Abstract
To prevent patient pain, the clinician may chose from opioid and nonopioid analgesics. It is rational for the practitioner to combine drugs from these classes when managing moderate to severe pain. To select combination regimens wisely, it is necessary to understand the significant pharmacological features of each category alone. Careful selection of an effective analgesic regimen based on the type and amount of pain the patient is expected to have can prevent the stress and anxiety associated with breakthrough pain. The clinician can and should develop a variety of effective, safe analgesic regimens, based on estimates of anticipated pain intensity that use sound pharmacological principles.
Keywords: Opioids, Nonopioids, Pain management, Continuing education
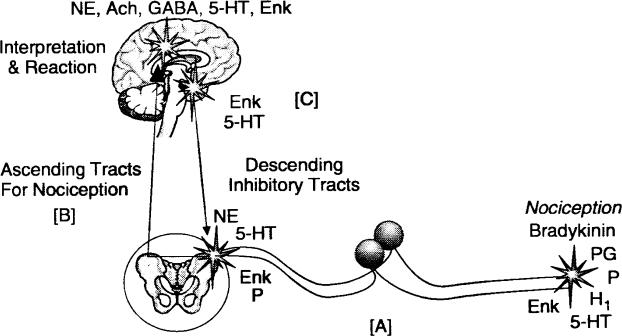
Analgesic drugs interrupt nociceptive pathways that transmit impulses to be interpreted as pain in the central nervous system. Conventional analgesics inhibit ascending impulses and/or their interpretation. Figure 1 offers a basic schematic of nociceptive transmission. A variety of so-called ‘‘analgesic adjuncts’’ have proven efficacy for managing chronic pain, but will not be addressed in this article. They include various antidepressants and anticonvulsants that either enhance descending inhibitory pathways or modulate excitatory neural traffic, which amplifies pain interpretation. These agents have marginal benefit in the management of acute pain, and they are not regarded as ‘‘analgesics’’ in the conventional sense.
Figure 1.
Nociceptive pathways. The drawing illustrates [A] incoming nociceptive impulses, [B] ascending nociceptive tracks, and [C] descending inhibitory tracts that act to blunt incoming pain signals. Abbreviations represent the myriad of neurotransmitters that contribute to pain transmission. They reflect potential targets for pharmacologic intervention to control pain.
Conventional analgesics are classified as opioids and nonopioids, but the older terms ‘‘narcotic’’ and ‘‘non-narcotic’’ continue to be used interchangeably. Current research has shown that both classes have varying degrees of central and peripheral action.1,2 The primary feature that distinguishes these 2 classes of analgesics is their mechanisms of action. Nonopioids include acetaminophen and the nonsteroidal anti-inflammatory drugs (NSAIDs), which interrupt prostaglandin synthesis and have a maximal dose or ceiling for their analgesic effect. Opioids, as represented by morphine, act as agonists at 3 specific receptors designated mu, kappa, and delta. The fact that opioids have no dose limit or ceiling permits their dose to be increased until relief is obtained, or limiting side effects occur. It is rational for the practitioner to combine drugs from these classes when managing moderate to severe pain. To select combination regimens wisely, it is necessary to understand the significant pharmacological features of each category alone.
NSAIDs
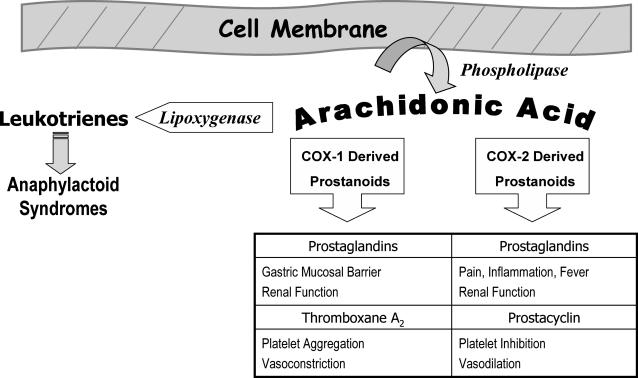
NSAIDs provide excellent analgesia for mild to moderate pain. They are particularly useful in the initial management of pain that has an inflammatory component. This includes pain associated with musculoskeletal trauma and dentistry. The analgesic, anti-inflammatory, and antipyretic effects of NSAIDs, as well as their most notable side effects, are attributed to inhibiting cyclooxygenases that catalyze the synthesis of prostaglandins, thromboxanes, and prostacyclin. These autacoids are collectively regarded as prostanoids, and their synthesis and functions are illustrated in Figure 2.
Figure 2.
Synthesis and functions of prostanoids.
The most frequent side effects attributed to NSAIDs are gastrointestinal in nature, and include dyspepsia, gastric erosions, and mucosal ulcerations. Patient complaint of dyspepsia does not necessarily correlate with mucosal injury. Although less likely to produce gastric upset, buffered aspirin carries similar risk for mucosal damage as regular aspirin.3 This is because NSAIDs inhibit prostaglandin synthesis in gastric mucosa following absorption and subsequent distribution to the gastrointestinal wall. Normally these prostaglandins are responsible for producing compounds that protect the gastric lining. NSAIDs available for parenteral administration produce gastric injury equivalent to those administered orally.
The antiplatelet effect of conventional NSAIDs is a consideration following surgical procedures, but aspirin is the only NSAID that significantly prolongs bleeding time. This is because aspirin’s antiplatelet action is irreversible, lasting the lifespan of the platelet (10–14 days). Other NSAIDs bind weakly and reversibly to platelet cyclooxygenases, which results in their mild antiplatelet influence being lost after drug elimination. Although these agents all prolong bleeding times to varying degrees, this does not correlate with clinical bleeding. Whether the antiplatelet influences of low-dose aspirin or other NSAIDs increases postsurgical bleeding to a significant degree remains unsettled, because case reports and clinical studies are at odds.4
Perturbation of cell membranes can be mediated by diverse endogenous and exogenous stimuli. This leads to release of arachidonic acid from the phospholipids comprising the membrane. Two families of cyclooxygenases (COX-1 and COX-2) convert this fatty acid to prostanoids that are unique to the particular cell or tissue and include prostaglandins, thromboxanes, and prostacyclin. Each of these has specific physiological functions, some of which are listed here. Arachidonic acid is also a substrate for lipoxygenase that catalyzes the formation of leukotrienes known for their anaphylactoid effects, including bronchospasm and upper airway edema. NSAIDs inhibit the activity of cyclooxygenases and lead to effects attributable to a reduction in the synthesis of the various prostanoids. This inhibition also allows a greater portion of arachidonic acid to be converted to leukotrienes, which may not be tolerated by patients with atopy because they experience pseudoallergic syndromes following NSAID administration. Selective COX-2 inhibition reduces pain and inflammation with little or no influence on gastric mucosa. However, this selective inhibition enhances the activity of thromboxane-mediated effects, leading to a greater potential for thrombotic events, eg, myocardial infarction and stroke.
NSAIDs should be avoided in patients taking anticoagulants, such as warfarin, or those suffering bleeding disorders. This is because gastric injury may result in extensive bleeding, not because of the added antiplatelet influences. Aspirin provides a maximum antiplatelet influence at 80 mg daily, and is frequently prescribed in combination with warfarin without consequence, because such doses seldom produce gastric insult.
In the healthy patient, nephrotoxicity attributed to NSAIDs requires high doses for extended periods, eg, a year or more.5 However, a patient with compromised renal function relies more heavily on prostaglandins for adequate function, and acute renal failure can occur within 24 hours of NSAID administration. NSAIDs should not be prescribed for patients having compromised or questionable renal function.
By inhibiting cyclooxygenases, NSAIDs shunt the arachidonic pathway toward leukotriene synthesis (Figure 2). These substances mediate a variety of tissue responses, including those associated with bronchospasm and anaphylaxis.6 Certain individuals may be extremely sensitive to even subtle elevation in leukotriene synthesis, which may result in signs and symptoms of allergic response. It is recommended that the term ‘‘aspirin or NSAID intolerance’’ should be used to distinguish this reaction from true hypersensitivity responses mediated by IgE. Acetaminophen is the conventional alternative for patients reporting allergic reactions to NSAIDs, unless the patient can identify a particular NSAID he or she has tolerated in the past without problem.
Based on these considerations, NSAIDs are contra-indicated for patients having a current history of nephropathy, erosive or ulcerative conditions of the GI mucosa, anticoagulant therapy, hemorrhagic disorders, intolerance or allergy to any NSAID, or pregnancy. In the developing fetus, prostaglandins maintain patency of the ductus arteriosus during fetal development, so they should not be inhibited. Although this concern is most relevant during the third trimester, NSAIDs should generally be avoided throughout pregnancy. In all cases in which NSAIDs are contraindicated, acetaminophen is the conventional nonopioid alternative.
Efficacy, Selection, and Dosages
All NSAIDs have greater potency as analgesics and antipyretics than as anti-inflammatory agents. For example, a single 325–1000 mg dose of aspirin may reduce pain and fever, but daily doses of 4–6 g are required to effectively suppress inflammation. The analgesic doseresponse curves for NSAIDs and acetaminophen demonstrate an upper limit or ceiling effect. A point is reached at which increasing the dose further provides no improvement in pain relief. For aspirin and acetaminophen, this ceiling response occurs at approximately 1000 mg, and for ibuprofen, 400 mg. A ceiling for their anti-inflammatory response cannot be ascertained because at higher dosages, side effects become prohibitive. Because higher NSAID doses are typically required to suppress inflammation than to provide analgesia, many NSAIDs are marketed in several dosages, ie, ibuprofen is available in dosages ranging from 200 to 800 mg. When prescribing a NSAID, the practitioner should select the lower dose range for noninflammatory pain and reserve the higher dosages for when inflammation and swelling are an issue.
All NSAIDs have the potential to produce side effects similar to aspirin, but most other NSAIDs have a lower frequency of side effects. Ibuprofen has one of the better safety profiles. It produces GI symptoms in >3% of patients treated and its antiplatelet activity is considerably less than that of aspirin and most other NSAIDs.7 There has been recent confirmation that ibuprofen is unique among NSAIDS in antagonizing the influence of aspirin’s antiplatelet effect. It should be avoided in patients taking aspirin for coronary artery disease or for prevention of transient ischemic attacks or stroke. No other NSAIDs have been confirmed in producing this interaction, but only diclofenac and selective COX-2 agents have been proven not to interact.8
Clinical trials comparing NSAIDs have not identified substantive differences in their anti-inflammatory or analgesic efficacy.9,10 The clinician must appreciate that there can be considerable variation among individual patients in terms of clinical response and GI tolerance. In a given patient, an unsatisfactory response with one NSAID does not preclude therapeutic success with another. Considering its low cost and side effect profile, ibuprofen is a logical first-line agent.
The preoperative use of NSAIDs has been demonstrated repeatedly to decrease the intensity of postoperative pain and swelling.11,12 This is not surprising, because NSAIDs inhibit the ‘‘formation’’ of prostaglandins. Recent insights into pain mechanisms indicate that benefits of this practice are evident so long as prostaglandin synthesis is inhibited before local anesthesia wanes. Otherwise, prostaglandins trigger nociceptive impulses that travel to the brain and ‘‘wind up’’ the brain’s interpretation of pain intensity. When an extensive surgical procedure is planned, optimal serum levels of an NSAID should be established either preoperatively or before patient discharge, and while tissues remain anesthetized.
Acetaminophen
The action of acetaminophen is poorly defined, but it is believed to interrupt the influence of prostaglandins within central nervous system pathways. Acetaminophen is approximately as active as aspirin in inhibiting prostaglandin synthesis within the central nervous system, but has little influence in peripheral tissues. This is one of several explanations for its lacking anti-inflammatory efficacy and sharing none of the peripheral side effects common to NSAIDs. As an analgesic and antipyretic, however, acetaminophen is equal in potency and efficacy to aspirin, achieving its analgesic ceiling at 1000 mg.
The major adverse effect of acetaminophen is hepatotoxicity. This is attributed to a metabolite that is not adequately conjugated following acute doses of 10–15 g (150–250 mg/kg). The conjugate for this toxic metabolite is provided by glycogen, and a lower dose of acetaminophen may be toxic for patients having depleted glycogen stores, such as are associated with dieting and anorexia, and for patients suffering primary liver dysfunction or receiving hepatotoxic medications. Patients suspected of chronic alcoholism should limit their daily acetaminophen intake to 2 g, rather than the normal daily maximum of 4 g.13
SUMMARY OF NONOPIOIDS
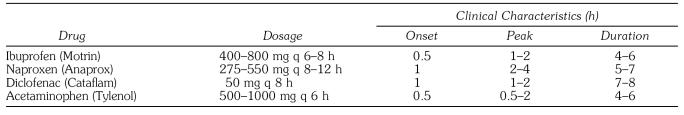
Data relevant for prescribing the more commonly used nonopioids are summarized in Table 1. An optimal dose of a nonopioid should be the initial component of all regimens prescribed for dental pain. The following are key features for the proper use of nonopioid analgesics:
Their analgesic efficacy exhibits a ceiling at which escalating doses offer no benefit unless the goal is to also suppress inflammation. When the clinician selects an agent having a range of manufacturer-suggested doses, the lower dose should be regarded as analgesic and higher dose anti-inflammatory.
All NSAIDs should be avoided in patients presenting a history of allergy to any NSAID, GI ulcerations, renal compromise, anticoagulant therapy or bleeding disorder, or pregnancy.
Ibuprofen is an ideal prototype for consideration unless a patient identifies a particular agent that has been effective previously.
It is acceptable to combine an NSAID with acetaminophen. They both inhibit prostaglandin synthesis but act at different sites. However, it is irrational to combine 2 or more NSAIDs.
Table 1.
Selected Nonopioid Analgesics Useful for Reducing Postoperative Pain and Inflammation (Adapted from Kastrup17)
OPIOID ANALGESICS
Opioids produce the majority of their therapeutic and adverse effects by acting as agonists at mu and/or kappa opioid receptors. Unlike the nonopioids, which exhibit a ceiling effect, the analgesic response to opioids acting at mu receptors continues to improve as their dose is increased. Although their analgesic efficacy is unlimited, side effects often preclude the use of doses adequate to completely relieve severe pain.14 These side effects include sedation, respiratory depression, dependence, nausea, miosis, and constipation. Following prolonged use, patients develop tolerance to most opioid effects. Constipation and miosis are notable exceptions, however. Patients suffering chronic and/or terminal illnesses may require astonishingly high opioid doses to achieve analgesia, but constipation and visual impairment may become troublesome. Similar doses, if administered to patients without opioid tolerance (‘‘opioid-naïve’’ patients), would be lethal because of resultant respiratory depression. The practitioner must use caution when considering the use of the newer high-dose, sustained-release opioids, because these are designed more specifically for opioid-tolerant patients.
THERAPEUTIC USE OF OPIOIDS
Patients and practitioners are often concerned with the potential for addiction, which may limit prescribing and use, leading to inadequate management of pain.14,15 This can be attributed to confusion regarding the concepts of ‘‘drug dependence’’ and ‘‘drug addiction.’’ Patients consuming opioids regularly for more than a week may develop some degree of dependence. This may require gradual tapering of the dosage to avoid withdrawal symptoms. However, drugs do not produce addiction. This is a compulsive pattern of behavior in which an individual continues to seek the drug for effects they perceive as pleasurable and not for legitimate medical conditions. Addictive behavior is a psychiatric condition that can be reinforced by a particular drug, but it is not a pharmacodynamic property. Obviously, opioids must be prescribed cautiously for patients who demonstrate addictive personality.
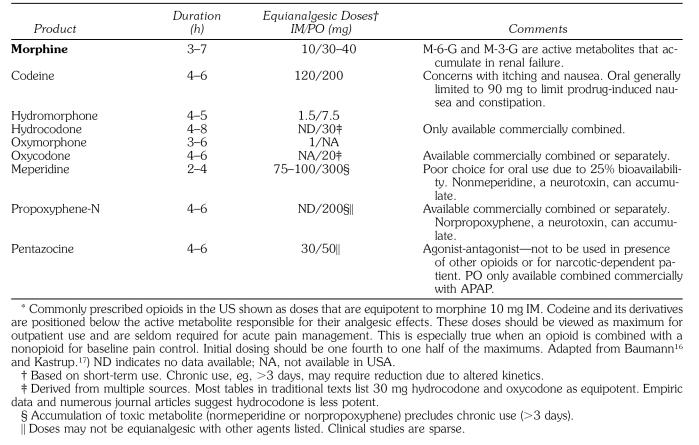
The practitioner should consider the use of opioid therapy for the short-term management of breakthrough pain that is not responsive to NSAIDs or acetaminophen. Despite common misconceptions, all opioids provide the same degree of pain relief provided that they are prescribed at equipotent doses. These doses have been confirmed for opioids administered by parenteral routes. However, precise oral equivalents are problematic because of altered bioavailability attributed to first-pass metabolism into inactive metabolites. Opioids used for treating moderate to moderately severe pain are compared by equipotent dose and clinical duration in Table 2.
Table 2.
Opioid Analgesics*
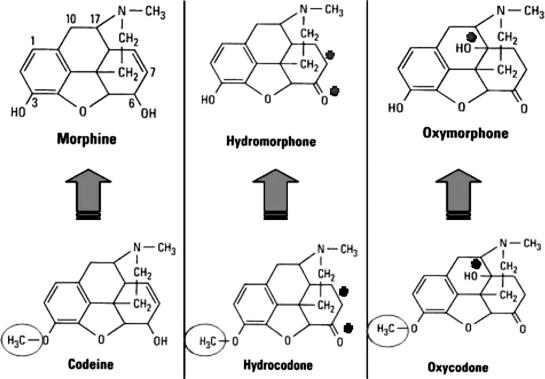
Genetic predisposition for biotransformation of opioids can lead to poor analgesia in certain patients. Codeine has very little affinity for the mu receptor and may be considered a prodrug because 10% of the parent drug is converted to morphine by cytochrome P450 CYP2D6. The same can be said for hydrocodone and oxycodone, which require demethylation to hydromorphone and oxymorphone, respectively (Figure 3). Approximately 7% of the Caucasian population metabolizes codeine and hydrocodone poorly, because they have inherited 2 nonfunctional alleles for cytochrome P450 CYP2D6. In these individuals, analgesia resulting from codeine, oxycodone, or hydrocodone will be less than expected with the general population. The influence of certain antidepressants on the effectiveness of codeine and its derivatives has significant clinical implications. Several of the selective serotonin reuptake inhibitor antidepressants can hinder the demethylation of codeine and its derivatives. This is established for fluoxetine (Prozac) and paroxetine (Paxil), but appears less likely with other agents of this class.18 Patients taking these antidepressants may experience reduced levels of analgesia from these opioids. In such cases, it is wise to prescribe the active parent drug, morphine or hydromorphone.
Figure 3.
Molecular structures of morphine, codeine, and derivatives. Codeine and its derivatives differ from their morphine-derived counterparts only in a methyl substitution (circled). This methyl group prevents adequate binding to the mu opioid receptor that mediates most opioid effects. However, these prodrugs are capable of producing nausea and constipation, which are problematic when high doses are administered to generate enough active metabolite for analgesia. (Asterisks indicate molecular alterations from morphine and codeine.)
SPECIFIC OPIOID CONSIDERATIONS
Hydrocodone and Oxycodone
Hydrocodone and oxycodone are attractive analgesics because they have an oral bioavailability comparable to codeine of 60%. Additionally, their greater potency reduces the portion of an administered dose of the parent drug that contributes to nausea and constipation. Unfortunately, equianalgesic doses were initially poorly understood, which spawned release of combination products that contain irrational formulations.
Like codeine, oxycodone and hydrocodone are methylated molecules having little or no analgesic efficacy. Presumably, 10% of a dose administered parenterally is demethylated to its respective morphine counterparts, hydromorphone and oxymorphone. This implies that the oral dose for codeine is approximately 20 times the IM dose of morphine (200 mg vs 10 mg). Table 2 indicates that 200 mg codeine, 30 mg hydrocodone, and 20 mg oxycodone are equipotent oral doses and are equianalgesic to morphine 10 mg IM. Clinical studies that have attempted to address equianalgesic doses of codeine derivatives are sparse, but they support this opinion. Beaver et al19 found that oxycodone 10 mg was comparable to codeine 100 mg, and that this would extrapolate to oxycodone 20 mg and codeine 200 mg. Studies by Hopkinson20 and by Beaver and McMillan21 have shown that hydrocodone 10 mg was approximately equipotent to codeine 60 mg, and this would extrapolate to 33 mg hydrocodone and 200 mg codeine.
Meperidine
Meperidine 75–100 mg is equianalgesic to morphine 10 mg following intramuscular (IM) administration. A significant portion of an IM dose of meperidine is converted to normeperidine, a metabolite that has no analgesic properties, but is a noted central nervous system stimulant. Furthermore, this metabolite has a 15–20 hour elimination half-life, compared to 3 hours for the parent drug. For hospitalized patients, meperidine is used only for a day or two; otherwise, normeperidine can accumulate. This issue becomes even more problematic following oral administration. The oral bioavailability for meperidine is approximately 25%, which requires a 300 mg dose to be equianalgesic to its IM dose of 75 mg. This introduces an even greater risk for accumulation of normeperidine. Poor oral absorption and accumulation of normeperidine make meperidine a very poor choice as an oral analgesic.
Propoxyphene
Propoxyphene is available only for oral administration. The equianalgesic dose compared to morphine has not been established, but its potency is low. By convention, 100 mg is considered equipotent to oral codeine 60 mg. It is similar to meperidine in that it is converted to norpropoxyphene, a central nervous system stimulant having an elimination half-life of 30 hours. Its use should be limited to short-term management of mild to moderate pain.
Pentazocine
Pentazocine is the only oral agonist-antagonist analgesic available in the United States. It produces its analgesic effect by acting as an agonist at kappa receptors, but is an antagonist at mu receptors. Therefore, it reverses all effects of traditional mu agonist opioids if taken concurrently. Unlike mu agonists, which provide unlimited analgesic efficacy, kappa agonists exhibit a ceiling to their analgesic effect and no benefit is derived by increasing doses beyond those listed in Table 2. Pentaz-ocine in the US is available for oral use compounded with naloxone, an antagonist at all opioid receptors, presumably to prevent parenteral injection abuse issues. If injected, the naloxone will block all effects of pentaz-ocine, rendering it useless. When taken by mouth, however, naloxone has no oral bioavailability and will not hinder pentazocine actions. Additionally, pentazocine is available compounded with acetaminophen. It should not be used in the presence of other opioids. When other opioids are present, pentazocine will serve as an opioid antagonist, thus reducing the patient’s analgesia. Additionally, it should not be prescribed for patients who are opioid-dependent and at risk for opioid withdrawal. It is an attractive choice for patients having a prior history of opioid abuse because it does not provide significant euphoric effects mediated by conventional mu agonists.
Tramadol
Tramadol is a centrally-acting analgesic with binary action. It is not classified as a controlled substance in the United States. The parent drug inhibits the reuptake of norepinephrine and serotonin. This resembles the action of tricyclic antidepressants and potentiates descending inhibitory pathways illustrated in Figure 1. This action has proven efficacy in the management of chronic pain. However, tramadol’s benefit in acute postoperative pain management is not as well defined. Tramadol’s principal metabolite, M1, demonstrates agonist action on mu receptors, providing analgesic efficacy approximating that of codeine 60 mg.
Tramadol is marketed as an effective and safe analgesic for moderate to moderately severe pain. Nausea, vomiting, and dizziness may occur with the use of tramadol. It should be used with caution in patients with a history of seizure disorder. Tramadol is not recommended for patients with a tendency to opioid abuse or dependence.22 Tramadol offers the clinician the potential to reduce the incidence of somnolence and constipation commonly seen with opioid use, and is available in combination with acetaminophen. However, it may not be safer or more effective than mild opioid/acetaminophen combinations.23 Consequently, the practitioner may find tramadol useful when added for breakthrough pain, to patients already receiving NSAIDs for baseline acute pain management.
ANALGESIC REGIMENS
Mild to moderate pain can frequently be managed effectively by first using optimal doses of nonopioids, ibuprofen 400–800 mg, or acetaminophen 1000 mg. Although it is unwise to combine NSAIDs, the addition of acetaminophen to an NSAID is an option, as acetaminophen has a different site of analgesic action.24,25 Regardless of pain severity, one should seek to optimize dosages of these agents, and then, if necessary, add an opioid to the regimen. This practice will generally reduce the amount of opioid required, sometimes to only a fraction of the maximum doses listed in Table 2. Ideally, one should maintain the regular nonopioid dosing schedule and add an opioid product as needed for ‘‘breakthrough’’ pain.
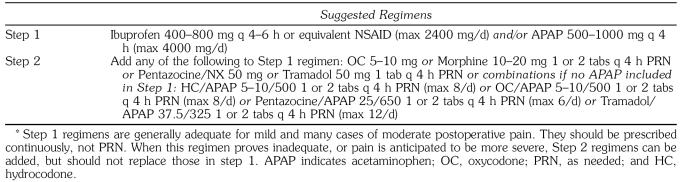
It is not surprising that such a large number of commercially compounded analgesics have been produced containing both a nonopioid and an opioid ingredient. The opioid contained in most of these products is either codeine or one of its derivatives, such as hydrocodone. Some of these combinations appear to have been formulated with little consideration given to equianalgesic dosage strategies. Additionally, several products contain large quantities of acetaminophen that preclude the use of multiple tablets to achieve an adequate amount of opioid for patients who experience severe pain. When prescribing combination products, the clinician must pay particular attention to the amount of acetaminophen used separately or compounded so that the maximum daily dose is not exceeded. In some cases, the clinician may choose to write separate prescriptions for the opioid and nonopioid analgesic needs of the patient to avoid acetaminophen overdose. Suggested regimens are presented in Table 3.
Table 3.
Stepped Approach for Managing Postoperative Pain*
CONCLUSION
Careful selection of an effective analgesic regimen based on the type and amount of pain the patient is expected to have can prevent the stress and anxiety associated with breakthrough pain.26,27 When analgesics fail, it is not unusual for patients to go to desperate lengths to seek relief. The clinician can and should develop a variety of effective, safe analgesic regimens based on estimates of anticipated pain intensity that use sound pharmacological principles. The following are key features for the proper management of acute postoperative pain:
Patients benefit from receiving optimal NSAID doses given on regular, ‘‘clock-based’’ time intervals. These agents are effective and relatively safe, and reduce the need for opioids. In situations where pain can be anticipated, the NSAID may be optimized by pre-operative administration and by continuing to dose the NSAID on a regular schedule to minimize pain and inflammation.
Acetaminophen’s site of action differs from that of NSAIDs. Therefore, acetaminophen’s analgesic effect is considered synergistic when combined with NSAIDs.
Once the dose of NSAID and/or acetaminophen has been optimized, but pain persists, opioid use may be a consideration. A commercially available combination product containing opioid and acetaminophen may be a good option, and is easy to prescribe. When prescribing combination opioid and acetaminophen analgesic products or acetaminophen alone, the practitioner must caution the patient not to exceed 4 g of acetaminophen per day because of concerns with hepatic injury.
As opioids have no ceiling dose, there are some situations where opioid dosing is better done with the opioid prescribed separately. This permits increasing the opioid to the needed analgesic dose and decreases concern for acetaminophen toxicity.
CONTINUING EDUCATION QUESTIONS
-
All of the following are correct statements regarding nonsteroidal anti-inflammatory drugs EXCEPT:
They produce useful and adverse effects by inhibiting the synthesis of various prostanoids.
Their analgesic ceiling is achieved at doses below those required to depress inflammation.
Nephrotoxicity is their most common side effect.
Compared to aspirin, other NSAIDS have less antiplatelet influence.
-
All of the following are correct statements regarding opioids EXCEPT:
Analgesia provided by opioids that act as mu agonists has an unlimited dose-response.
Codeine and its derivatives may be less effective for patients medicated with selective serotonin re-uptake inhibitor antidepressants.
Patients managed chronically with opioids develop tolerance to analgesia and respiratory depression, but not to constipation.
When an opioid is indicated for postoperative pain, the agonist-antagonist opioids are ideal for patients medicated with transdermal morphine patches.
-
Which of the following statements regarding analgesics for postoperative pain is most correct?
All regimens should commence with an NSAID and/or acetaminophen (APAP) prescribed on an as needed (PRN) schedule.
Pentazocine is an acceptable step 2 agent for patients having a current history of opioid abuse.
The efficacy of hydrocodone (HC) is diminished in patients medicated with tricyclic antidepressants.
Opioid preparations should be reserved for situations in which optimal nonopioid regimens have failed, or the intensity of postoperative pain is predicted to be severe.
-
The following analgesic regimens have been prescribed following various dental procedures, and for patients having different tolerances for pain. Assuming no drug allergies or contraindications, which regimen is least rational?
HC/APAP 10/500 q 4 h PRN.
Ibuprofen 400 mg q 4 h.
Ibuprofen 400 mg q 4 h plus APAP 1000 mg q 6 h.
Ibuprofen 400 mg q 4 h plus HC/APAP 7.5/ 500 q 4 h.
REFERENCES
- Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- Stein CS. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Kimmey MB. Cardioprotective effects and gastrointestinal risks of aspirin: maintaining the delicate balance. Am J Med. 2004;117(5A):72s–78s. doi: 10.1016/j.amjmed.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Goldenberg NA, Jacobson MT, Manco-Johnson MJ. Duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med. 2005;142:506–509. doi: 10.7326/0003-4819-142-7-200504050-00009. [DOI] [PubMed] [Google Scholar]
- DeBroe ME, Elseviers MM. Analgesic nephropathy. N Engl J Med. 1998;338:446–452. doi: 10.1056/NEJM199802123380707. [DOI] [PubMed] [Google Scholar]
- Babu KS, Salvi SS. Aspirin and asthma. Chest. 2000;118:1470–1476. doi: 10.1378/chest.118.5.1470. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Bridge HS, Nolan J, Stoddart PA. Double-blind, placebo-controlled analgesic study of ibuprofen or rofecoxib in combination with paracetamol for tonsillectomy in children. Br J Anaesth. 2002;88(1):72–77. doi: 10.1093/bja/88.1.72. [DOI] [PubMed] [Google Scholar]
- MacDonald TM, Wei L. Effect of ibuprofen on cardio-protective effect of aspirin. Lancet. 2003;361:573–574. doi: 10.1016/s0140-6736(03)12509-3. [DOI] [PubMed] [Google Scholar]
- Watcha MF, Issioui T, Klein KW, White PF. Costs and effectiveness of rofecoxib, celecoxib, and acetaminophen for preventing pain after ambulatory otolaryngologic surgery. Anesth Analg. 2003;96:987–994. doi: 10.1213/01.ANE.0000053255.93270.31. [DOI] [PubMed] [Google Scholar]
- Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs—differences and similarities. N Engl J Med. 1991;324:1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- Jackson DL, Moore PA, Hargreaves KM. Preoperative nonsteroidal anti-inflammatory medication for the prevention of postoperative dental pain. J Am Dent Assoc. 1989;119:641–647. doi: 10.1016/s0002-8177(89)95018-6. [DOI] [PubMed] [Google Scholar]
- Camu F, Beecher T, Recker DP, Verburg KM. Valde-coxib, a COX-2 specific inhibitor, is an efficacious, opioid-sparing analgesic in patients undergoing hip arthroplasty. Am J Ther. 2002;9:43–51. doi: 10.1097/00045391-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Block GD. Association of acetaminophen toxicity when fasting and ethanol. JAMA. 1994;272:1845–1850. doi: 10.1001/jama.1994.03520230055038. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Akil H. Opioid analgesics. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001. [Google Scholar]
- Savage SR. Opioid use in the management of chronic pain. Med Clin North Am. 1999;83:761–786. doi: 10.1016/s0025-7125(05)70133-4. [DOI] [PubMed] [Google Scholar]
- Baumann TJ. Pain management. In: DiPiro JT, Talbert RL, Yee GC, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. Stamford, CT: Appleton and Lange; 1997. [Google Scholar]
- Kastrup EK, editor. Drug Facts and Comparisons. St. Louis, Mo: Facts and Comparisons Div, JB Lippincott Co; 2005. [Google Scholar]
- Abramowicz M, editor. Med Lett. 1999;41(1056):61–62. [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer I: comparisons of oral with intramuscular codeine and of oral and intramuscular oxycodone. J Pharmacol Exp Ther. 1978;207:92–100. [PubMed] [Google Scholar]
- Hopkinson JH. Hydrocodone—a unique challenge for an established drug: comparison of repeated or doses of hydrocodone (10mg) and codeine (60mg) in the treatment of postpartum pain. Curr Ther Res. 1978;24:503–516. [Google Scholar]
- Beaver WT, McMillan D. Methodological considerations in the evaluation of analgesic combinations: acetaminophen (paracetamol) and hydrocodone in postpartum pain. Br J Clin Pharmacol. 1980;10:215s–223s. [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Adams EH, Geller A, et al. A postmarketing surveillance program to monitor Ultram (tramadol) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Moore PA, Crout RJ, Jackson DL, et al. Tramadol hydrochloride: analgesic efficacy compared with codeine, aspirin with codeine, and placeo after dental extraction. J Clin Pharmacol. 1998;38:554–560. doi: 10.1002/j.1552-4604.1998.tb05794.x. [DOI] [PubMed] [Google Scholar]
- Breivik EK, Barkvoll P, Skowlund E. Combining diclofenac with acetaminophen or acetaminophencodeine after oral surgery: a randomized, double-blind, single-dose study. Clin Pharmacol Ther. 1999;66:625–635. doi: 10.1053/cp.1999.v66.103629001. [DOI] [PubMed] [Google Scholar]
- Issioui T, Klein KW, White PF, et al. The efficacy of premedication with celecoxib and acetaminophen in preventing pain after otolaryngologic surgery. Anesth Analg. 2002;94(5):1188–1193. doi: 10.1097/00000539-200205000-00025. [DOI] [PubMed] [Google Scholar]
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR. The efficacy of combination analgesic therapy in relieving dental pain. J Am Dent Assoc. 2002;133:861–871. doi: 10.14219/jada.archive.2002.0300. [DOI] [PubMed] [Google Scholar]