Abstract
A 57-year-old male with a documented history of obstructive sleep apnea with loud snoring received deep intravenous sedation with midazolam, fentanyl, ketamine, and propofol infusion and a left interscalene brachial plexus nerve block for a left biceps tendon repair. Loud snoring during the case was noted. On the second postoperative day, he was observed to have significant uvular edema. After due consideration of the various elements in the differential diagnosis, it was concluded that negative pressure trauma from deep snoring during the sedation was the most likely etiology.
Keywords: Uvular edema, Obstructive sleep apnea, Deep sedation, Negative pressure edema
Airway complications are typically not associated with regional anesthesia and monitored anesthetic care. In fact, these anesthetic strategies are often specifically used in patients with known difficult airways. However, anesthesiologists need to be cognizant that airway misadventures can and do occur when using monitored anesthetic care or regional anesthesia.
Brachial plexus nerve blocks (BPNBs), particularly the interscalene approach, can result in dyspnea or respiratory compromise. Hemidiaphragmatic paresis secondary to unilateral phrenic block is thought to accompany virtually all successful interscalene BPNBs. Recurrent laryngeal nerve block can lead to laryngeal musculature dysfunction. Cervical epidural, total spinal anesthesia, or seizures secondary to vertebral artery injection have all been reported during attempted interscalene BPNBs.
Patients with obstructive sleep apnea (OSA) are at increased perioperative risk of hypoxia especially after general anesthesia. Regional anesthesia is ideally suited for this patient population in that both intraoperative anesthesia and postoperative analgesia can be accomplished, thus avoiding general anesthesia and limiting the use of respiratory depressant medications (ie, opioids).
We present herein a patient with OSA who had a painful upper extremity procedure performed under interscalene BPNB. Although the intraoperative course was without incident, postoperatively the patient developed an airway complication that required medical intervention.
CASE REPORT
A 57-year-old Caucasian man with a body mass index of 28 (height 178 cm, weight 88.6 kg) presented for a left biceps tendon repair. His medical history was significant for asthma, OSA with loud snoring documented by a previous sleep study, hypercholesterolemia, and frequent alcohol use (8 beers daily). Medications at the time of admission were atorvastatin, aspirin, and albuterol inhaler. The patient denied any drug allergies. His surgical history was notable for an umbilical herniorraphy under subarachnoid block and molar teeth extraction without complications.
Vital signs at admission were blood pressure of 137/81 mm Hg, heart rate of 88 beats/min, respiratory rate of 16 breaths/min, and a room air pulse oximetry of 98%. The cardiac and pulmonary exams were unremarkable. The patient's airway was notable for a short neck with good range of motion, normal thyromental distance, a Malampati class II airway, multiple crowns, no pharyngeal erythema or exudates, and no rhinorrhea or recent upper respiratory tract infections.
After consent, and intravenous and monitor placement, midazolam (2 mg) and fentanyl (100 μg) were administered for sedation before placement of a peripheral nerve block. A left interscalene BPNB was placed by the neurostimulator technique with an insulated, 25-mm Stimuplex needle (B. Braun, Bethlehem, Pa). Aspiration for cerebrospinal fluid or blood was negative. A deltoid motor response was obtained at less than 0.6 mA. The motor response was extinguished with injection of a 1-mL local anesthetic test dose followed by 30 mL of a 1 : 1 solution (2% mepivacaine and 0.5% bupivacaine with 1 : 200,000 epinephrine) in divided doses with multiple negative aspirations. To ensure cutaneous anesthesia, a superficial cervical plexus block was placed with 5 mL of 2% lidocaine. Propofol (30 mg) was administered during block placement for supplemental sedation. Fifteen minutes after peripheral nerve block placement, the patient had complete motor and sensory nerve blockade (including the ulnar nerve). There were no signs or symptoms of dyspnea (secondary to phrenic nerve block). The patient was noted to have a marked change in phonation (recurrent laryngeal nerve blockade) and Horner syndrome. Vital signs were stable with pulse oximetry of 98% on 2 L/min nasal cannula oxygen.
The patient was transported to the operative theater, and the American Society of Anesthesiologists standard monitors and a BIS monitor (Aspect Medical Systems Inc, Newton, Mass) were reapplied. The patient requested additional sedation at this time, and a small dose of ketamine (25 mg) was administered while a propofol infusion (100 μg/kg/min) was initiated. An upper extremity tourniquet was inflated to 275 mm Hg. The patient was comfortable at skin incision. Sedation was maintained with the propofol infusion and intermittent ketamine bolus doses. A total of 90 mg ketamine was administered during the 130-minute case. No antibiotics or other medications were administered throughout the case. Although loud snoring was observed throughout the case, the respiratory rate remained stable at 10–14 breaths/min. No periods of apnea were noted. Pulse oximetry varied between 96% and 98% intraoperatively. The BIS monitor analysis was maintained between 86 and 90. There were no airway manipulations (eg, nasal or oral airway device) or suctioning of the airway during the case. The patient complained of a hoarse voice and mild difficulty swallowing while in the postoperative care unit. Oral and pharyngeal examination, including of the uvula, was without change compared with the preoperative examination. The patient required no supplemental analgesia or other medications in the postoperative care unit and was discharged home in stable condition.
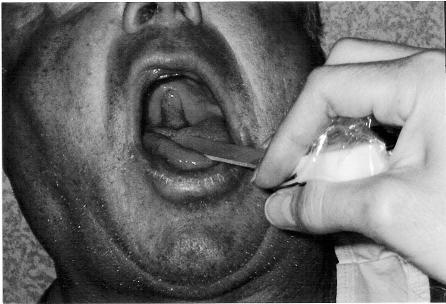
The patient returned on the second postoperative day with a chief complaint of a painless mass in the back of his throat. The hoarseness had resolved on the first postoperative day. However, he had noticed that a pharyngeal mass had progressively enlarged since resolution of the pharyngeal sensory nerve blockade. The patient denied dyspnea or any other complaints. He was afebrile with blood pressure of 139/81 mm Hg, heart rate of 96 beats/min, respiratory rate of 16 breaths/ min, and a pulse oximetry of 97% on room air. Physical examination demonstrated an elongated, erythematous, and edematous uvula (see Figure). The remainder of the oropharyngeal tissue was normal and without erythema or exudates. There was no cervical lymphadenopathy. The interscalene BPNB had complete resolution after 24 hours. In the interim, the patient had taken 2 acetaminophen-hydrocodone tablets (Vicodin) for analgesia on the evening of the first postoperative day. He reported that the pharyngeal swelling was present before the Vicodin.
The patient's uvular edema and erythema on postoperative day 2. Note that the uvula has been retracted anteriorly and inverted superiorly for photographic purposes.
The patient was treated with nebulized aerosol of albuterol and racemic epinephrine along with dexamethasone (8 mg intravenously). The uvula was unchanged in appearance after this intervention. The patient was issued prescriptions for azithromycin (500 mg orally followed by 250 mg orally for 5 days) and methylprednisolone (4 mg orally for 7 days). He refused hospital admission from the ambulatory surgery center and was therefore discharged to home with soft diet and hydration instructions. He was reevaluated on the seventh postoperative day. The uvula had returned to normal appearance and architecture without erythema or exudates. The patient reported no respiratory difficulties during the entire postoperative period.
DISCUSSION
Edema of the uvula or pharyngeal tissues can result in a compromised airway. We report a case of uvular edema that occurred without any airway manipulation or known pharyngeal trauma. The differential diagnosis consisted of trauma, infectious disease, drug reaction, substance abuse, and mechanical etiologies (eg, snoring). Interscalene BPNB can result in respiratory compromise caused by phrenic nerve paresis. It is unlikely that the interscalene BPNB played a role in the complication observed in this case because uvular edema is not associated with this anesthetic technique. However, the anesthetic management (ie, sedation) probably did contribute to the airway complication.
The uvula is susceptible to both mechanical and thermal trauma. Anesthesiologists must be vigilant during airway manipulation and insertion of devices to prevent injury to the uvula. Additionally, head positioning, especially forced neck flexion, during surgery has been reported to cause airway edema and compromise.1,2 Patients may cause uvula or pharyngeal injury by tooth brushing. Furthermore, the uvula may exhibit thermal trauma by contact with either very hot or very cold food and drinks. Sensory blockade of the oropharnyx may increase the susceptibility of patients to trauma. Trauma is not likely in this case because there was no airway manipulation of any kind.
Uvulitis resulting from infectious agents is a rare cause of life-threatening airway obstruction. The most common cause of uvulitis is epiglottitis, and both are frequently associated with dyspnea, pharyngitis, erythema of all pharyngeal structures, fever, and a toxic-appearing patient. The patient described herein did not exhibit these characteristics. Although an infectious etiology was considered not as likely as other causes, antibiotic therapy was instituted. Because of the consequences of airway compromise, the treating physician believed that the misdiagnosis of uvulitis and delaying of antibiotic therapy outweighed the risk of empiric antibiotics.
Angioedema of the uvula is most commonly associated with drug reactions. Angiotensin-converting enzyme inhibitors are known to produce life-threatening angioedema of the upper airway structures when administered to patients with C1 esterase inhibitor deficiency. Individuals with alcohol addictions can develop angioedema by mechanisms that are not yet elucidated. Recreational drug use when self-administered via inhalation of hot gases can result in thermal trauma that can be isolated to the uvula. Uvula edema has been well documented after marijuana use.3 Virtually any allergic reaction can result in angioedema and must be included in our differential diagnosis. Angioedema resulting from immune mechanisms does not seem likely in this case.
OSA is associated with significant morbidity and mortality. Snoring is considered the most prominent sign of sleep apnea, though snoring alone indicates only a variable partial airway obstruction. Morbidly obese patients have a greater prevalence of OSA, but obesity is not required to develop OSA. Early airway closure and hypoxia result in hypoxic pulmonary vasoconstriction, eventually resulting in pulmonary hypertension and right ventricular failure. Although continuous positive airway pressure devices can alleviate OSA, patient tolerance and compliance is problematic.4 In severe OSA, tracheostomy may be the only therapeutic option because of cardiopulmonary failure resultant from chronic, severe OSA.4 OSA can result from anatomic narrowing at various sites, with approximately a 75% decrease reported in the asleep state when compared with the awake state.5 Normally, the anterior uvula surface is keratinized and thus resistant to mechanical stresses, whereas the nonkeratinized posterior uvula is more susceptible to tissue edema and fibrosis.6
Airway edema during OSA is facilitated by Starling forces governing capillary fluid shifts. Typically, Starling forces create a gradient of 2 mm Hg favoring transudation of fluid from the vascular spaces into the interstitial space. The interstitial fluid is then returned to the circulation via lymphatic drainage. In patients with OSA, negative pressures of −28 cm H2O have been reported,7 a 10-fold increase in transudative forces. This is supported histiologically by the observation that edema8 and plasma cell infiltrates9 are noted in upper airway tissue from OSA patients. It is not known whether airway edema is from a causative mechanism of the obstruction or a consequence of OSA.8
In the case presented herein, the most likely explanation for the patient's postoperative presentation is that OSA and high negative mechanical pressure induced uvular edema. The primary mechanical cause of the uvular edema in this patient may be attributed to the sedative agents that allowed an exacerbation of airway collapse and pronounced snoring. The clinical evidence available does not support the other possibilities with this study's differential diagnosis. The lesson in this case is that patients with a history of snoring are at risk for airway compromise from (partial) airway obstruction even without any airway device or manipulation. Like negative pressure pulmonary edema, mechanical forces favor the formation of edema in upper airway structures. Sedation may increase this risk because the airway in the asleep state is smaller and more collapsible than in the awake state.
REFERENCES
- Bennett RL, Lee TS, Wright BD. Airway-obstructing supraglottic edema following anesthesia with the head positioned in forced flexion. Anesthesiology. 1981;54:78–80. doi: 10.1097/00000542-198101000-00016. [DOI] [PubMed] [Google Scholar]
- Choi JK, Goldman M, Koyal S, Clark G. Effect of jaw and head position on airway resistance in obstructive sleep apnea. Sleep Breath. 2000;4:163–168. doi: 10.1007/s11325-000-0163-1. [DOI] [PubMed] [Google Scholar]
- Mallat A, Roberson J, Brock-Utne JG. Preoperative marijuana inhalation—an airway concern. Can J Anaesth. 1996;43:691–693. doi: 10.1007/BF03017953. [DOI] [PubMed] [Google Scholar]
- Barthel SW, Strome M. Snoring, obstructive sleep apnea, and surgery. Med Clin North Am. 1999;83:85–96. doi: 10.1016/s0025-7125(05)70089-4. [DOI] [PubMed] [Google Scholar]
- Choi JK, Kee WC, Lee JM, Ye MK. Variable site of oropharyngeal narrowing and regional variations of oropharyngeal collapsibility among snoring patients during wakefulness and sleep. Cranio. 2001;19:252–259. doi: 10.1080/08869634.2001.11746176. [DOI] [PubMed] [Google Scholar]
- Olofsson K, Mattsson C, Hammarstrom ML, Hellstrom S. Structure of the human uvula. Acta Otolaryngol. 1999;119:712–717. doi: 10.1080/00016489950180685. [DOI] [PubMed] [Google Scholar]
- Farmer WC, Giudici SC. Site of airway collapse in obstructive sleep apnea after uvulopalatopharyngoplasty. Ann Otol Rhinol Laryngol. 2000;109:581–584. doi: 10.1177/000348940010900609. [DOI] [PubMed] [Google Scholar]
- Anastassov GE, Trieger N. Edema in the upper airway in patients with obstructive sleep apnea syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:644–647. doi: 10.1016/s1079-2104(98)90197-4. [DOI] [PubMed] [Google Scholar]
- Sekosan M, Zakkar M, Wenig BL, Olopade CO, Rubinstein I. Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope. 1996;106:1018–1020. doi: 10.1097/00005537-199608000-00021. [DOI] [PubMed] [Google Scholar]