of forced abstinence (white) (n = 10). (c) Daily weight fluctuations of rats self-administering sucrose pellets: Mean (± SEM) daily weight fluctuations of rats allowed to self-administer sucrose pellets (black) alternating with days of forced abstinence (white) (n = 10).
During training, the rats demonstrated reliable pellet self-administration (Fig. 2a) with a 3-fold increase in active lever presses for NSC pellets, and a 2-fold increase in active lever presses for sucrose pellets (Fig 2b). However, rats demonstrated only a 1.4-fold increase in active lever presses for the fiber pellets (Fig. 2b). Moreover, there was an escalation in active lever presses across session days for sucrose and NSC pellets, but not for fiber pellets (Fig. 2b). A two-way mixed factorial ANOVA using session day as a within-subject factor, pellet type as a between-subject factor, and active lever presses as the dependent variable revealed a significant interaction (F16,200=4.1, P < 0.01), indicating that the rate of active lever presses across training days varied by pellet type. There was also a significant main effect of pellet type (F2,25=33.6, P < 0.01); Fisher’s PLSD tests revealed that rats lever pressed more for NSC and sucrose pellets than for fiber pellets. A two-way mixed factorial ANOVA using session day as a within-subject factor, pellet type as a between-subject factor, and number of pellets earned (i.e., number of reinforced lever presses) as a dependent variable revealed that there was a significant main effect of pellet type (F2,25=20.1, P < 0.01); Fisher’s PLSD tests revealed that rats earned more NSC and sucrose pellets than fiber pellets, however, there was no significant interaction (F16,200=1.4, P > 0.1). For all groups, inactive-lever presses were very low and did not change over time (P > 0.1, Fig. 2d).
Figure 2.
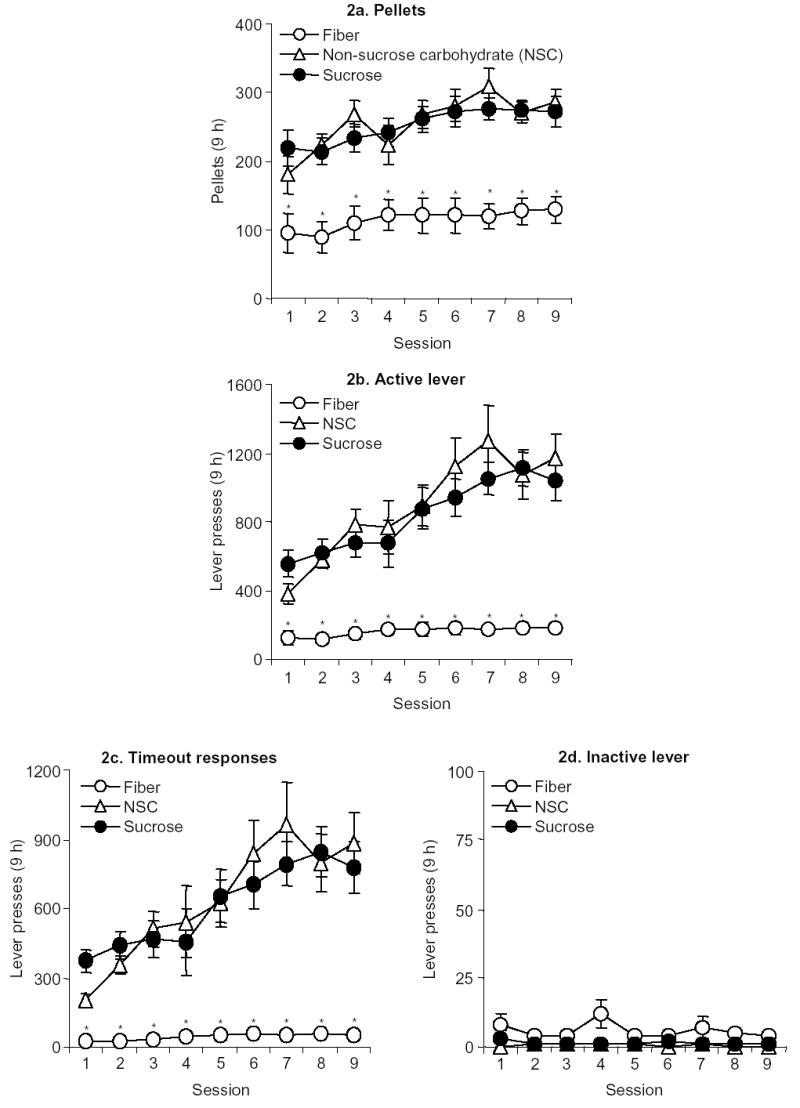
Self-administration training of fiber, NSC and sucrose pellets (a) Pellets: Mean (± SEM) fiber, NSC and sucrose pellets during 9-h training sessions over 9 days (sessions 48 h apart). (b) Active lever presses: Mean (± SEM) presses on the active lever during 9-h (9 days) sessions (n=8–10 per group). (c) Timeout responses: Mean (±SEM) non-reinforced presses on the active lever during the 20-sec timeout period. (d) Inactive lever presses: Mean (± SEM) non-reinforced presses on the inactive lever during the 9-h (9 d) sessions. * Different from the NSC and sucrose conditions, P < 0.05.
Since the difference between active lever presses and reinforced lever presses is equal to the number of non-reinforced lever presses during the 20-s timeout period (termed timeout responses), we investigated whether the number of these timeout responses varied by food-pellet type across session days. A two-way mixed factorial ANOVA using session day as a within-subject factor, pellet type as a between-subject factor, and non-reinforced lever presses during the 20-s timeout as the dependent variable revealed a significant interaction (F16,200=3.7, P < 0.01), indicating that NSC pellet- and sucrose pellet- but not fiber pellet-trained rats exhibited a progressive increase in timeout lever responding across session days (Fig. 2c). A two-way mixed factorial ANOVA using session day as a within-subject factor, pellet type as a between-subject factor, and the ratio of timeout presses to reinforced lever presses as the dependent variable also revealed a significant interaction (F16,200=2.9, P < 0.01) confirming that the number of non-reinforced lever presses across session days varied by pellet type.
Lever pressing was extinguished over six 9-h extinction sessions followed by three to four 3-h extinction sessions (Fig. 3a). A repeated-measures ANOVA revealed that active lever presses decreased significantly over those days (F5,25 =63.9, P < 0.01), and remained low over the subsequent three to four 3-hr sessions (P > 0.1) (Fig. 3b). A two-way mixed factorial ANOVA using extinction day as a within-subject factor, pellet type as a between-subject factor, and active-lever presses as the dependent variable revealed a significant interaction during the first 6 extinction sessions (F10,125=12.0, P < 0.01), indicating that the rate of active presses across these sessions varied by pellet type (Fig. 3a). There was also a significant main effect of pellet type (F2,25=12.4, P < 0.01); Fisher’s PLSD tests revealed a difference between the sucrose versus fiber pellet, and between the NSC versus fiber pellet conditions. For all groups, inactive-lever presses were very low and did not change over time (P > 0.1) (Fig. 3b).
Figure 3.
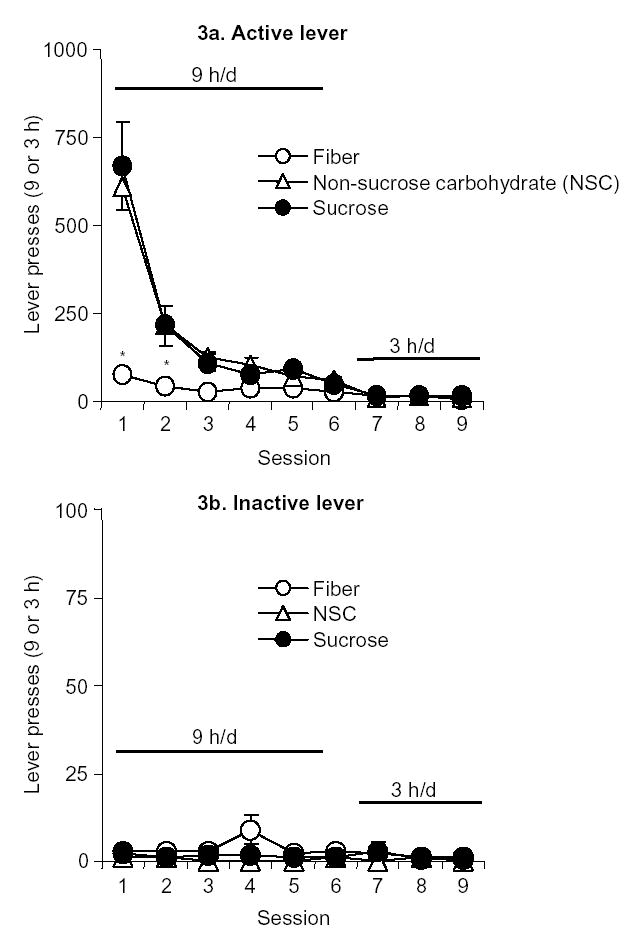
Extinction of self-administration behavior. (a) Active lever presses: Mean (± SEM) presses on the previously active lever during the 9-h (6 d) or 3-h (3–4 d) extinction sessions (n = 8–10 per pellet condition). * Different from the NSC and sucrose conditions, P < 0.05. (b) Inactive lever presses: Mean (± SEM) presses on the inactive lever during 9-h (6 days) or 3-h (3–4 days) sessions.
Reinstatement tests
Yohimbine
A skewed distribution of non-reinforced active-lever presses during reinstatement tests was converted into a normal distribution by use of a log-linear transformation. Three outlier rats, one from each experimental group, which showed an unusually high lever responding following yohimbine administration (195, 235, 327 presses/3 h; more than 3 standard deviations above their group mean) were excluded from the analyses. The effect of yohimbine on reinstatement of active lever responding was more pronounced in the NSC- and sucrose-trained rats than in the fiber-trained rats (Fig. 4a). A two-way mixed factorial ANOVA with a within-subject factor of yohimbine dose (0, 0.5, 1, 2 mg/kg, i.p.) and a between-subject factor of pellet type revealed significant main effects of pellet type (F2,23=4.4, P < 0.05) and yohimbine dose (F3,23=23.2, P < 0.01); the interaction between the pellet type and yohimbine was not statistically significant (P > 0.05). In all experiments, inactive-lever presses were very low and did not change over time (P > 0.1) (Fig. 4b). Post-hoc differences between group within each dose of yohimbine and within groups across the different yohimbine doses are indicated in Fig. 4.
Figure 4.
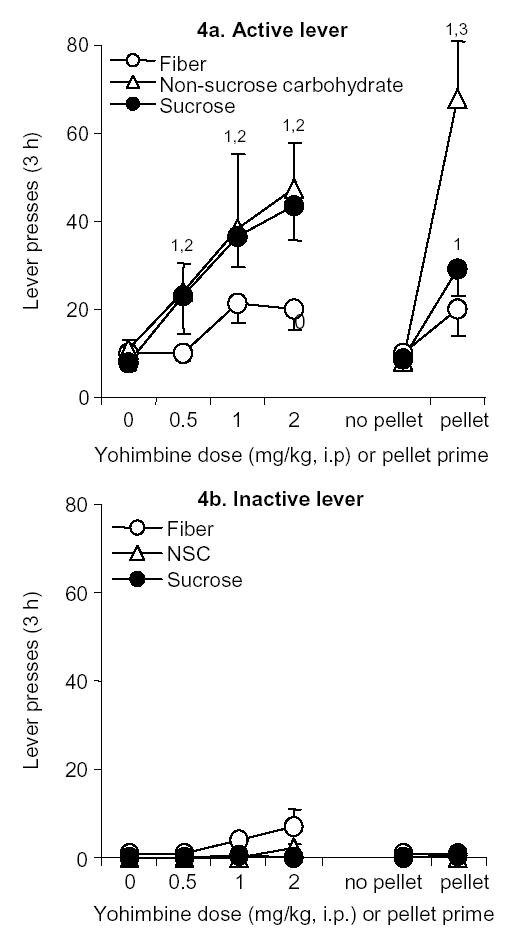
Reinstatement of fiber, NSC and sucrose seeking (a) Active lever presses: Mean (± SEM) presses on the previously active lever performed under extinction conditions. During these test days, rats were given injections of yohimbine (0.5, 1 or 2 mg/kg, i.p.) or its vehicle 30–45 min prior to the start of the 3-h session(s). Pellet prime, or non-contingent pellet delivery, occurred immediately prior to the start of the session. 1--Different from the Vehicle condition, P < 0.05; 2--Different from the fiber condition, P < 0.05; 3--Different from the fiber and sucrose conditions, P < 0.05, n=8–10 per pellet condition. (b) Inactive lever presses: Mean (+ SEM) presses on the inactive lever during 3-h test sessions.
Pellet priming
The effect of a single exposure to 45 mg food pellet on reinstatement of active lever responding was substantially stronger in the NSC pellet-trained rats than in the sucrose pellet- or fiber-pellet-trained rats (Fig. 4b). A two-way mixed factorial ANOVA included the between-subjects factor of pellet type and the within-subjects factor of pellet priming (priming, no priming). This analysis revealed significant main effects of pellet priming (F1,23=28.7, P < 0.01) and pellet type (F2,23=4.6, P < 0.05) and a significant interaction between pellet priming and pellet type (F2,23=6.3, P < 0.01). Post-hoc differences are indicated in Fig. 4.
4. DISCUSSION
In the present study, we evaluated whether yohimbine and pellet priming-induced reinstatement generalizes to other food types (i.e. non-sucrose carbohydrate (NSC) pellets (5.5% fat, 60% carbohydrate, 4.5% fiber, 3.64 kcal/g), fiber pellets (0% fat, 0% carbohydrate, 91% fiber, 0 kcal/g) and sucrose pellets (0% fat, 91% carbohydrate, 4% fiber, 3.64 kcal/g)) or whether this reinstatement behavior is dependent on the dietary composition of the pellets used during training. We found that yohimbine reinstated food seeking previously reinforced by NSC and sucrose pellets, whereas its effect on reinstatement in rats previously trained to lever press for fiber pellets was relatively weak. Pellet-priming produced a greater number of lever presses in the test for reinstatement in rats previously trained on NSC pellets than in rats trained on fiber or sucrose pellets. This suggests that the effect of yohimbine and pellet-priming on reinstatement of food seeking depends, in part, on the composition of the food pellets used during training. During training, we also observed that rats progressively increased timeout and total lever presses for the NSC and sucrose pellets, but not fiber pellets. Thus, rats given intermittent access to palatable food (NSC and sucrose pellets) during extended (i.e., 9 h) food self-administration sessions escalate non-reinforced food seeking across days, an effect we interpret to indicate the development of a ‘compulsive’ food-seeking habit (see below).
The choice of the different pellets used in the present study was primarily based on the variable composition of carbohydrate, sucrose and fiber contents as well as pellet availability for operant conditioning experiments. The micronutrient composition of these pellets was similar. Foods with high carbohydrate and fat contents have been reported to be highly palatable, as compared to foods with high fiber content which are less palatable [19]. Thus, it is reasonable to classify the high caloric, energy dense NSC and sucrose pellets as ‘palatable’ and non-caloric fiber pellets as ‘unpalatable’ reinforcers.
Reinstatement of food seeking by yohimbine and pellet priming
It is well known that stress and anxiety-like states induce relapse to maladaptive eating habits. Humans seem to be particularly vulnerable to these effects of stress and anxiety during dieting [16,23]. Surprisingly, this phenomenon has not been closely studied in preclinical animal models. Recently, we developed a reinstatement model to study anxiety-induced relapse to palatable-food seeking [20] that was based on the rat reinstatement procedure that has widely been used to study relapse to drugs of abuse [7,34,36,38,39,40]. Ghitza et al. [20] trained rats to lever press for palatable food pellets (25% fat, 48% carbohydrate) on a fixed ratio-1 reinforcement schedule. Following extinction of lever pressing, reinstatement of the extinguished lever responding was induced by exposure to the anxiogenic drug yohimbine or by non-contingent exposure to a palatable food pellet.
In the present study, yohimbine dose-dependently increased reinstatement of food seeking across the three different food types. Previous studies have indicated that central noradrenergic systems play an important role in mediating footshock stress-induced reinstatement of drug-seeking [17,29,35]. Yohimbine also has significant affinities for the D2, 5-HT1A, α-1 and benzodiazepine receptors. But it is unlikely that the effect of yohimbine on the reinstatement of palatable food-seeking is mediated by these receptors types [27,37]. Thus, the results from the present study have been interpreted to indicate that brain noradrenaline release plays a role in yohimbine-induced reinstatement. Interestingly, we found significant differences in the magnitude of food seeking in yohimbine-induced reinstatement that was dependent on the type of pellet used during training. Rats trained to lever press for NSC and sucrose pellets produced a greater degree of reinstatement across all doses of yohimbine, as compared to rats trained on fiber pellets (Figure 4a).
Reinstatement of food-seeking was also determined after non-contingent exposure to a food pellet, which has been known to reinstate extinguished food-responding [13]. Pellet-priming produced a greater degree of reinstatement of lever pressing in rats previously trained on NSC pellets than in rats trained on fiber or sucrose pellets (Figure 4a). In a food preference test conducted across the different pellet types, rats exhibited a greater preference for NSC pellets (over 90%) than for sucrose or fiber pellets. This observation may account for the greater magnitude of pellet-priming induced reinstatement in rats primed with NSC pellets. Furthermore, these data suggest that the neurochemical mechanisms that mediate yohimbine and pellet-priming induced reinstatement are influenced, at least in part, by the dietary composition of pellets used during training.
Progressive increase in non-reinforced lever responding but not pellet intake during training
During training, food-restricted rats given intermittent access to the palatable (NSC and sucrose) pellets that lead to significant alterations in body weights (Figures 1b and 1c), progressively increased lever pressing. It is unlikely that this increase is related to an enhanced learning capability, because these rats discriminated between active and inactive levers early in training (Figure 2d). Moreover, there was a progressive escalation of the non-reinforced lever presses in rats trained on NSC and sucrose, but not on fiber pellets. The escalation in lever responding is consistent with the results obtained from other operant [20] and non-operant [8,9,21,22] studies where food-restricted rats given intermittent access to palatable food progressively increase lever responding and food intake, an effect that suggests the development of binge-eating habits [8,9,21,22]. Thus, the data from the present study suggest that only exposure to high caloric, energy dense palatable pellets (NSC and sucrose pellets), but not to non-caloric, unpalatable fiber pellets, during training leads to the development of compulsive-like eating behavior in our animal model.
In the present study, while NSC and sucrose pellet intake only marginally increased over the first 5 days of training before stabilizing, the total number of non-reinforced lever presses during the 20 s timeout period increased progressively by approximately 3 fold over the training period (Figure 2c). This is interesting in the light of findings of Deroche-Gamonet et al. [14] who reported that rats that developed an ‘addiction’-like behavior following prolonged exposure to cocaine, lever pressed at much higher rates during drug non-reinforced periods than rats that did not develop this phenotype. The persistence of non-reinforced responses, which developed following prolonged exposure to cocaine, might reflect the development of ‘compulsive’ drug-taking behavior, as suggested by these authors. Therefore, in our study, a progressive increase in timeout lever responding during training in the rats trained on palatable pellets might indicate that these rats develop ‘compulsive’ food-taking habits. In contrast, the rats training on fiber pellets did not show a progressive escalation in timeout responses on the active lever, suggesting that the development of ‘compulsive’ food-seeking behavior may be restricted to rats trained only on palatable (NSC and sucrose) food pellets.
Conclusions
The results of the present study indicate that both the anxiogenic drug yohimbine and pellet-priming reinstate food seeking in our rat reinstatement model across different food types. However, the degree of yohimbine and pellet-priming-induced reinstatement of extinguished food-seeking behavior is dependent on the dietary composition of the pellets used during training. In addition, it appears that only rats trained on high-caloric, palatable food develop a ‘compulsive’ food-taking behavior. Thus, our model of stress- and anxiety-induced reinstatement of food-seeking behavior in the rat may be relevant to delineate the mechanisms that mediate relapse to maladaptive eating habits to high caloric palatable food. Furthermore, this model may also be used to study the neurochemical parameters that are involved in the development of ‘compulsive’ food-taking behaviors in humans.
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the NIH, the National Institute on Drug Abuse. We thank Dr. Yavin Shaham for helpful comments on the manuscript.
References
- 1.Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- 3.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine . Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- 7.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 8.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3452. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 9.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Crowther JH, Sanftner J, Bonifazi DZ, Shepherd KL. The role of daily hassles in binge eating. Int J Eat Disord. 2001;29:449–454. doi: 10.1002/eat.1041. [DOI] [PubMed] [Google Scholar]
- 11.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci (U S A) 2003;100:11696–701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- 13.de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- 14.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 15.Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants II: Cocaine intravenous self-administration and reinstatement depend on glucocorticoids levels. J Pharmcol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- 16.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 17.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- 18.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein DE, Woodward-Lopez G, Evans AE, Kelsey K, Drewnowski A. Clarifying concepts about macronutrients’ effects on satiation and satiety. J Am Diet Assoc. 2004;104:1151–1153. doi: 10.1016/j.jada.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1300964. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 22.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 23.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84:66–72. [PubMed] [Google Scholar]
- 24.Holmberg G, Gershon S, Beck LH. Yohimbine as an autonomic test drug. Nature. 1962;193:1313–1314. doi: 10.1038/1931313b0. [DOI] [PubMed] [Google Scholar]
- 25.Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch Int Pharmacodyn Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- 26.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 27.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 29.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced, but not cocaine-induced reinstatement, by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 31.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 32.Morley JE, Levine AS, Rowland NE. Minireview. Stress induced eating Life Sci. 1983;32:2169–2182. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- 33.Peterson CB, Mitchell JE. Psychosocial and pharmacological treatment of eating disorders: a review of research findings. J Clin Psychol. 1999;55:685–697. doi: 10.1002/(sici)1097-4679(199906)55:6<685::aid-jclp3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 34.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 35.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 36.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 39.Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]


