ABSTRACT
Objective: To evaluate the feasibility of using a voice-controlled robot Automated Endoscopic System for Optimal Positioning (AESOP) for holding and maneuvering the endoscope in the trans-sphenoidal approach to the pituitary. Design: To compare the manual approach to the voice-activated robotic scope holder in maneuvering the endoscope and resecting pituitary lesions using a two-handed technique. Setting: Robotic laboratory at Louisiana State University Health Sciences Center, Shreveport. Cadavers: Ten fresh cadaver heads. Main Outcome Measures: To determine the feasibility, advantages, and disadvantages of a single neurosurgeon maneuvering the endoscope, visualizing key anatomical features in the sphenoid, and resecting skull base lesions after the approach by an otolaryngologist. Results: The learning curve for utilization of the voice-controlled robotic arm was short. The compact cart with the AESOP took up little space and allowed the standard setup for this procedure. The elimination of the need for manual stabilization of the endoscope permitted the use of both hands for the actual procedure. The elimination of the tremor inherent with holding the endoscope manually allowed the scope to be placed closer to the target organ with fewer collisions. The most significant advantage was the ability of AESOP to save three anatomical positions, which could be returned to with a single voice command. Conclusions: Recently, the endoscopic-endonasal approach to the sella has gained popularity. The voice-activated robotic scope holder is safe and has several advantages over current scope holders. Its utility may reduce operating time and eliminate the need for a second surgeon to hold the endoscope.
Keywords: AESOP, minimally invasive surgery, skull base
Advances in technology during the past decade have helped propel the transition to minimally invasive surgery. The next generation of minimally invasive surgery is evolving through technological innovation and robotics. Although robots have been readily used in various industries for almost 40 years, medical robots designed to support surgeons have existed for only a decade and a half.1 The number and the range of robotic applications in surgery are growing rapidly. Robots are used in neurosurgery, urology, orthopedics, general surgery, and ophthalmology.2,3,4 The use of robots in the field of otolaryngology is recent.5,6 The most commonly performed minimally invasive surgery in otolaryngology is endoscopic sinus surgery. In 2002 we performed the first voice-controlled robotic assist for endoscopic sinus surgery in a patient after perfecting the technique in cadavers.7
Recently, the endoscopic-endonasal approach to the sella has gained popularity.8,9,10 The applications of the endoscopic approach to the sella turcica have been extended beyond that of pituitary tumors to include a large number of skull base tumors.11,12,13 This approach has several advantages.14,15 However, the inherent difficulty with holding the endoscope and thus having only one hand available for the surgical procedure, or the need for a second surgeon to hold the endoscope, has hindered its widespread use by neurosurgeons. We wanted to evaluate the feasibility of using a voice-controlled robot for holding and maneuvering the endoscope. One of the most established commercial robotic systems is the FDA-approved AESOP, the Automated Endoscopic System for Optimal Positioning (Intuitive Surgical, Inc., Sunnyvale, CA), which actively positions an endoscope. AESOP uses voice recognition to control a robotic arm with seven degrees of freedom. It provides direct control of the endoscope using simple spoken voice commands.
Recently, a robot-guided device combined with redundant navigational surgery was developed to determine the feasibility of its use for skull base surgery.16 The article focused on the design of the instrument and not on the advantages of the robot as a scope holder. We describe the use of the affordable, compact, FDA-approved robot AESOP and the advantages this system offers with special reference to improving the learning curve for the endoscopic approach to the skull base.
MATERIALS AND METHODS
The procedure was performed on 10 fresh cadaveric heads in the robotic laboratory at Louisiana State University Health Sciences Center, Shreveport. Computed tomography (CT) axial scans of the paranasal sinuses (1 mm thick) were obtained after the InstaTrak® (GE Medical Systems, Lawrence, MA) headset was placed on the cadaveric head. The InstaTrak® system automatically reformatted the coronal and sagittal images.
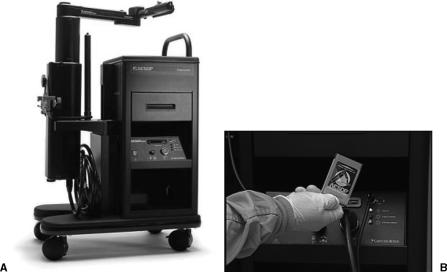
We used our standard operating room setup for the video cart and instrument table. The compact system cart for AESOP is a storage, transportation, and loading device used to hold and move the position arm before the procedure (Fig. 1A). The positioning arm for the robot was lifted off the cart and attached to the operating room table by hand. The surgeon wore the lightweight headset microphone, which was connected to the controller on the transport cart, and is the primary interface for the surgeon to control the arm by the use of the personal voice card for simple verbal commands (Fig. 1B).
Figure 1.
(A) The compact transport cart with the mounting arm on which the AESOP central processing unit is placed and (B) the personal voice card required for verbal commands.
The positioning arm was first adjusted by using the 3-D manual mode button to position the arm right above the cadaveric head. The endoscope collar holder, which houses the endoscope, was then attached to the positioning arm by simply snapping it in place. The 0-degree endoscope was slid through the 3-mm collar holder. With the endoscope in place, the lower limit was set. This safety feature sets the lowest point the positioning arm can travel without making contact with the patient. It is typically set about one finger-breadth above the patient. The lower limit can be set and cleared using the verbal commands “Set lower limit” and “Clear lower limit.”
There are three modalities to control the positioning arm: the 3-D manual mode button, voice control interface, and hand control. We used the 3-D manual mode to slide the scope into the naris until we identified the sphenoethmoid recess using the median approach to the sella, between the nasal septum and the middle and superior turbinates. Then the voice control interface was enabled. To control the positioning arm, the system must be placed in a command mode by uttering the keyword AESOP. A sharp audible tone can be heard each time the system is placed in command mode. Once the voice control interface is enabled, the AESOP positioning arm can maneuver and position the endoscope incrementally or continuously in response to simple verbal commands (up, down, left, right, in, out). The word “Stop” is used to exit the command mode. The system also exits the command mode automatically if 15 seconds lapse without the utterance of a verbal command.
Next, the natural ostium of the sphenoid was identified. In some cases the superior turbinate was resected to expose the ostium. At this point, we used a helpful feature of AESOP called positioning with the Re-view feature. The Re-view feature can be used to store a specific endoscope position. The surgeon can then return to the same operative view any time during the procedure with a single voice command. For example, once the sphenoid ostium was identified, the verbal command “Save One” stored the specific location in the system's computer memory. AESOP plays back the audible message “Position 1 Saved” to confirm the stored memory position. The surgeon can return to this position anytime during the procedure by uttering the command “Return 1.”
There are three Re-view memory positions available using AESOP's voice control interface. The system computer retains the previously saved Re-view positions if the patient is moved during surgery, as long as the relationship between the arm and the patient has not changed. Similarly, if the endoscope is detached from the arm and reconnected, the saved Re-view positions will be retained if the relationship between the arm and the patient has not changed significantly. The view of the natural ostium was saved as Position 1.
Incremental voice commands were used to enter the sphenoid sinus, and the microdebrider and Kerrison rongeurs were used to enlarge the ostium medially and inferiorly. The endoscope was further positioned using voice commands to visualize the carotid and optic nerve, and this position was saved as Position 2. The image guidance navigational system was used to localize the sella, and this position was saved as Position 3. After the extent of pneumatization of the sella was determined, the mushroom punch was used to enter the sella.
RESULTS
The approach to the sella was feasible with the robotic scope holder. The scope holder and position of the endoscope permitted enough room for dual instrumentation in the nose (Fig. 2). We used the microdebrider to remove the superior turbinate and to identify the ostium. This maneuver also could be performed without the robotic scope holder making contact with the instruments. The setup required for AESOP was easy, as was draping and maneuverability. Furthermore, it did not occupy too much space. The normal setup of the operating room for this procedure was the same because the robotic arm can be mounted on the operating table. Unlike master-slave design robots, the AESOP cart is also compact.
Figure 2.
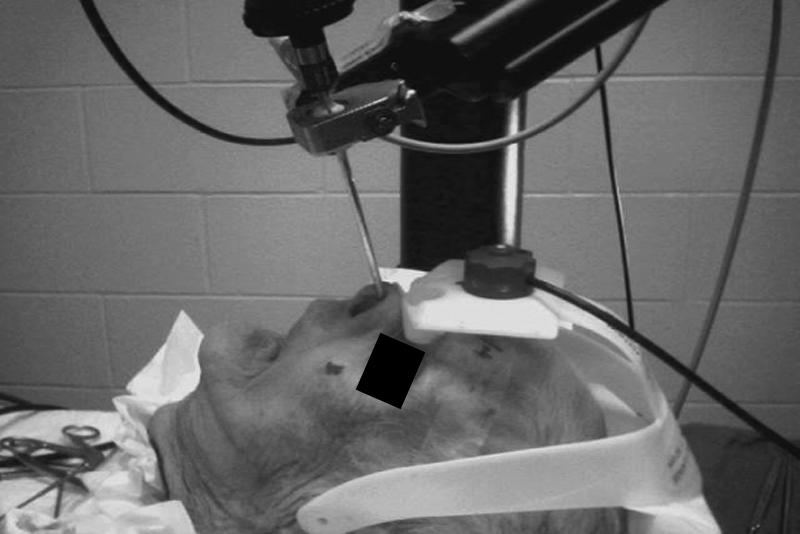
This figure shows the attachment of the endoscope to the robotic arm via a magnetic collar. The amount of room available for dual instrumentation in the nose is adequate.
The voice-activated scope holder added no significant length to the procedure. Magnetic coupling of the endoscope collar and collar holder allowed the endoscope to be removed quickly and easily during the case. We determined that the lower limit had to be set only after the endoscope was attached. If set before the endoscope is attached, the robot identifies the positioning arm without the scope as the lower limit and nasal structures can be damaged with the endoscope. To shorten the length of the procedure, it was important to use the 3-D manual mode button to bring the endoscope into the nose until the sphenoethmoid recess was identified and then switch to the voice-controlled interface to identify the ostium.
We used the incremental move commands that cause fine movements of the endoscope for close-up viewing of the anatomy within the sphenoid sinus. The continuous move commands that cause large movements in the endoscope position and that are used to pan or zoom the endoscope when necessary to view broad regions of anatomy were not used during the procedure. Once the sphenoid sinus ostium who identified, only close-up viewing was required during the procedure.
The advantage of the robotic arm was that it automated the critical tasks of positioning the endoscope and provided the surgeon with direct control over a smooth, precise, stable view of the internal surgical field. It mimicked the human arm in form and function, with seven degrees of freedom. It never fatigued or misinterpreted the surgeon's commands. The elimination of the need for manual stabilization of the endoscope permitted the use of both hands for the actual procedure. Consequently, suction could be used at all times to obtain a clean operative field. It also eliminated the fine tremor. It reduced eye fatigue, provided a steady field when instruments were passed back and forth to the scrub nurse, and allowed the scope to be positioned closer to the target tissue with fewer collisions. Thus, it also eliminated the frequent need to clean the endoscope.
The robotic scope holder eliminated the need for a second surgeon to be present for the entire procedure. Another significant advantage was the ability of AESOP to save three anatomical positions and return to them with a single voice command. It is especially useful in skull base procedures when repositioning the scope and identifying critical structures typically require the otolaryngologist to be present for the entire procedure.
At a teaching institution, this advantage allows the teaching surgeon to control the scope view. The saved Position 1 was the view of the natural ostium located in the sphenoethmoid recess, medial to the superior turbinate. We also could save the view of the lateral sphenoid wall with the two important bulges (i.e., the optic nerve prominences above and carotid prominences below) to visualize the optocarotid recess between these prominences. This was saved as Position 2. The entrance into the sella with the mushroom punch was saved as Position 3. We were able to return to each position with a single voice command after the scope was withdrawn from the sphenoid sinus.
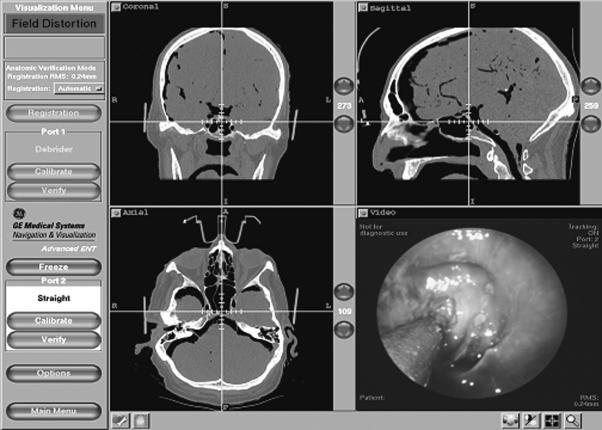
The only difficulty encountered during the procedure was interference of the magnetic collar with the image guidance system. This interference resulted in a warning field distortion sign on the computer. Interestingly, we could still track accurately despite the distortion sign on the screen (Fig. 3). If the positioning arm with the magnetic endoscope holder was brought into the field at the time of registration, difficulty was noted with registration of the InstaTrak® head set.
Figure 3.
Endoscopic view of the suction probe in the sphenoid sinus and its localization on the CT scan using the InstaTrak® computer navigational system. Although the tracking appears to be accurate, a warning field distortion sign appears on the screen. The warning indicates a potential problem with compatibility of the electromagnetic tracking system and robotic magnetic clasp holder for the endoscope.
DISCUSSION
The endoscopic approach was first used to supplement the transrhinoseptal approach to microsurgical exploration of the sella in 1963 by the French neurosurgeon Gerard Guiot.17,18 Advances by otolaryngologists in the endoscopic treatment of sinus surgery led Carrau and colleagues to propagate the “pure” endoscopic approach to the pituitary in 1993.19 However, a decade later the endoscopic approach has yet to gain the overwhelming support one would expect for a technique associated with low morbidity.
One disadvantage has been that the endoscope provides a two-dimensional image, unlike the three-dimensional, stereoscopic image obtained through a microscope. The learning curve associated with the endoscopic approach has also limited its widespread use. This may reflect the need for the endoscope to be handled with the nondominant hand and the surgical instruments with the other.
The endoscope and the instruments must enter parallel to each other in a single nostril. Castelnuovo20 describes the approach as a “four-handed” technique where the intervention is performed through one nostril using the endoscope and an instrument, while another instrument operated by a second surgeon is passed through the other nostril. Hence, having a third hand available (an otolaryngologist), although possible, occupies space and requires coordination of two different specialties and surgeons' time.21,22 The endoscope-holding device enables the surgeon to use both hands and simultaneously provides a continuous view on the monitoring screen. The AESOP scope holder also allows the otolaryngologist to find and save landmarks so that the neurosurgeon can easily return to any of the key landmarks with a single voice command. The Pittsburgh group of skull base surgeons have been proponents of the endoscopic approach not only for pituitary surgery but also for areas of the skull base that previously were not addressed by the endoscopic approach.11,12,13 Advances in technology with both the endoscopes and navigational surgery have made the endoscopic approach feasible. This approach is a natural extension for the experienced endoscopic sinus surgeon. It requires neither incisions nor speculum retraction. More importantly, the angled telescopes facilitate excellent visualization of the sellar and parasellar regions from various angles.
Although scope-holding devices have been used, they have not gained popularity because of the constant need to change the position of the endoscope to view various areas of the sella. A holder free of flexible tension arms for rigid Karl Storz telescopes and a Mataka pneumatic scope holder have been described.23 However, the cost of the scope holder is high, and it still requires that the scope be detached when the field is blurry. The otolaryngologist is also needed to obtain the endoscopic view of the required field. Hence the Re-view feature with AESOP that allows the surgeon to store specific positions of the endoscope, which allows the surgeon to return to the same operative view at any time during the procedure with a single voice command, is a significant advantage. The otolaryngologist can identify the key positions in the surgery at the start of the case and have the neurosurgeon use the voice-activated card to save the positions. The neurosurgeon can thus return to the key positions in the case.
The mortality and morbidity rates associated with the endoscopic approach, although small, include vascular complications some of which are fatal due to injury to the carotid artery.24,25 Although the endoscopic view has improved visualization to the lateral recesses compared with the standard microsurgical view, risks still exist because of bleeding from the tumor.26,27,28 Blood in the field can blur the endoscope lens impeding vision and forcing the surgeon to repeatedly wash and reposition the scope. The newer irrigating scopes that wash the distal lens have dispensed with constant withdrawing and reinsertion of the scope into the nasal cavity. However, a second hand is still useful because many tumors involving the skull base are vascular, and excision requires continuous suction to further debride the tumor. This problem inherently improves with the robotic approach as the operating surgeon now has an extra hand to perform suction simultaneously, making the operating field less bloody.
In this study we used the median approach to the sella. However, for certain areas of the skull base where wide exposure of the lateral sphenoid wall is needed or the full length of the optic nerve must be decompressed, the transethmoidal approach was used. This approach was just as user-friendly with the robot.
AESOP (Fig. 1) is a compact, inexpensive system approved by the FDA in 1996. Some of the disadvantages noted with the master-slave design robots (Intuitive Surgical's da Vinci® Surgical System), such as high cost, excessive bulk or dimensions, and lack of user-friendliness, are not issues with AESOP. Because this procedure is in the developmental stage, the equipment cost can not be reimbursed. One other shortcoming is the lack of a built-in mechanism to stop the endoscope if the voice is not recognized. One of the safety features is a manual shut-off, which is important should the robot fail to respond to the voice command “Stop.” Pressure sensors would help to provide automatic shut-off if pressure on the robotic arm or drill is too great. In this application the lack of tactile feedback recognition and proprioception was not an issue. Nonetheless, tactile proprioception for the endoscope itself would be useful.
The use of a navigational system is becoming state of the art for treatment of skull base tumors. These systems help locate the optimal site for safely entering the sinus, indicating proximity to critical structures. We used the InstaTrak® system in our study. The InstaTrak® is based on electromagnetic tracking while allowing real-time endoscopic visualization. The robotic scope holder is clasped with a magnetic holder. Hence, there was occasional field distortion on the imaging systems. This issue requires further evaluation to determine the accuracy of this system in the presence of the robot or during the use of the optical image guidance systems, which are often used by neurosurgeons.
Robotic surgery has been described for otolaryngologic uses. However, most of these studies have been on cadavers and for uses other than skull base and sinus surgery. A recent study was performed to determine the accuracy of combining the navigational, robotics, and endoscopic features for skull base surgery.15 That team had earlier used an Evolution 1, Universal Robotic System (Schwerin, Germany), which was cumbersome, time consuming, and not on the market. Our system used two separate standard FDA-approved pieces of equipment to combine two existing and well-studied technologies (i.e., AESOP and InstaTrak®).
The other advantage of this system is that no stabilization of the head is required unlike the systems that require the robot to be oriented to the head frame and used to navigate a probe or biopsy instrument to the target. Kwoh and associates combined the two technologies and measured the accuracy of computer tomography- (CT-) guided stereotactic brain surgery using a PUMA 2000 robot.29 Although the relative accuracy of some of these systems has been 0.05 mm, a minute error in the skull base with major vessels attached to the tumor could be detrimental. Hence, true robotic surgery as described for prostatectomy is infeasible in the skull base at present.30
Robots offer multiple advantages in the surgical field. They increase the accuracy and dexterity of the surgeon and reduce the tremor of the human hand. They can amplify or reduce movements or forces applied by the surgeon. We wanted to validate the use of the robotic assist AESOP as a scope holder in skull base surgery with respect to the key requirements, namely, safety, accuracy, sterility, integration in the operating room, and measurable benefits. Robotic-assisted scope holding eliminated tremor of the human hand and fatigue, especially during prolonged skull base resections. Although precision, accuracy, and the potential for telesurgery are some of the prime advantages of robotic surgery, the simple use of having a robotic scope holder has definite advantages in the combined otolaryngologic/neurosurgical approach to the skull base. This is the first report of using a simple, FDA-approved robotic scope holder for the endoscopic approach to the pituitary. We expect further studies to show an actual decrease in operating time.
CONCLUSION
A video-assisted voice-controlled robot is safe and efficient for an endoscopic approach to the pituitary. It can serve as a good training tool in a teaching institution. Further studies are needed to establish its effectiveness in clinical settings in terms of operative time, inadvertent neurovascular injury, and postoperative complications.
PAPER PRESENTED
Presented at the 16th Annual NASBS Meeting, Toronto, Ontario, Canada, April 2005.
REFERENCES
- Satava R M. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech. 2002;12:6–16. doi: 10.1097/00129689-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Satava R M. Robotic surgery: from past to future—a personal journey. Surg Clin North Am. 2003;83:1491–1500. doi: 10.1016/S0039-6109(03)00168-3. xii. [DOI] [PubMed] [Google Scholar]
- Kappert U, Cichon R, Schneider J, et al. Closed-chest coronary artery surgery on the beating heart with the use of a robotic system. J Thorac Cardiovasc Surg. 2000;120:809–811. doi: 10.1067/mtc.2000.109543. [DOI] [PubMed] [Google Scholar]
- Hollands C M, Dixey L N. Applications of robotic surgery in pediatric patients. Surg Laparosc Endosc Percutan Tech. 2002;12:71–76. doi: 10.1097/00129689-200202000-00012. [DOI] [PubMed] [Google Scholar]
- Haus B M, Kambham N, Le D, Gourin C, Terris D J. Surgical robotic applications in otolaryngology. Laryngoscope. 2003;113:1139–1144. doi: 10.1097/00005537-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Gourin C G, Terris D J. Surgical robotics in otolaryngology: expanding the technology envelope. Curr Opin Otolaryngol Head Neck Surg. 2004;12:204–208. doi: 10.1097/01.moo.0000122309.13359.af. [DOI] [PubMed] [Google Scholar]
- Nathan C O, Dixie L, Stucker F. Voice controlled robotic assist for endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2002;127:57. [Google Scholar]
- Gandhi C D, Post K D. Historical movements in transsphenoidal surgery. Neurosurg Focus. 2001;11:E7. doi: 10.3171/foc.2001.11.4.8. [DOI] [PubMed] [Google Scholar]
- Collins W F. Hypophysectomy: historical and personal perspective. Clin Neurosurg. 1974;21:68–78. doi: 10.1093/neurosurgery/21.cn_suppl_1.68. [DOI] [PubMed] [Google Scholar]
- Hardy J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582–594. doi: 10.3171/jns.1971.34.4.0582. [DOI] [PubMed] [Google Scholar]
- Jho H D, Ha H G. Endoscopic endonasal skull base surgery: Part 1—The midline anterior fossa skull base. Minim Invasive Neurosurg. 2004;47:1–8. doi: 10.1055/s-2003-812538. [DOI] [PubMed] [Google Scholar]
- Jho H D, Ha H G. Endoscopic endonasal skull base surgery: Part 2—The cavernous sinus. Minim Invasive Neurosurg. 2004;47:9–15. doi: 10.1055/s-2004-818346. [DOI] [PubMed] [Google Scholar]
- Jho H D, Ha H G. Endoscopic endonasal skull base surgery: Part 3—The clivus and posterior fossa. Minim Invasive Neurosurg. 2004;47:16–23. doi: 10.1055/s-2004-818347. [DOI] [PubMed] [Google Scholar]
- Koren I, Hadar T, Rappaport Z H, Yaniv E. Endoscopic transnasal transsphenoidal microsurgery versus the sublabial approach for the treatment of pituitary tumors: endonasal complications. Laryngoscope. 1999;109:1838–1840. doi: 10.1097/00005537-199911000-00022. [DOI] [PubMed] [Google Scholar]
- Moses R L, Keane W M, Simeone Frederick, et al. Endoscopic transseptal transsphenoidal hypophysectomy with three-dimensional intraoperative localization technology. Laryngoscope. 1999;109:509–512. doi: 10.1097/00005537-199903000-00031. [DOI] [PubMed] [Google Scholar]
- Bumm K, Wurm J, Rachinger J, et al. An automated robotic approach with redundant navigation for minimal invasive extended transsphenoidal skull base surgery. Minim Invasive Neurosurg. 2005;48:159–164. doi: 10.1055/s-2005-870903. [DOI] [PubMed] [Google Scholar]
- Tucker H M, Hahn J F. Transnasal transseptal sphenoidal approach to hypophysectomy. Laryngoscope. 1982;92:55–57. doi: 10.1288/00005537-198201000-00011. [DOI] [PubMed] [Google Scholar]
- Wilson W R, Khan A, Laws E R., Jr Transseptal approaches for pituitary surgery. Laryngoscope. 1990;100:817–819. doi: 10.1288/00005537-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Carrau R L, Jho H D, Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914–918. doi: 10.1097/00005537-199607000-00025. [DOI] [PubMed] [Google Scholar]
- Castelnuovo P, Pistochini A, Locatelli D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique.”. Rhinology. 2006;44:2–7. [PubMed] [Google Scholar]
- Kennedy D W. Functional endoscopic sinus surgery. Technique. Arch Otolaryngol. 1985;111:643–649. doi: 10.1001/archotol.1985.00800120037003. [DOI] [PubMed] [Google Scholar]
- Sethi D S, Pillay P K. In: Friedman M ed, editor. Endoscopic surgery for pituitary tumors. Operative Techniques in Otolaryngology—Head and Neck Surgery. 1996. pp. 264–268.
- Arnholt J L. Capt, Mair, Eric. A “third hand” for endoscopic skull base surgery. Laryngoscope. 2002;112:2244–2249. doi: 10.1097/00005537-200212000-00021. [DOI] [PubMed] [Google Scholar]
- Laws E R., Jr Vascular complications of transsphenoidal surgery. Pituitary. 1999;2:163–170. doi: 10.1023/a:1009951917649. [DOI] [PubMed] [Google Scholar]
- Ciric I, Ragin A, Baumgartner C, et al. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Jho H D, Alfieri A. Endoscopic endonasal pituitary surgery: evolution of surgical technique and equipment in 150 operations. Minim Invasive Neurosurg. 2001;44:1–12. doi: 10.1055/s-2001-13590. [DOI] [PubMed] [Google Scholar]
- Jho H D. Endoscopic endonasal approach to the optic. Minim Invasive Neurosurg. 2001;44:190–193. doi: 10.1055/s-2001-19927. [DOI] [PubMed] [Google Scholar]
- Cappabianca P, Cavallo L M, Colao A, et al. Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedure. Minim Invasive Neurosurg. 2002;45:193–200. doi: 10.1055/s-2002-36197. [DOI] [PubMed] [Google Scholar]
- Kwoh Y S, Hou J, Jonckheere E A, Hayati S. A robot with improved absolute positioning accuracy for CT-guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35:153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- Davies B L, Hibberd R D, Coptcoat M J, et al. A surgeon robot prostatectomy—a laboratory evaluation. J Med Eng Technol. 1989;13:273–277. doi: 10.3109/03091908909016201. [DOI] [PubMed] [Google Scholar]