Abstract
The aim of this study was to investigate the properties of di-rhamnolipid [α-L-rhamnopyranosyl-(1–2)-α-L-rhamnopyranosyl-3-hydroxydecanoyl-3-hydroxydecanoic acid, also referred to as di-rhamnolipid BAC-3] relating to the process of cutaneous wound healing. Di-rhamnolipid was prepared in a eucerin ointment and applied topically on full-thickness burn wounds in normal Sprague–Dawley rats covering 5% of the total body surface area. The rate of wound closure was measured over the period of 45 days. The collagen content was evaluated microscopically, by performing densitometric analysis on Verhoeff’s stained histopathological slides of wound biopsies taken at the end of 45th day of di-rhamnolipid treatment. Di-rhamnolipid toxicity was assessed with the subcutaneous multi-dose study in Swiss–Webster mice. The treatment of full-thickness-burn wounds with topical 0.1% di-rhamnolipid accelerated the closure of wounds on day 21 of the treatment by 32% compared to the control ( p < 0.05). On day 35, the wounds closed in all animals-treated with 0.1% di-rhamnolipid ointment while some rats in the control group had open wounds on days 35 and even 45. Histologic comparisons have shown that di-rhamnolipid significantly decreased collagen content in burn wounds (47.5%, p < 0.05) as compared to the vehicle-treated (control) wounds. Di-rhamnolipid was well-tolerated. The results of this study raise the possibility of potential efficacy of di-rhamnolipid in accelerating normal wound healing and perhaps in overcoming defects associated with healing failure in chronic wounds.
Keywords: Di-rhamnolipid, Subcutaneous toxicity, Full-thickness burn wounds, Rate of wound closure, Fibrosis, Animal model
1. Introduction
The wound healing process involves a highly coordinated cascade of cellular responses encompassing the interaction of many cell types over long periods of time. The early phase of normal cutaneous wound healing is characterized by the influx of inflammatory cells from the circulation to the site of injury. Monocytes which become activated macrophages at the injured site, play multiple roles in wound healing, including release of proteases for wound debridement, phagocytosis of debris, and secretion of various cytokines and growth factors which, in turn, regulate the activity and interactions of other cell types involved in tissue repair [1]. Later on, these growth factors trigger activation of fibroblasts and keratinocytes. The inflammatory phase of the wound healing is followed by the proliferative phase. During that phase, vascular integrity is restored, the soft tissue defect is filled with new connective tissue produced by fibroblasts, and the wound surface is covered with the new epithelium. These processes are interdependent, i.e. collagen synthesis and angeogenesis occur simultaneously, thus forming the granulation tissue which consists of capillary loops; fibroblasts, inflammatory cells and matrix proteins [2]. Fibroblasts migrate into the wound bed from the surrounding tissues in response to cytokines and growth factors produced by the platelets, activated neutrophils and activated macrophages. A provisional ‘‘support’’ matrix is replaced by a matrix of collagen, glycosaminoglycans (GAGs), proteoglycans (PGs) and elastin, with collagen becoming the predominant protein. The final step of the proliferative phase is the epithelization. It involves migration, proliferation and differentiation of epithelial cells from the wound edges to resurface the defect. In open full-thickness burn wounds, epithelization is delayed until a bed of granulation tissue is established to allow migration of epithelial cells [1].
In general, topical growth factors have not been effective in accelerating healing of full-thickness burn wounds. For example, neither platelet derived growth factor (PDGF) nor the epidermal growth factor (EGF) stimulated closure [3]. In another study, wounds treated with EGF appeared to close rapidly (due to epithelization), but reopened after the treatment was discontinued [4].
The aim of this study was to investigate the properties of di-rhamnolipid relating to the process of cutaneous wound healing. Di-rhamnolipid, [α-L-rhamnopyranosyl-(1–2)-αL-rhamnopyranosyl-3-hydroxydecanoyl-3-hydroxydecanoic acid], also referred to as di-rhamnolipid BAC-3] has been isolated from Pseudomonas aeruginosa and characterized (Fig. 1) [5]. Previously, in vitro studies have shown that at certain BAC-3 concentrations, proliferation of fibroblasts is inhibited and the proliferation of keratinocytes in the presence of serum is stimulated [6,7]. It has been determined that di-rhamnolipid BAC-3, in the absence of fetal calf serum and at a concentration of 0.1 mg/ml, inhibits DNA synthesis in A 431 human epidermal cells in vitro [8]. Additional tests on di-rhamnolipid, at a concentration of 10 μmol/kg, showed T-cell immunosuppressive activity after stimulation with Concavalin A [8]. In vivo data relates specifically to the treatment of autoimmune diseases and includes the effects of di-rhamnolipid on cellular immunosuppression, oxazolone-induced delayed type hypersensitivity, immunorestoration, etc. Lastly, clinical data on the treatment of psoriasis and lichen ruber planus have confirmed long lasting ameliorative effects of BAC-3 when compared to conventional therapy using corticosteroids [5].
Fig. 1.
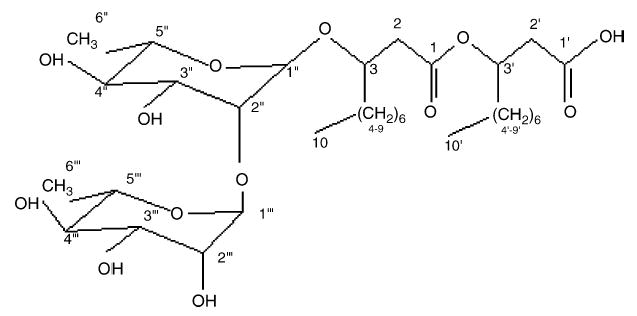
Structure of di-rhamnolipid BAC-3: α-L-rhamnopyranosyl-(1–2)-α-L-rhamnopyranosyl-3-hydroxydecanoyl-3-hydroxydecanoic acid.
At higher concentrations (500 μg/ml and above in the culture medium), BAC-3 is toxic and causes cell necrosis due to it’s hemolytic effect, i.e. ‘‘detergent effect’’. The hemolytic ability of di-rhamnolipid appears to be associated with the insertion of two apolar fatty acid residues into the phospholipid bilayer of the cell membrane [9]. At lower concentrations (below 200 μg/ml) BAC-3 induces programmed cell death in fibroblasts and keratinocytes. However, in the presence of fetal calf serum and at concentration of 50 μg/ml di-rhamnolipid was shown to stimulate the formation of differentiated keratinocyte colonies 34% above the untreated control ( p = 0.04). The same di-rhamnolipid concentration inhibited fibroblast proliferation 23% below controls ( p = 0.04) [6]. These results indicated that it is possible to have di-rhamnolipid concentrations at which proliferation of fibroblasts is inhibited and proliferation of keratinocytes is stimulated. Additionally, the effect of BAC-3 on the proliferation of keratinocytes in the presence of serum served as a predictor of the possible effect of di-rhamnolipid in vivo, on the healing of wounds, since in full-thickness wounds blood vessels are disrupted and epidermal cells are exposed to serum components, as opposed to intact skin [1]. Therefore, we tested di-rhamnolipid topically on the healing of burn wounds in normal Sprague–Dawley rats covering 5% of the whole skin area. We measured the rate of wound closure and the amount of fibrosis present at the end of 45th day of treatment. In parallel, we also present a subcutaneous multi-dose toxicity study of di-rhamnolipid BAC-3 on Swiss–Webster mice.
2. Materials and methods
2.1. Subcutaneous multi-dose toxicity study with di-rhamnolipid BAC-3
The toxicity of a 98.7% pure BAC-3 (TajCo Inc., VA, USA), administered subcutaneously once daily for 7 days followed by a 7-day recovery period, was determined in young female Swiss–Webster mice. The objective was to assess the repeat-dose toxicity by relevant route with regard to a possible use of di-rhamnolipid as a topical dermatologic agent. Fifteen 9-week-old female Swiss–Webster mice were used in the study. The mice were healthy and had no prior exposure to drugs. Mice were quarantined for 3 days prior to the start of the study and housed three per cage. Food and water were provided ad libidum. Mice were handled following principles described in the 1996 National Research Council ‘‘Guide for the Care and Use of Laboratory Animals’’.
A 10 mg/ml stock solution of BAC-3 was prepared for delivery by dissolving in Dulbecco’s sodium phosphate buffered saline (DPBS, Gibco, Invitrogen, USA). The solution was sterilized by filtration through a 0.2 μm filter. One day before the start of the study, the stock solution was diluted into sterile vials-for-injection at concentrations 6.0, 0.10 and 0.001 mg/ml. The concentration range of the tested di-rhamnolipid was selected based on our previous determination of the intraperitoneal dose, which was 135 mg/kg in Swiss–Webster mice (unpublished data). The vehicle control group was injected with sterile DPBS.
2.1.1. Study design
The animals were given a subcutaneous injection once daily for 7 days at different sites on the back (clipped of hair). The injections were started at the lower back, and moved bilaterally from tail to head (Fig. 2). All animals were weighed and observed throughout the study for the clinical signs of toxicity, including the skin injection sites.
Fig. 2.
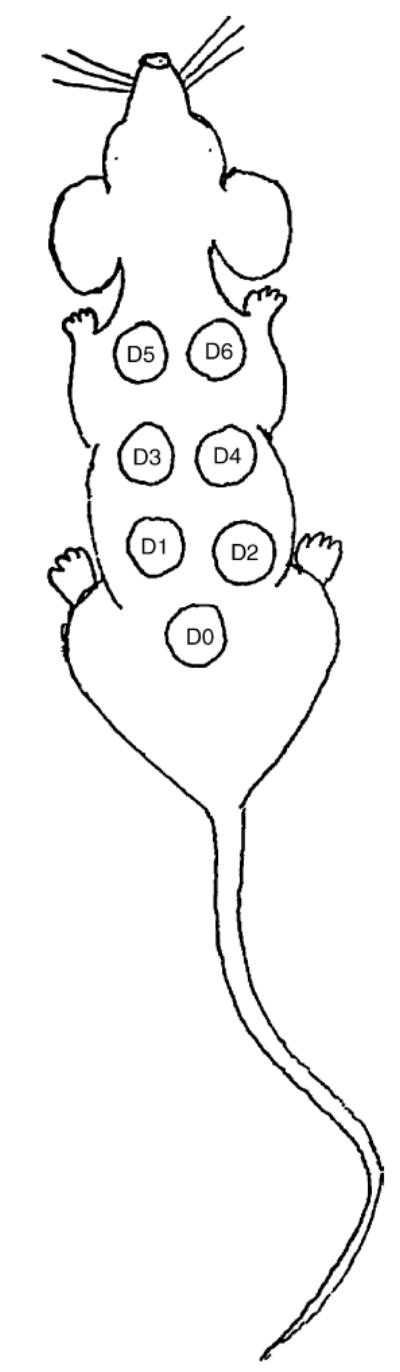
Mouse di-rhamnolipid skin injection sites.
Five groups (three mice per group) received BAC-3 at doses of 0.012, 1.20, 60 or 120 mg/(kg day) (Groups 2–5). A control group received vehicle (Group 1). Treatment of animals in the 60 mg/kg group started 1 day after the others, and was terminated as in all other groups, after 13 study days (Table 1). All animals were weighed and observed daily for clinical signs of toxicity. On study day 14, the animals were euthanized by carbon dioxide inhalation and cervical dislocation. Autopsy was performed and the following organs were weighed and preserved in 10% formalin: heart, lungs, liver, kidneys, thymus, spleen and skin lesions. Organs (kidney, spleen, liver and skin) with obvious lesions (adhesions, enlargements or scabbing on skin sites) were sent for histopathologic analysis. Thymus, spleen, liver and selected skin injection sites were routinely sent for histopathology. The tissues were fixed in formaldehyde, embedded in paraffin, sectioned at 5 μm and rountinely stained with hematoxylin and eosin according to the procedure described in the ‘‘Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology’’ [10].
Table 1.
Subcutaneous di-rhamnolipid toxicity—study design
| Group number | Animal labels | Dose (mg/kg) | Concentration (mg/kg) | Dose volume (mg/kg) | Dose regimen | Study period |
|---|---|---|---|---|---|---|
| 1 | 98-0030 | Once daily for 7 days | 14 days | |||
| 98-0031 | 0.00 | None (vehicle) | 12.0 | |||
| 98-0032 | ||||||
| 2 | 98-0033 | Once daily for 7 days | 14 days | |||
| 98-0034 | 0.012 | 0.001 | 12.0 | |||
| 98-0035 | ||||||
| 3 | 98-0036 | Once daily for 7 days | 14 days | |||
| 98-0037 | 1.20 | 0.10 | 12.0 | |||
| 98-0038 | ||||||
| 5 | 98-0039 | Once daily for 7 days | 13 days | |||
| 98-0040 | 60.0 | 6.0 | 12.0 | |||
| 98-0041 | ||||||
| 4 | 98-0042 | Once daily for 7 days | 14 days | |||
| 98-0043 | 120.0 | 10.0 | 12.0 | |||
| 98-0044 |
2.2. Wound healing study with di-rhamnolipid BAC-3
The effect of di-rhamnolipid BAC-3, administered topically twice daily on full-thickness burn wounds covering 5% of the whole skin area over the period of 45 days was determined in female Sprague–Dawley rats. The objective was to assess BAC-3 assisted wound healing in vivo.
2.2.1. Study design
Wounding of animals was performed according to the ‘‘Protocol of Animal Use and Care at UC Davis’’ confirming to standards set by U.S. National Institutes of Health Bethesda, MD, USA. Experimental burn wounds should ideally be identical in depth and extent. A standard method requires exactly defining the size and location of the wounds, the temperature gradient, the duration of exposure and the method of applying the burns. The standard burns were performed by techniques and device described by Walker [11]. The burning device was a model ordered from the U.S. Army Institute of Surgical Research, Fort Sam Houston, TX USA. The device was a 29 cm long plastic cradle accommodating the rat, with an aperture of 7 cm × 3 cm that enabled exposure of 5% total skin surface equivalent to the heat. The correct wound dimension was obtained by measuring the average whole skin area of all animals using Mech’s formula: A = kW2/3, in which A is the surface area in cm2, W the body weight in grams and k = 10. Based on an average animal weight (257 g) and according to Mech’s formula, the average whole skin area is A = 404 cm2 and 5% of total skin area is then 20.2 cm2, which corresponds to a wound dimension of approximately 7 cm length × 3 cm width.
Each animal was anesthetized with 50 mg/kg body weight sodium pentobarbital administered i.p. (Thiopental-Nycomed, Nycomed Arzneimittel GmbH, Munich). The hair over the dorsum was clipped with animal clipper (Golden A5, Oster). Each animal was placed supine in the burning device. A flexible sponge (22 cm × 7 cm) was placed on top of the animal with metal holders (7 cm) and secured snugly with plastic straps. The pressure served to immobilize the animal and did not affect its ability to breathe. The entire device was picked up by metal holders and the exposed skin area was immersed in boiling water (water bath Köttermann, Uetze-Hänigsen, Germany). Ten seconds of the exposure produced a full-thickness burn. On removal from water, the dorsum was quickly dried by rolling on a towel and the animal was released and individually caged. This procedure produces a uniform burn with sharp margins. In this animal model burning 5% of total skin surface area causes no major systemic problems, as confirmed by undisturbed weight gain of the animals following the wounding.
Di-rhamnolipid ointment formulation was prepared in a standard manner. White petrolatum 71.5% (w/w) (D.E.A. Hamburg, Germany), lanolin 23.8% (w/w) (I.O.I. Hamburg, Germany) and cholesterol 4.7% (w/w) (Solvay Pharma GmbH, Klosterneuburg, Austria) were mixed separately to make the ointment base, i.e. eucerin. Chemical analysis of ointment base indicated Water Number 320. Ointment base without di-rhamnolipid served as a placebo. In a mortar dish, 1 g of BAC-3 (98.7% pure powder) and 10 g of paraffin oil (Merkur Vaseline GmbH&Co., Hamburg, Germany) were mixed with pestle until even suspension was obtained. Following, 89 g of basic ointment was added and mixed clockwise firmly. The ingredients were mixed continuously until the components were evenly blended (approximately after 5 min of hard mixing on the bench). The rest of the ointment base was added in four 100 g portions, with thorough mixing between each addition. In such a manner, 500 g of 1.0 and 0.1% (w/w)—di-rhamnolipid with or without the antiseptic chlorhexidine hydrochloride (Pliva, Zagreb, Croatia) was prepared in ointment base (eucerin) and kept at +4 °C.
Rats were divided into six groups of three animals. Immediately after wounding, the burns were treated topically with 1.0% (w/w) (Groups 1-A and 2-A) and 0.1% (w/w) (Groups 1-B and 2-B) BAC-3 ointments (applied to wound as thin film). The rate of wound closure was assessed by measuring distance across wound edges at days 14, 21, 28, 35 and 45. Every third day during the course of the experiment, animal body weight and behavior was recorded. Photographs were taken periodically (Table 2).
Table 2.
Burn wounds—study design
| Group number | Animal labels | Di-rhamnolipid preparation |
|---|---|---|
| 1-A | 1, 2, 5 | 1% di-rhamnolipid in eucerin |
| 1-B | 16, 19, 20 | 0.1% di-rhamnolipid in eucerin |
| 1-D | 21, 24, 25 | eucerin |
| 2-A | 8, 10, 33 | 1% di-rhamnolipid and 1% chlorhexidine hydrochloride in eucerin |
| 2-B | 4, 11, 14 | 0.1% di-rhamnolipid and 1% chlorhexidine hydrochloride in eucerin |
| 2-D | 26, 27, 28 | 1% chlorhexidine hydrochloride in eucerin |
On the post burn day 45, the animals were sacrificed by an overdose of sodium pentobarbital (300 mg/kg) and skin specimens were taken for histopathology evaluation. Specimens included the wound bed as well as the healthy skin of the wound margins. The tissues were fixed in formaldehyde [12], embedded in paraffin, sectioned at 5 μm and rountinely stained with two stains: (1) Hematoxylin and eosin, according to the procedure described in the ‘‘Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology’’ [9] for routine microscopic evaluation and (2) Verhoeff’s stain, according to the procedure described in the ‘‘Theory and Practice of Histology’’ [13] for a better visualization of the crust, i.e. of the regenerating dermis and the alpha smooth muscle actin to identify the myofibroblasts. During the microscopic and macroscopic observation, four wound healing parameters were evaluated: crust formation, re-epithelization, formation of the granulation tissue and inflammation.
Measurements of total wound area were taken for all 18 rats sacrificed on the 45th day of treatment. Three measurements of collagen area and density of the burned skin were taken from all 18 histopathological Verhoeff’s stained slides for rats sacrificed on the 45th day of treatment. Wound area was measured using a Leitz Diplan microscope with normal skin determining the wound margins (Leitz GmbH & Co., Oberkochen,Germany) and Issa Version 3.1 software—an integrated system for archiving patient data, examination data, including pictures (Copyright 1995–2000 Vamstec, Vamstec Analysis and Measurement System Technologies, Zagreb, Croatia). Collagen density was measured using densitometric analysis and special program Sform Version 1.0 (Vamstec Analysis and Measurement System Technologies, Zagreb, Croatia). Three measurements of collagen area and density of the normal untreated skin were also taken from each histopathological slide. Total collagen content was obtained by multiplying collagen area with the corresponding collagen density (OD, mm2). Percent difference in collagen values of the wounded skin to the normal skin was calculated by substracting the control readings (normal untreated skin) from the average of three wound readings (burned skin), and then dividing by control reading. Average percent difference in collagen content of BAC-3-treated burn wounds from collagen content of normal untreated skin was calculated per each group. Measurements of collagen and density were carried out by an independent blinded observer.
2.3. Data analysis
Animals were weighed on a Sartorius balance model LC6201-00MS (Sartorius AG, Goettingen, Germany). The balance was connected directly to a computer via standard software. Animal body weight data was recorded and data files were processed for means, standard deviations and graphics using Microsoft Excell Version 7.0 (Microsoft Corporation, Redmond, WA, USA).
2.4. Statistical analysis
Statistical significance was evaluated by one-way analysis of variance (ANOVA) and performed using the Microsoft Excell Version 7.0 (Microsoft Corporation).
3. Results
3.1. Subcutaneous multi-dose toxicity of di-rhamnolipid BAC-3
3.1.1. Animal body weights
There was no significant difference in the body weights between the groups at any time during the study.
3.1.2. Clinical observations
No mortality was observed in the study. Abnormal clinical observations were limited to skin injection site lesions, which were seen as early as day 1 in the high dose group (120 mg/(kg day), Group 4). The lesions were red colored, moist spots approximately the size of the initial intradermal injection (1 cm in length and 0.5 cm in width). In this group, the lesions were observed in four to six injection sites and were still apparent (but appeared to be healing) at the end of experiment on day 14. Due to the occurrence of the lesions on day 1 in the 120 mg/(kg day) dose group (Group 4), an intermediate dose group was added to the study (60 mg/(kg day), Group 5). Skin lesions were observed in Group 5 animals, but appeared later (day 5) and affected no more than one to two injection sites per animal until the day of termination. Representative skin lesions were photographed and biopsied for histopathology at the end of the study. It was proposed that the lesions might have been caused by the toxic effect of BAC-3 due to its ‘‘detergent effect’’ at the site of skin injection. While these were not tested the lesions had a red, hemolytic appearance at 24 h post administration, which is suggestive of di-rhamnolipid causing the cell lysis at high concentrations.
3.1.3. Necropsy findings
In addition to the skin lesions, the only abnormal gross findings were seen in one mouse from the high dose group (Animal 98–0040, Group 4, 120 mg/(kg day)), which had a moderate enlargement of the spleen and liver. The liver weighed 1.79 g as compared to the mean 1.17 g in the control group. The spleen weighed 0.17 g as compared to the mean 0.08 g in the control group. Adhesions between the liver, spleen, kidneys and peritoneum were also noted in this animal. Mean organ weights are presented in Table 3. There was no significant difference in the organ weights to body weight ratios between the controls and any of the treatment groups.
Table 3.
Body and organ weights of mice-treated with di-rhamnolipid at the end of experiment (day 14)
| Mouse number | Body weight (g) | Heart | Lungs | Liver | Kidney | Thymus | Spleen | Skin site collected |
|---|---|---|---|---|---|---|---|---|
| Group 1: Vehicle | ||||||||
| 98-0030 | 21.9 | 0.1504 | 0.1512 | 1.1281 | 0.3507 | 0.0707 | 0.0880 | No |
| 98-0031 | 20.6 | 0.1365 | 0.1574 | 1.1912 | 0.2920 | 0.0718 | 0.0621 | No |
| 98-0032 | 21.8 | 0.1547 | 0.1862 | 1.1875 | 0.3477 | 0.0541 | 0.1002 | No |
| Mean | 21.4 | 0.1472 | 0.1649 | 1.1689 | 0.3301 | 0.0655 | 0.0834 | |
| S.D. | 0.7 | 0.0095 | 0.0187 | 0.0354 | 0.0331 | 0.0099 | 0.0195 | |
| Group 2: 0.012 mg/(kg day) | ||||||||
| 98-0033 | 20.3 | 0.1279 | 0.1507 | 1.2729 | 0.3045 | 0.0743 | 0.0782 | No |
| 98-0034 | 22.0 | 0.1380 | 0.1762 | 1.2064 | 0.3573 | 0.0627 | 0.0760 | No |
| 98-0035 | 21.2 | 0.1442 | 0.1662 | 1.1755 | 0.3583 | 0.0892 | 0.0992 | No |
| Mean | 21.2 | 0.1367 | 0.1644 | 1.2183 | 0.3400 | 0.0754 | 0.0845 | |
| S.D. | 0.9 | 0.0082 | 0.0128 | 0.0498 | 0.0308 | 0.0133 | 0.0128 | |
| Group 3: 1.2 mg/(kg day) | ||||||||
| 98-0036 | 24.5 | 0.1449 | 0.2062 | 1.4937 | 0.3988 | 0.0605 | 0.1027 | No |
| 98-0037 | 20.9 | 0.1204 | 0.1792 | 1.0268 | 0.2862 | 0.0640 | 0.0889 | No |
| 98-0038 | 22.9 | 0.1443 | 0.1961 | 1.3002 | 0.3651 | 0.0730 | 0.0946 | No |
| Mean | 22.8 | 0.1365 | 0.1938 | 1.2736 | 0.3500 | 0.0658 | 0.0954 | |
| S.D. | 1.8 | 0.0140 | 0.0136 | 0.2346 | 0.0578 | 0.0064 | 0.0069 | |
| Group 4: 120 mg/(kg day) | ||||||||
| 98-0039 | 21.4 | 0.1910 | 0.1849 | 1.4325 | 0.4327 | 0.0604 | 0.0786 | 1, 2, 4, 6 |
| 98-0040 | 25.6 | 0.1529 | 0.2036 | 1.7887 | 0.4467 | 0.0567 | 0.1704 | 0, 1, 3, 5 |
| 98-0041 | 25.0 | 0.1182 | 0.1522 | 1.3370 | 0.3949 | 0.0455 | 0.1402 | 0, 3, 4, 6 |
| Mean | 24.0 | 0.1540 | 0.1802 | 1.5194 | 0.4248 | 0.0542 | 0.1297 | |
| S.D. | 2.3 | 0.0364 | 0.0260 | 0.2381 | 0.0268 | 0.0078 | 0.0468 | |
| Group 5: 60 mg/(kg day) | ||||||||
| 98-0042 | 19.8 | 0.1275 | 0.1377 | 1.0620 | 0.2581 | 0.0930 | 0.0701 | 6 |
| 98-0043 | 23.3 | 0.1503 | 0.2056 | 1.3253 | 0.3559 | 0.0703 | 0.0610 | 1, 3 |
| 98-0044 | 21.9 | 0.1327 | 0.2008 | 1.2690 | 0.3515 | 0.0616 | 0.0856 | 1, 5 |
| Mean | 21.7 | 0.1368 | 0.1814 | 1.2188 | 0.3218 | 0.0750 | 0.0722 | |
| S.D. | 1.8 | 0.0119 | 0.379 | 0.1387 | 0.0552 | 0.0162 | 0.0124 | |
3.1.4. Histopathology
Tissues from all animals in treatment Group 4 (120 mg/kg) and from one animal in the vehicle control group (Group 1) were sent out for histology evaluation by a veterinary histopathologist. Skin lesions (except from 98-0030, vehicle treated), liver, spleen and thymus were evaluated. Animal 98-0041 (120 mg/kg di-rhamnolipid, Group 4) was terminated earlier, on day 7, so that histology of the skin lesions could be performed immediately, after the completion of the 7 days BAC-3 treatment. The rest of the animals were left to recover for additional 7 days after which they were sacrificed (day 14 since the start of experiment).
In summary, liver and thymus were all within a normal range of appearance, with the following exception. One mouse (98-0041) from the high dose group (Group 4, 120 mg/kg) which was euthanized on day 7 rather than day 14, showed a lower thymus weight and organ weight as a percentage. The spleen showed increased extramedullary hematopoiesis. A similar observation was made on the spleen of mouse 98-040; however, to a lesser extent. Extramedullary hematopoiesis was not seen in other animals killed at day 14. That significant observation might have been a result of an acute toxicity effect of BAC-3, which regressed when the treatment was terminated, because the di-rhamnolipid dose given subcutaneously was very high, close to determined lethal i.p. dose. Since, these findings were significantly present in only one mouse (out of three per group) it was also suggested that this animal might have been ill prior to entering the study. However, more animals should be evaluated in order to confirm that possibility.
The skin lesions displayed signs of a chronic active inflammation, either co-existing with ulceration or progressing as a sequel to ulceration. All skin sections had acanthosis and dermal fibrosis, while others had some surface exudates, hyperkeratosis and other changes including epidermal inclusion cysts. Overall, it appeared that the lesions were in an intermediate stage of healing, with residual changes, such as acanthosis, loss of adnexal structures and fibrosis of the dermis and or subcutis. We concluded that with time the skin might be expected to return to nearly normal architecture in most or all cases. However, no extension of recovery was performed in this study.
Thus, di-rhamnolipid BAC-3 was generally well-tolerated, if given subcutaneously once daily for 7 days to female Swiss–Webster mice in doses up to 120 mg/(kg day). Skin lesions developed at the injection sites in some animals in the 60 and 120 mg/kg dose groups.
3.2. Wound healing study with di-rhamnolipid BAC-3
3.2.1. Animal weights
Weight gain of di-rhamnolipid-treated animals was the same compared to the control animals treated with vehicle.
3.2.2. Histopathology
During wound healing, we observed that inflammation was pronounced in vehicle groups, with and without antiseptic chlorhexidine hydrochloride (Groups 1-D, 2-D). There were no significant differences in crust formation between all groups (treatment and control). Granulation tissue was significantly developed in wounds treated with 1% BAC-3 (Groups 1-A and 2-A). The rate of wound closure in the middle part of the burn wounds was faster in all rats treated with 0.1% (Groups 1-B and 2-B) (Fig. 4).
Fig. 4.
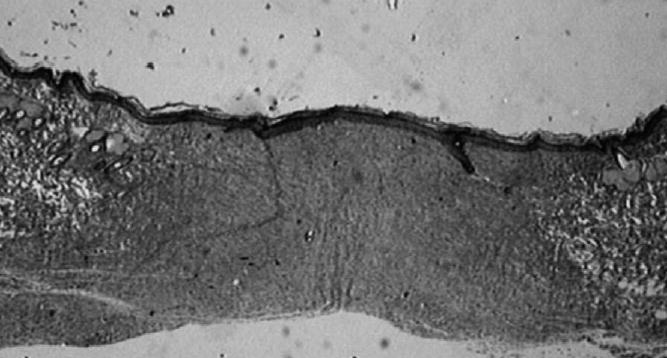
Verhoeff’s staining of wound area and its wound margin in rat treated for 45 days with 1% di-rhamnolipid in eucerin ointment (2× magnification). Evidence of developed fibrosis in wound area with maturing collagen fibers around wound margins. Photograph of histopathology slide created with Issa Version 3.1 software (Vamstec, Vamstec Analysis and Measurement System Technologies, Zagreb, Croatia).
Histologic comparisons have shown that on the 45th day of treatment all animals had uniform wound areas with differences reflected in the collagen content (indicator of fibrosis) present within the wound (Fig. 3). Animals receiving 0.1% topical BAC-3 twice daily showed a significant overall decrease (47.5%, p < 0.05) in the collagen content in the wound area as compared to the animals receiving vehicle. Similarly, treatment with 1% BAC-3 also decreased the collagen content although to a less extent (24.1%, p < 0.05). A small difference in reduction of collagen content was observed in favor of vehicle containing the antiseptic as compared to placebo without antiseptic (Fig. 5).
Fig. 3.
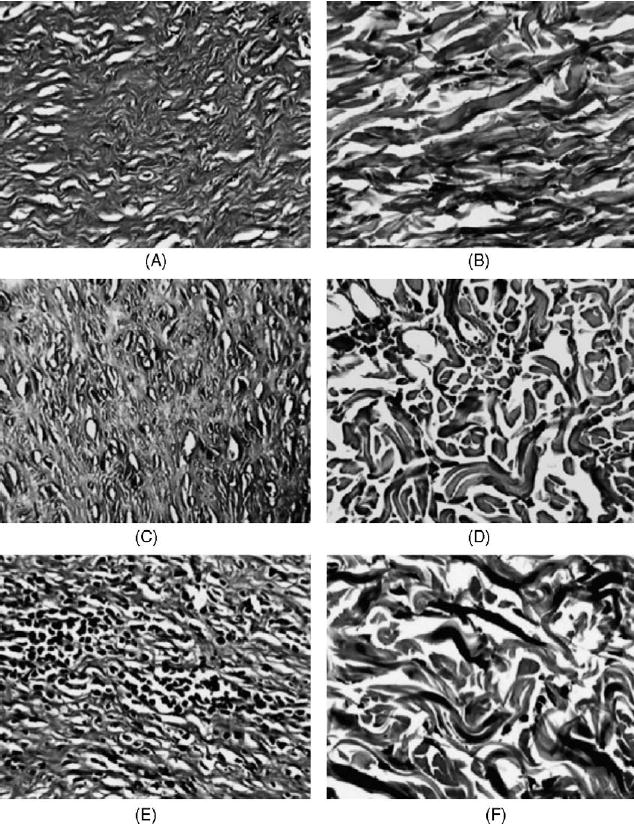
Visualization of the treated burn wounds (left) and the corresponding untreated normal skin of the same rat (right). Verhoeff’s stain of collagen (25×magnification). Left (top to bottom): Collagen fibers in the center of burn wound in rats treated with (A) 1% di-rhamnolipid, (C) 0.1% di-rhamnolipid and (E) vehicle ointments. Right (top to bottom): Collagen fibers from the normal untreated skin of the same animals. Note islets of inflammatory infiltrate in vehicle-treated burn wound (F). Photographs of histopathology slides created with Issa Version 3.1 software.
Fig. 5.
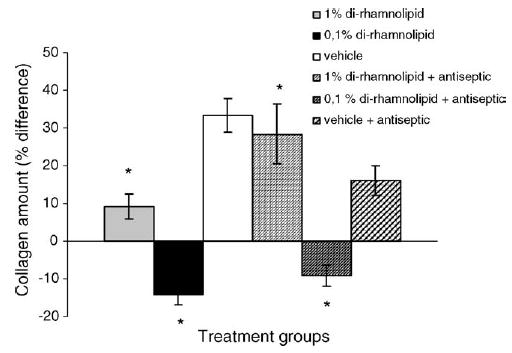
Percent difference in collagen amount (fibrosis index) of the treated burn wounds as compared to collagen amount of the normal skin. T-bars represent standard deviations. *p < 0.05.
3.2.3. Rate of closure
As shown in the Fig. 6, burn wound closure measured on days 14, 21 and 28 was significantly faster in rats administered 0.1% di-rhamnolipid (with or without standard antiseptic) than in rats receiving the vehicle alone ( p < 0.05 by ANOVA). We point out that burn wounds treated with vehicle alone did not achieve full-closure by day 45, while wounds treated with vehicle containing antiseptic did. In addition, even with the presence of the antiseptic, wound closure occurred first in the group treated with 0.1% di-rhamnolipid.
Fig. 6.
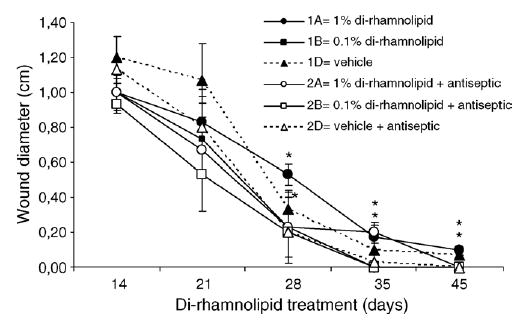
Effects of topical di-rhamnolipid and antiseptic chlorhexidine hydrochloride on the rate of burn wound closure. Mean values for each time point are shown as indicated by designated symbols; T-bars represent standard deviations. *p < 0.05.
Overall, treatment of full-thickness-burn wounds covering 5% of whole body skin area in normal Sprague–Dawley rats with topical applications (2 day−1) of 0.1% di-rhamnolipid in eucerin significantly accelerated wound closure. Di-rhamnolipid-treated wounds, on average, were closed 32% more than the control wounds on day 21 of treatment (*p < 0.01). Higher concentration of di-rhamnolipid (1%) had less effect on stimulating the rate of closure than lower most likely due to the toxic (‘‘detergent’’) effect of di-rhamnolipid at the site of treatment. On day 35 of treatment, the wounds closed in all rats treated with 0.1% di-rhamnolipid ointment while some rats in the control group had open wounds on days 35 and even 45.
4. Discussion
Full thickness wounds, by definition, involve total loss of the epidermal and dermal layers, extending at least to the subcutaneous tissue layer and possibly as deep as the fascia-muscle layer and the bone. There are several processes involved in full-thickness repair, but they are commonly conceptualized as three phases—inflammatory, proliferative, and remodeling. There is a considerable overlap among the phases, and the cells involved in one phase produce the chemical stimuli that serve to move the wound healing process into the next phase. Thus, normal repair is a complex and well-orchestrated series of events [1].
In the United States, approximately 1% of the population (2.5 million people) are seen by a physician each year for a burn related injuries [14]. As all burns should be monitored for at least 6 weeks for hypertrophic scars, it is inevitable that a proportion of burns would benefit from therapies that may reduce the incidence of red, thick, raised and often itchy or painful hypertrophic scars. An ideal therapy should not only promote the rapid healing process, but should act as an antiscarring therapy. Scar formation and over production of extracellular matrix by connective tissue characterize pathologic process called fibrosis, which occurs as a consequence of deranged healing in response to tissue damage caused among other by burning. The molecular process leading to fibrosis is not different from normal formation of connective tissue and extracellular matrix in normal organs. The context, the environment and the overproduction make the difference [15]. Exaggeration in inflammatory phase of wound healing process, in an open or infected burn, increases the concentration of growth factors known to produce increased fibroblast numbers and excess amounts of collagen and extracellular matrix [16]. The predominant proteins in fibrotic lesions are collagens types I and III. The letter protein is the major form in early lesions while type I predominates in ‘‘mature’’ or advanced lesions. The linear fibrillar properties of these proteins are responsible for the tough, fibrous nature of the lesions. A reduction in the amount of collagen deposited in fibrosis can be achieved by manipulating the various cytokines and hormones, which regulate collagen metabolism [15]. Current, in vitro models for studying the mechanism behind fibrosis include fibroblast cultures (from human and murine origin and from both healthy and fibrotic tissues). Histologic and biochemical measures of fibrosis include evaluating several histological parameters (collagen deposition, infiltrating cells, proliferation and resident cells) biochemical parameters (collagen content, collagen modifications, collagen cross-links and proteolytic enzyme activity) as well as gene expression profiling (real-time PCR quantification of gene expression for extracellular matrix proteins, chemokines/cytokines, and transcription factors) [17].
Although fibrosis severely affects the functioning of organ or tissue, no effective therapy is currently available to attenuate the process. Therefore, the recent widespread interest in novel pharmacological agents efficient in stimulating tissue repair, precisely targeting fibroproliferative disorders, is not surprising.
Rhamnolipids, as a class of compounds, are rhamnose-containing glycolipid biosurfactants produced by P. aeruginosa. Since there are no reported clinical uses of rhamnolipids in wound healing, it is not possible to compare the results of the present study with the efficacy of a similar, available wound-healing product. The relevant information exists only for biosurfactants in general, which have been used in cosmetic and healthcare industries for a wide variety of products including insect repellents, antacids, acne pads, anti-dendruff products, deodorants, nail care products, lipstick, eyeshadow, mascara, toothpaste, tooth paste, denture cleaners, antiprespirants, footcare products, antiseptics, shaving and depilatory products and moisturizers. Biosurfactants are considered to have some advantages over synthetic surfactants due to their low irritancy and even anti-irritating effects, and compatibility with skin [18].
The early phase of normal cutaneous wound healing is characterized by the influx of inflammatory cells from the circulation to the site of injury. In our study, microscopic observations of the histopathological slides, prepared after the completion of treatment, revealed that the wounds treated with 0.1% di-rhamnolipid ointment contained fewer inflammatory cells than the untreated control (data not shown). The inflammatory cells consist mainly of neutrophils and monocytes/macrophages, with smaller numbers of mast cells and lymphocytes. Majority of cells infiltrating the cutanous wounds has been shown to originate from blood, except for the tissue mast cells, which are abundant in the dermal and subdermal compartments of rodent skin and may migrate to the wound site from the local sources [19]. Since Pseudomonas di-rhamnolipids stimulate, both chemotaxis and chemokinesis of leukocytes [20], it could be proposed that tested di-rhamnolipid increased the early recruitment of inflammatory cells to the wound site without triggering a persistent, abnormal accumulation of neutrophils and mononuclear cells within injured tissue.
The inflammatory phase of the wound healing is followed by the proliferative phase. During that phase, vascular integrity is restored, the soft tissue defect is filled with new connective tissue produced by fibroblasts, and the wound surface is covered with new epithelium. These processes are interdependent, i.e. collagen synthesis and angeogenesis occur simultaneously, thus forming the granulation tissue which consists of capillary loops; fibroblasts, inflammatory cells and matrix proteins. Since fibroblasts are responsible for the synthesis of collagen and other connective tissue substances, it is clear that di-rhamnolipid, being capable of inhibiting the activity of fibroblasts, i.e. proliferation and collagen production, could affect the wound repair process [6].
Wound contraction begins almost concurrently with collagen synthesis. The rate of contraction depends on the degree of tissue laxity and shape of the wound. Loose tissue-contract more than tissues with poor laxity and square wounds tend to contract more than circular wounds. Contraction does not seem to depend on collagen synthesis [21]. Rat skin wound healing does not perfectly mimic human skin wound healing because the skin morphology is different (rats are described as loose-skinned animals) and ‘‘loose’’ skin allows wound contraction to play a significant role in closing rat skin wounds. Consequently, wound contraction, which is usually more rapid than epithelization, causes decrease in the overall healing time of rat wounds [22,23]. Humans have tight skin, and this difference makes comparison with loose-skinned animals difficult. Although there are inherent drawbacks in using rats for comparisons with human skin wound healing, there are also advantages in the use of rats as a research model, such as the availability of a broad knowledge based on rat wound healing gained from years for previous research.
Complying to the current recommendations of the Food and Drug Administration in human clinical trials, we used 3 months old female Sprague–Dawley rats with a faster rate of wound healing and higher wound contraction rate comparable to males. According to the Harlan Laboratory Animal Company reports 3 months old rats are be comparable to young adults [24]. We have used rats in our studies because they are large enough to provide a suitable area of skin for burn wounding. The results of this study have shown that di-rhamnolipid accelerated wound closure by at least 10 days as compared to the control. The inability of growth factors to accelerate healing in full-thickness open wounds in healthy rodents has led to a general belief that normal, unimpaired healing proceeds at a nearly maximal rate in these animal models [3,4]. Thus, it is highly significant that di-rhamnolipid is able to increase the rate of wound closure as well as to decrease the collagen content.
The final step of the proliferative phase is epithelization. It involves migration, proliferation and differentiation of epithelial cells from the wound edges as to resurface the defect. In open full-thickness burn wounds, epithelization is delayed until a bed of granulation tissue is established. We have shown that di-rhamnolipid stimulates the proliferation of differentiating keratinocytes in the presence of fetal calf serum [6], it is reasonable to expect that this feature could assist the process of wound re-epithelization.
Di-rhamnolipid clearly influences the process of healing by accelerating the wound closure and by decreasing the collagen content in the wound area. Subcutaneously, di-rhamnolipid is well-tolerated. Fig. 7 represents key events in wound healing with the possible di-rhamnolipid interference.
Fig. 7.
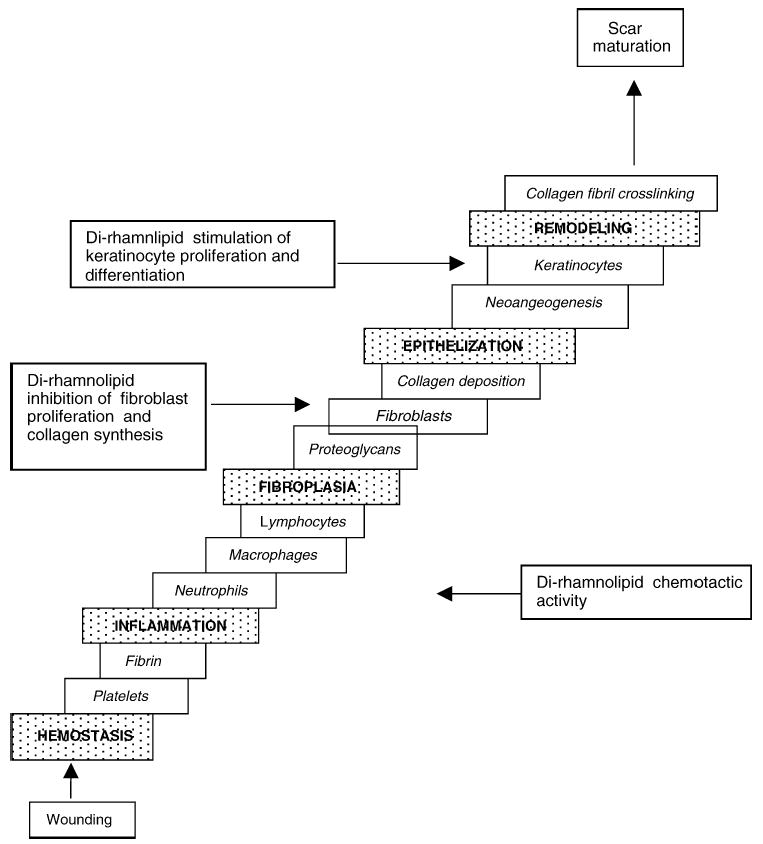
Possible involvement of di-rhamnolipid in the process of normal wound healing.
In conclusion, the results of this study raise the possibility of potential efficacy of di-rhamnolipid in accelerating normal wound healing and perhaps in overcoming defects associated with healing failure in chronic wounds. Demontrated biological effects of di-rhamnolipid, in skin cell cultures and in animal model of full-thickness burn wounds, point out the possible use of di-rhamnolipid as therapeutic agent for treatment of pressure, diabetic and venous stasis ulcers. Better understanding of di-rhamnolipid mechanism of action at the cellular level is required further to delineate exactly its effects on the wound healing cascade of events. Studies with BAC-3 designed at the downstream cellular and biochemical events, which sustain the fibrotic process should be conducted. With respect to that, relationship of BAC-3 with some fibrogenic cytokines, such as transforming growth factor beta (TGF-β) and connective tissue growth factor (CTGF) should be investigated. Additionally, more in vivo tests investigating the effect of di-rhamnolipid on the healing of burn wounds should be performed. For example, the most potent concentration of di-rhamnolipid to be used topically in wound healing as well as biodegradation of topical BAC-3 in the wound following application should be determined as closely as possible. Animals with full-thickness burn wounds covering 10% of the whole body skin, which are unable to heal spontaneously (chronic wounds by definition) should be used as the experimental models as well.
Acknowledgments
This study was entirely funded by TajCo Inc., a biotech company from VA, USA, and partially NIH SBIR grant A1 R43 AR44443-01A1. The authors would like to thank U.S. Army Institute of Surgical Research in Fort Sam Houston, TX, for technical support, and Connetics Corporation in Palo Alto, CA, USA, for help with di-rhamnolipid toxicity study. We also thank Dr. Nikolaos A. Papadopulos, M.D., Department of Plastic and Reconstructve Surgery, Technical University Munich, for critical reading of the manuscript.
References
- 1.Waldorf H, Fewkes J. Wound healing. Adv Dermatol. 1995;1:77. [PubMed] [Google Scholar]
- 2.Martin P. Wound healing. Aiming for perfect skin regeneration Science. 1997;276(4):75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Leitzel K, Cano C, Marks JGJ, Lipton A. Growth factors and wound healing in the hamster. J Dermatol Surg Oncol. 1985;11:617–22. doi: 10.1111/j.1524-4725.1985.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 4.Singh G, Foster CS. Epidermal growth factor in alkali-burned corneal epithelial wound healing. Am J Opthalmol. 1987;103:802–7. doi: 10.1016/s0002-9394(14)74397-1. [DOI] [PubMed] [Google Scholar]
- 5.Piljac G, et al. Pharmaceutical preparation based on rhamnolipid. 5,455,232. United States. 1995
- 6.Stipcevic T, et al. Di-rhamnolipid from Pseudomonas aeruginosa displays differential effect on keratinocyte and fibroblast cell cultures. J Dermatol Sci. doi: 10.1016/j.jdermsci.2005.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stipcevic T, et al. Use of rhamnolipid in wound healing, treatment and prevention of gum disease and periodontal regeneration. WO 01/10447 A1. PCT Patent Application. 2001
- 8.Piljac G, et al. Immunological activity of rhamnolipids. 5,466,675. United States. 1995
- 9.Johnson MK, Boese-Marazzo O. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980;29(3):1028–33. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luna LG. 3rd ed. Mc Graw-Hill: 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology; pp. 32–9. [Google Scholar]
- 11.Walker HL, Mason AD. A standard animal burn. J Trauma. 1968;8(6):1049–51. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J histochem Cytochem. 1985;(33):845–53. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. St. Louis: Mosby Company; 1978. p. 115–6; Rheinwald JG, Greem H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–43. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 14.Barillo DJ, Goodwin CW. Dermatologists and the burn center. Dermatol Clin 1999;17(1):61–75; Peate WF. Outpatient management of burns. Am Fam Physician. 1992;45:1321–30. [PubMed] [Google Scholar]
- 15.Scriven JM, Taylor LE, Wood AJ, Bell PR, Naylor AR, London NJ. A prospective randomised trial of four/layer versus short stretch compression bandages for the treatment of venous leg ulcers. Ann R Coll Surg 1998;80:215–20; Franklin TJ. Therapeutic approaches to organ fibrosis. Int J Biochem Cell Biol. 1997;29:79–89. [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel DH, Ash K, Lord J, Newman J, Zukowski M. Accelerated laser resurfacing wound healing using a triad of topical antioxidants. Dermatol Surg. 1998;24:661–4. doi: 10.1111/j.1524-4725.1998.tb04224.x. [DOI] [PubMed] [Google Scholar]; Molina V, Blank M, Shoenfeld Y. Fibrotic diseases. Harefuah. 2002;141:973–8. 1009. [PubMed] [Google Scholar]
- 17.Zamboni WA, Wong HP, Stephenson LL, Pfeifer MA. Evaluation of hyperbaric oxygen for diabetic wounds: a prospective study. Undersea Hyperbaric Med. 1997;24:175–9. TNO Pharma, Netherlands. http://www.tno.nl/kwaliteit_van_leven/markten/pharma/disease_models_and_expert/fibrosis/. [PubMed] [Google Scholar]
- 18.Reich JD, Cayyaniga AL, Mertz PM, Kerdel FA, Eaglstein WH. The effects of electrical stimulation on the number of mast cells in healing wounds. J Am Acad Dermatol 1991;25:40–6; Maier RM, Soberon-Chavez G. Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications . Appl Microbiol Biotechnol. 2000;54:625–33. doi: 10.1007/s002530000443. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez OM, Mertz PM, Smerbeck RN, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol 1983;81:144–8; van Furth R, Nibbering PH, van Dissel JT, DiesselhoffdenDulk MM. Transient functional expression of alpha Vbeta 3 on vascular cells during wound repair. J Invest Dermatol. 1985;85:398–402. doi: 10.1111/1523-1747.ep12543498. [DOI] [PubMed] [Google Scholar]
- 20.Beck LS, Deguzman L, Lee WP, Xu Y, McFatridge LA, Amento EP. TGFβ[capiota] accelerates wound healing: reversal of steroid-impaired healing in rats and rabbits. Growth Factors 1991;5:295–304; Shryocok R, Scott A, Silver M, Banschbach W, Kramer JC. Effect of Psudomonas aeruginosa rhamnolipid on human neutrophil migration . Curr Microbiol. 1984;10:323–8. [Google Scholar]
- 21.Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219:688–91. doi: 10.1097/00000658-199406000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rohrich RJ. Wound healing and closure, abnormal scars, envenomation and extravasation injuries. In: McCarthy JG, editor. Plastic surgery. Philadelphia, PA: WB Saunders; 1990. pp. 2–17. [Google Scholar]
- 22.Cross SE, Naylor IL, Coleman RA, Teo TC. An experimental model to investigate the dynamics of wound contraction. Br J Plast Surg. 1995;48:189–97. doi: 10.1016/0007-1226(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 23.Davidson JM. Animal models for wound repair. Arch Dermatol Res. 1998;290(Suppl):S1–S11. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]
- 24.Harlan product guide. 2003. Harlan Laboratory Animals. [Google Scholar]


