Abstract
A case is reported in which a patient developed methemoglobinemia-induced cyanosis while under general anesthesia during surgery for multiple fascial space infections. The cause of methemoglobinemia was 20% benzocaine spray used for local anesthesia before intubation. Acutely developing methemoglobinemia is infrequently encountered in clinical practice. When confronted with cyanosis in the absence of cardiac or pulmonary disease, one must seriously consider the diagnosis of methe-moglobinemia. The etiology of methemoglobinemia, the causative agents, the diagnosis, and the emergency treatment required are discussed.
Keywords: Methemoglobinemia, Benzocaine, Cyanosis
The presence of cyanosis is disturbing even in cases when its cause is known. When a patient under general anesthesia suddenly develops cyanosis, it may be particularly alarming for the surgeon and anesthesiologist. The sudden onset of cyanosis in patients under general anesthesia is most often caused by airway obstruction, cardiovascular collapse, massive embolism, or pneumothorax. In these cases, the cyanosis is caused by hypoxia. In some rare instances, cyanosis is the result of methemoglobinemia, where the hemoglobin’s oxygen-carrying capacity is greatly decreased due to an increased amount of methemoglobin in the blood. The hallmark of methemoglobinemia is cyanosis unresponsive to high-flow oxygen in the absence of cardiac or pulmonary disorders. Acutely developing methemoglobinemia is infrequently encountered in clinical practice. Several drugs used in surgery and medicine can cause methemoglobinemia. A patient is described who developed methemoglobinemia after topical administration of benzocaine during surgery for multiple fascial space infections.
CASE REPORT
A 48-year-old female patient was admitted with multiple fascial space infections that required incision and drainage. Her medical history was significant for cervical cancer and hysterectomy. She denied taking any medications and had no known allergies to medication. Her social history included tobacco use, 1 pack per day for 30 years, and ethanol abuse. She denied intravenous drug abuse. The findings of her physical examination were unremarkable, except for a tender left buccal and submandibular moderate edema with erythema and sub-mandibular fluctuation. The maximal incisal opening was 25 mm. Intraoral examination findings were significant for a left vestibular fluctuant edema and for a severely displaced uvula to the right. Admission laboratory results were within normal limits, except for a white blood cell count of 17.3 × 109/L. Chest x-ray examination and electrocardiography results were normal. Computed tomography of the neck showed left para-pharyngeal abscess that compromised the airway and originated in the left peritonsillar region and left sub-mandibular and buccal space edema with collection. The patient was taken to the operating room and, while the patient was awake, nasal intubation was attempted after using 2% lidocaine spray local anesthesia without success. Fiberoptic nasoendotracheal intubation was then performed, and incision and drainage of the buccal and submandibular spaces were established with the patient under general anesthesia. On the third postoperative day, the patient developed persistent trismus without shortness of breath or stridor. Subsequent neck computed tomography indicated possible fluid recollection in the left peritonsillar and lateral pharyngeal spaces, and the patient returned to the operating room for incision and drainage of a left peritonsillar abscess.
Hemoglobin.
On arrival to the operating room, a continuous intravenous infusion was established and all monitors were placed. The patient was prepared for fiberoptic nasal intubation with lidocaine nebulizer and 2 sprays of 20% benzocaine to both nares while awake. While the patient was awake, fiberoptic nasal intubation was accomplished without difficulty through the right naris with a 7.0 endotracheal tube. General anesthesia was induced and maintained with oxygen, propofol, midazolam, fentanyl, and sevoflurane. Ventilation was controlled. Approximately 2 minutes after induction, the patient was noted to have decreasing oxygen saturation (90%) and she was becoming cyanotic. Bilateral breath sounds, chest expansion, oxygen flow, and vital signs were immediately checked and found to be normal. Despite oxygenation through the endotracheal tube with 100% oxygen, the patient’s oxygen saturation remained 88% to 90%. Immediate bronchoscopy showed no evidence of mucus or bronchopulmonary lesion, and the endotracheal tube was in a good position above the carina. A portable chest x-ray film was obtained and was negative, with no signs indicative of pulmonary edema or pneumothorax. Arterial line was established, and a specimen was immediately obtained for arterial blood gas analysis. The blood obtained was dark chocolate in color. The results were as follows: pH, 7.42; PaO2, 261 mm Hg; PaCO2, 40 mm Hg; and methemoglobin, 41%. The diagnosis of methemoglobinemia was established, and 70 mg of 1% methylene blue was administered intravenously over 5 minutes. A subsequent arterial blood gas determination 15 minutes later showed a methemoglobin concentration of 11.6%. Serial arterial blood gas analyses showed progressive decrease in methemoglobin levels: 41%, 11.6%, 6.3%, 4.1%, 2.9%, and 2.1%. The patient did not require further methylene blue therapy. After an uneventful postoperative period, the patient was discharged on the fourth postoperative day.
DISCUSSION
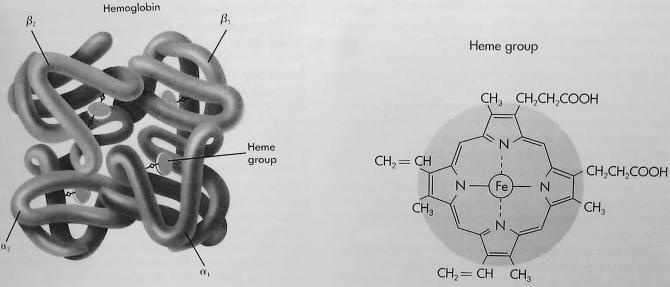
Hemoglobin consists of 4 protein chains and 4 heme groups. Each protein chain is called a globin, and it is bound to 1 heme, a red pigment molecule. Each heme contains 1 iron atom (Figure). Under normal circumstances, adult blood contains 4 types of hemoglobin: oxygenated hemoglobin or oxyhemoglobin, hemoglobin that contains no oxygen (reduced hemoglobin) or deoxyhemoglobin, hemoglobin transporting carbon dioxide or carboxyhemoglobin, and oxidized hemoglobin or methemoglobin. The last 2 are usually found in small amounts in blood.
Under normal circumstances, most of the hemoglobin iron exists in the ferrous (Fe+2) state, and a small fraction of the hemoglobin present in erythrocytes undergoes oxidation. As a result of this process, some of the hemoglobin iron is converted to the ferric (Fe+3) state, forming methemoglobin. Oxidized hemoglobin cannot bind or carry oxygen. The physiologic level of methemoglobin is less than or equal to 1% of total hemoglobin concentration, and when greater than 1% of hemoglobin is oxidized to methemoglobin, a hemoglobin deficiency occurs. In normal blood, the methemoglobin reductase system maintains methemoglobin in equilibrium with deoxygenated hemoglobin.1,2
Methemoglobinemia can be either inherited or acquired. Inherited methemoglobinemia is a rare disorder caused by a deficiency of the enzyme methemoglobin reductase, by the synthesis of an abnormal enzyme, or by the presence of an abnormal hemoglobin (hemoglobin M). Although cyanotic from birth, patients who have inherited methemoglobinemia are otherwise well. The fact that the patient was not known to be cyanotic in the past decreases the likelihood of an inherited form of methemoglobinemia in this case. Acquired methemoglobinemia results from exposure to chemicals that oxidize the ferrous iron in hemoglobin to the ferric state at a rate that exceeds the reducing capacity of the methemoglobin reductase enzyme in erythrocytes.1,2
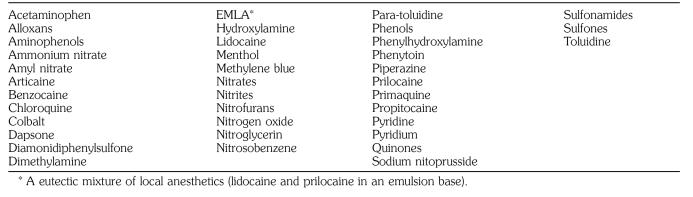
A wide variety of agents are known to induce methemoglobinemia (Table), including lidocaine. However, the patient did not develop cyanosis from the first operation when lidocaine spray was used alone. It was only after the benzocaine and lidocaine combination that methemoglobinemia developed during the second anesthesia, which implicates benzocaine as the etiologic factor. Benzocaine has been reported to cause methemoglobinemia when applied to infants as an ointment or a rectal suppository2,3 and when used topically to the perineal area.4 It has also been associated with methemoglobinemia after its use as a lubricant on endotracheal, bronchoscopic, and esophageal tubes.5,6 The particular preparation of benzocaine used in this case contained 20% benzocaine (Hurricane).3
Methemoglobinemia has also been observed after the use of other local anesthetics. Prilocaine has been implicated most frequently.7 Cyanosis can develop after administration of a dose as low as 500 mg for major nerve block.8 Methemoglobinemia has been also reported after topical use of a eutectic mixture of the local anesthetics lidocaine and prilocaine in children.9 Other commonly used drugs known to cause methemoglobinemia are phenacetin, acetaminophen, vasodilators, sulfonamides, and dapsone.
Oxidizing Agents Known to Induce Methemoglobinemia
The diagnosis of methemoglobinemia in our patient was suggested by the occurrence of cyanosis that did not improve with increasing the percentage of inspired oxygen, as well as the incompatibility between the elevated arterial partial pressure of oxygen and the gross appearance of the patient’s blood. Cyanosis without cardiorespiratory change is the cardinal sign of methemoglobinemia. For definitive diagnosis, spectrophotometry is used. Methemoglobin is not stable in samples of blood and should be measured promptly. Otherwise the measured level may be lower than the values at the time cyanosis was observed.
The onset of methemoglobinemia may be immediate or delayed. Cyanosis with a gray-brown hue that is unresponsive to oxygen occurs when the fraction of hemoglobin existing as methemoglobin exceeds 15%. Most patients are asymptomatic until the methemoglobin fraction is greater than 20% to 30%. At this point fatigue, headache, tachycardia, dizziness, and weakness develop. At fractions greater than 45%, dyspnea, bradycardia, hypoxia, metabolic acidosis, seizures, coma, and cardiac arrhythmias may occur. Fractions above 70% are rapidly fatal.1,2
Intravenous administration of methylene blue (1–2 mg/kg) as a 1% solution over 5 minutes quickly relieves cyanosis due to methemoglobinemia.Intravenous methylene blue is indicated for methemoglobin fractions over 30% and at lower fractions in patients with anemia or cardiovascular disease. The dose may be repeated if no clinical response is observed within 1 hour. A dose greater than 7 mg/kg of methylene blue by itself can cause methemoglobinemia.10 Supplemental oxygen should also be administered. Ascorbic acid has also been used in the treatment of methemoglobinemia, but its action is slower than that of methylene blue.
A patient is described who developed cyanosis while under general anesthesia due to methemoglobinemia. The cause of methemoglobinemia was benzocaine used topically. When confronted with cyanosis in the absence of cardiac or pulmonary disease, one must seriously consider the diagnosis of methemoglobinemia.
REFERENCES
- Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol. 1993;42:7–12. doi: 10.1002/ajh.2830420104. [DOI] [PubMed] [Google Scholar]
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- Spielman FJ, Anderson JA, Terry WC. Benzocaine-induced methemoglobinemia during general anesthesia. J Oral Maxillofac Surg. 1984;42:740–743. doi: 10.1016/0278-2391(84)90424-5. [DOI] [PubMed] [Google Scholar]
- Ferraro-Borgida MJ, Mulhern SA, DeMeo MO, Bayer MJ. Methemoglobinemia from perineal application of an anesthetic cream. Ann Emerg Med. 1996;27:785–788. doi: 10.1016/s0196-0644(96)70203-2. [DOI] [PubMed] [Google Scholar]
- Novaro GM, Aronow HD, Militello MA, Garcia MJ, Sabik EM. Benzocaine-induced methemoglobinemia: experience from a high-volume transesophageal echocardiography laboratory. J Am Soc Echocardiogr. 2003;16:170–175. doi: 10.1067/mje.2003.5. [DOI] [PubMed] [Google Scholar]
- Clary B, Skaryak L, Tedder M, Hilton A, Botz G, Harpole D. Methemoglobinemia complicating topical anesthesia during bronchoscopic procedures. J Thorac Cardiovasc Surg. 1997;114:293–295. doi: 10.1016/S0022-5223(97)70163-6. [DOI] [PubMed] [Google Scholar]
- Klos CP, Hays GL. Prilocaine-induced methemoglobinemia in a child with Shwachman syndrome. J Oral Maxillofac Surg. 1985;43:621–623. doi: 10.1016/0278-2391(85)90133-8. [DOI] [PubMed] [Google Scholar]
- Warren RE, Van de Mark TB, Weinberg S. Methemoglobinemia induced by high doses of prilocaine. Oral Surg. 1974;37:866. doi: 10.1016/0030-4220(74)90439-3. [DOI] [PubMed] [Google Scholar]
- Sinisterra S, Miravet E, Alfonso I, Soliz A, Papazian O. Methemoglobinemia in an infant receiving nitric oxide after use of eutectic mixture of local anesthetic. J Pediatr. 2002;141:285–286. doi: 10.1067/mpd.2002.125732. [DOI] [PubMed] [Google Scholar]
- Allegaert K, Miserez M, Lerut T, Naulaers G, Vanhole C, Devlieger H. Methemoglobinemia and hemolysis after enteral administration of methylene blue in a preterm infant: relevance for pediatric surgeons. J Pediatr Surg. 2004;39:35–37. doi: 10.1016/j.jpedsurg.2003.09.045. [DOI] [PubMed] [Google Scholar]